Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database hold information on periodic tables, the discovery of the elements and elucidation of atomic weights (and more).
The nineteenth century saw great progress in chemistry, but there were problems with atomic weight and stoichiometry data. Oxygen was thought to have a weight of 7 or 8 (rather than 16), and this caused many systematic errors.
Cannizzaro's Letter of 1858 (below) contained largely correct atomic weight data and resolved most of the issues. However, the influential German-language reference journal Der Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften (The Annual Report on the Progress of Chemistry and Related Parts of Other Sciences) which systematically summarized international research findings took until 1873 to adopt the correct atomic weight values.
Once correct atomic weight values and the corresponding stoichiometric relationships were known and accepted, it was possible for other researchers to construct periodic tables.
| Year: 1803 | PT id = 4, Type = formulation element weight |
Dalton's Postulates About The Elements
Around the year 1803 in Manchester, John Dalton gave a series of lectures in which he presented his postulates:
- Elements are made of tiny particles called atoms.
- The atoms of a given element are different from those of any other element, and the atoms of different elements can be distinguished from one another by their respective relative atomic weigh/mass.
- All atoms of a given element are identical.
- Atoms of one element can combine with atoms of other elements to form chemical compounds, and a given compound always has the same relative numbers of types of atoms.
- Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process, and a chemical reaction simply changes the way atoms are grouped together.
From a very early notebook from around this time:

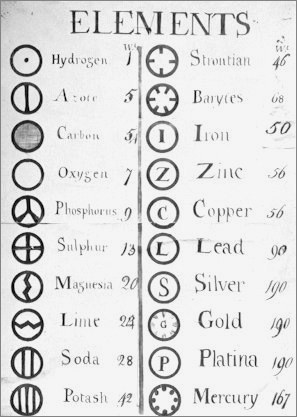
| Year: 1808 | PT id = 5, Type = formulation data element weight |
Dalton's Elements
Two pages from John Dalton's A New System of Chemical Philosophy in which he proposed his version of atomic theory based on scientific experimentation (see the scanned book, page 219):
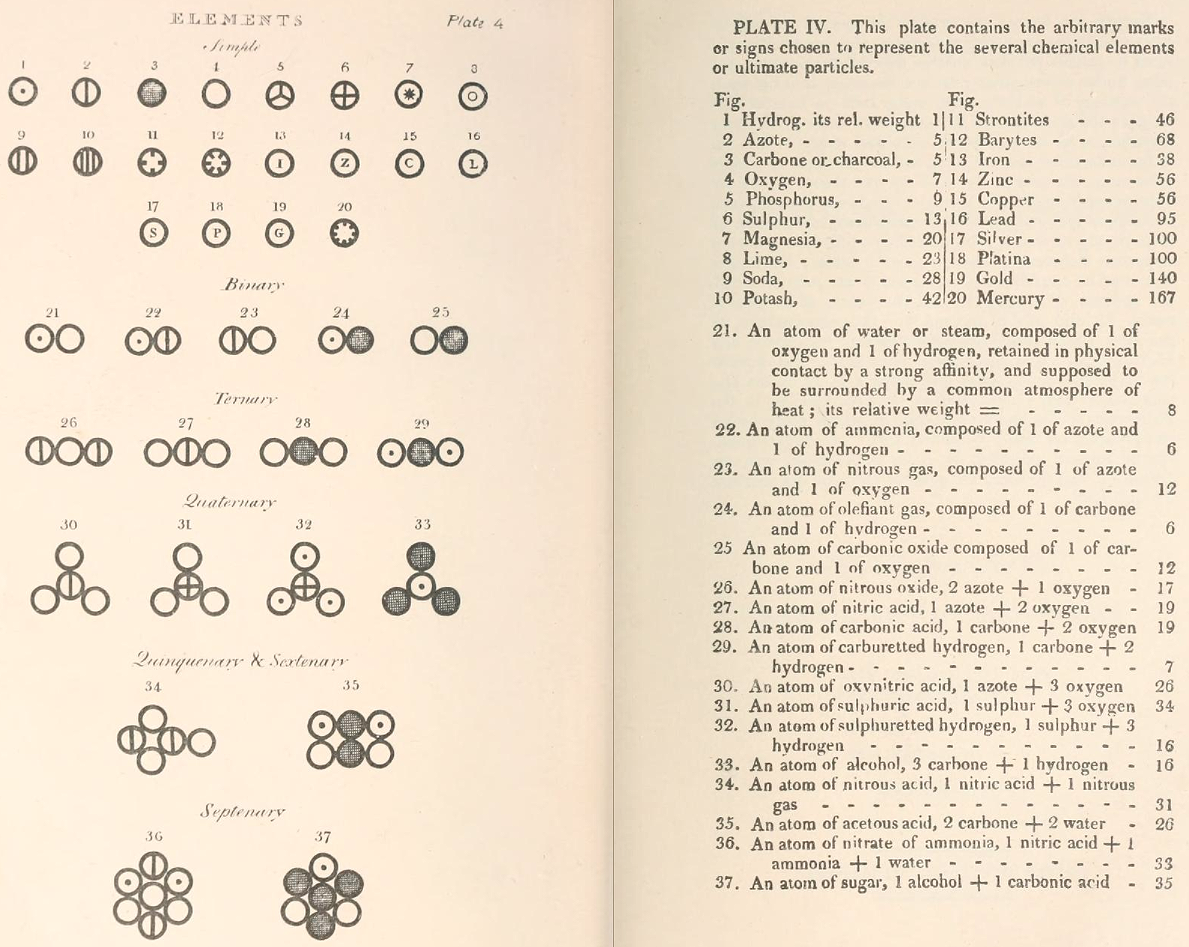
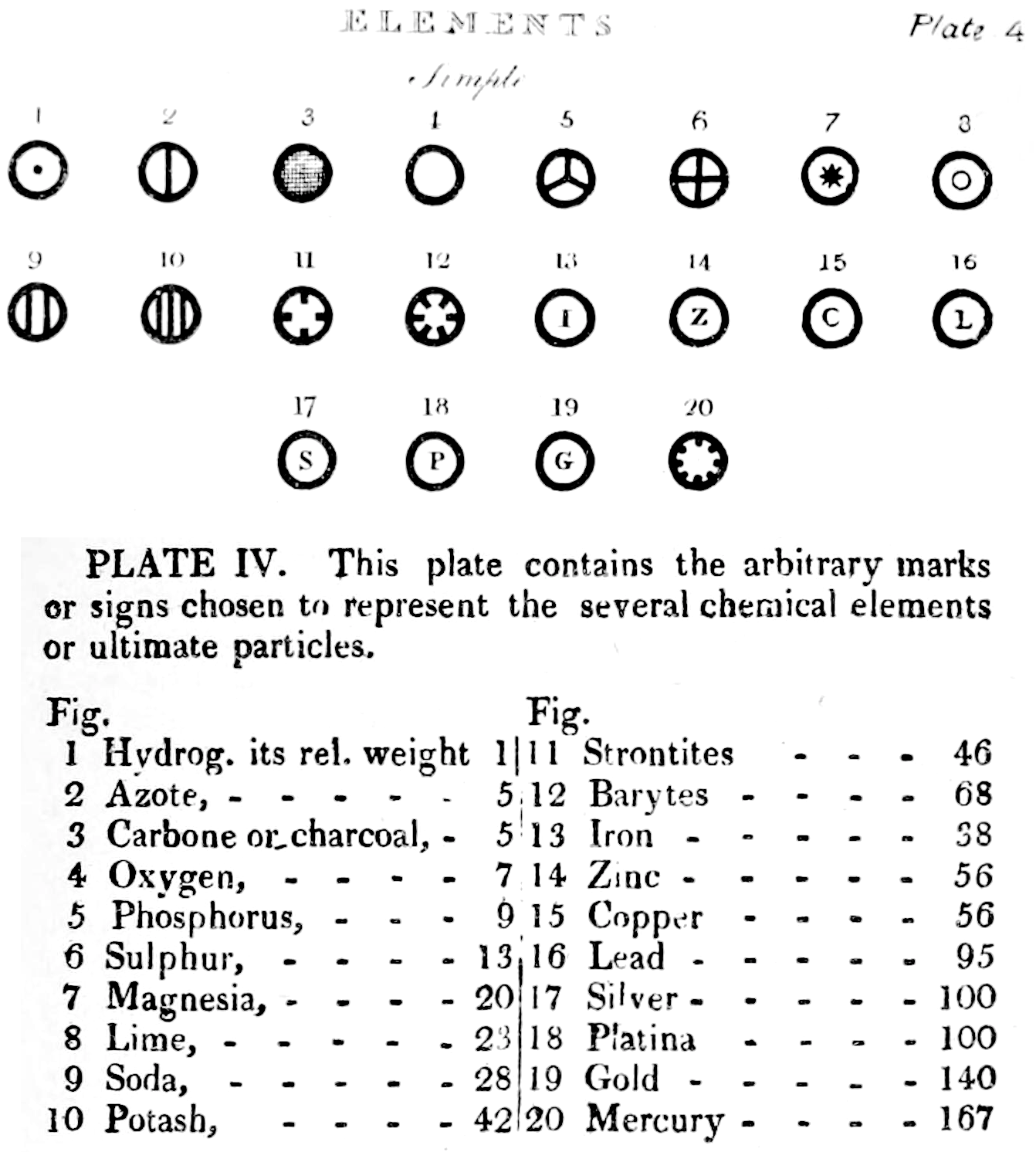
| Name | Modern Symbol | Dalton's Data | Modern Values | % error |
| Hydrog. | H | 1 | 1 | 0% |
| Azote | N | 5 | 14 | -180% |
| Carbone | C | 5 | 12 | -140% |
| Oxygen | O | 7 | 16 | -129% |
| Phosphorus | P | 9 | 31 | -244% |
| Sulphur | S | 13 | 32.1 | -147% |
| Magnesia | Mg | 20 | 24.3 | -22% |
| Lime | Ca | 24 | 40.1 | -67% |
| Soda | Na | 28 | 23 | 18% |
| Potash | K | 42 | 39.1 | 7% |
| Strontites | Sr | 46 | 87.6 | -90% |
| Barytes | Ba | 68 | 137.3 | -102% |
| Iron | Fe | 50 | 55.8 | -12% |
| Zinc | Zn | 56 | 65.4 | -17% |
| Copper | Cu | 56 | 63.5 | -13% |
| Lead | Pb | 90 | 200.6 | -123% |
| Silver | Ag | 190 | 107.9 | 43% |
| Gold | Au | 190 | 197 | -4% |
| Platina | Pt | 190 | 195.1 | -3% |
| Mercury | Hg | 167 | 200.6 | -20% |
- Dalton states that he is considering "chemical elements or ultimate particles"
- Dalton assigns hydrogen as having a relative weight of 1.
- Note the seemingly huge % errors in the atomic weights, compared with modern values.
- These errors occurred because while Dalton had deduced that atoms combine in fixed (stoichiometric) ratios in compounds, he not always know what the ratios were. Thus there were two unknowns: the atomic weights (masses) and the stoichiometric ratios.
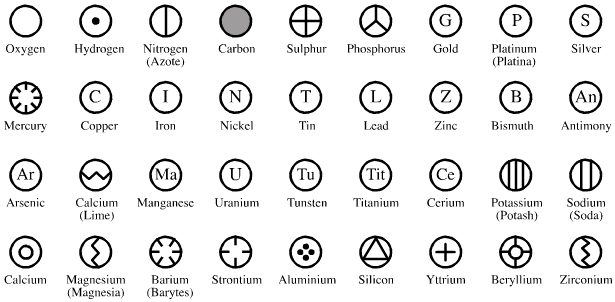
By Mark Leach
| Year: 1813 | PT id = 1044, Type = formulation element weight |
Wollaston's Synoptic Scale of Chemical Equivalents
Philosophical Transactions: A Synoptic Scale of Chemical Equivalents by William Hyde Wollaston, M.D. Sec. R.S., or from here.
It is apparent that chemistry the years 1810 to 1850 was largely concerned with discovering the whole number stoichiometric ratios of atoms in chemical compounds.
Wollaston writes in the text above:
"It is impossible in several instances, where only two combinations of the same ingredients are known, to discover which of the compounds is to be regarded as consisting of a pair of single atoms, and since the decision of these questions is purely theoretical, and by no means necessary to the formation of a table adapted to most practical purposes, I have not been desirous of warping my numbers according to an atomic theory, but have endeavored to make practical convenience my sole guide, and have considered the doctrine of simple multiples, on which that of atoms is founded, merely as a valuable assistant in determining, by simple division, the amount of those quantities that are liable to such definite deviations from the original law of Richter."
"Mr. Dalton in his atomic views of chemical combination appears not to have taken much pains to ascertain the actual prevalence of that law of multiple proportions by which the atomic theory is best supported [however] it is in fact to Mr. Dalton that we are indebted for the first correct observation of such an instance of a simple multiple in the union of nitrous gas with oxygen."
"[I have] computed a series of supposed atoms, I [have] assumed oxygen as the decimal unit of my scale [ie. oxygen = 10], in order to facilitate the estimation of those numerous combinations which it forms with other bodies. Though the present table of Equivalents, I have taken care to make oxygen equally prominent on account of the important part it performs in determining the affinities of bodies by the different proportions in which it is united to them.."
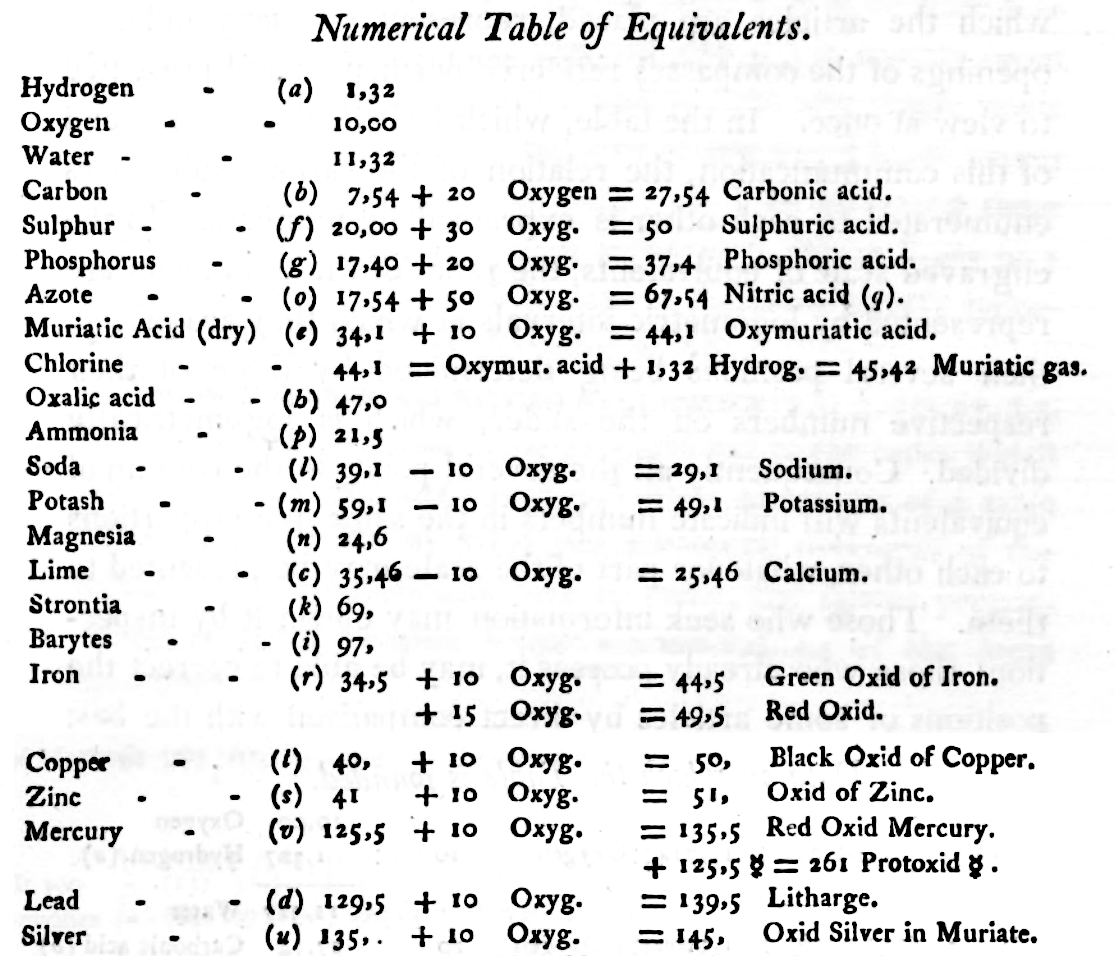
Mark Leach writes:
"When Wollaston's equivalent weights are converted from O = 10.00 to the modern value of O = 15.999, the atomic weight values can be seen to be astonishingly accurate.
"However, the language of the article is quite difficult as the meaning of many of the terms is unclear (to me, at least). For example, in modern usage adding 'ia' to a metal implies the oxide: 'magnesia' is magnesium oxide, MgO. I am not clear if this historical usage is consistent. 'Azote' is nitrogen and 'muriatic acid (dry)' is hydrogen chloride gas. I have only analyses/re-calculated the elements and a couple of common/obvious compounds:"
| Wollaston's data | Scaled to O = 15.999 | Modern Values | % error | |
| H (as H2) | 1.32 | 2.112 | 2.016 | 5% |
| O | 10.00 | 15.999 | 15.999 | ref. value |
| H2O | 11.32 | 18.111 | 18.015 | 1% |
| C | 7.74 | 12.383 | 12.011 | 3% |
| S | 20.00 | 31.998 | 32.060 | 0% |
| P | 17.40 | 27.838 | 30.974 | -11% |
| N (as N2) | 17.54 | 28.062 | 28.014 | 0% |
| Cl (as Cl2) | 44.10 | 70.556 | 70.900 | 0% |
| Fe | 34.50 | 55.197 | 55.845 | -1% |
| Cu | 40.00 | 63.996 | 63.546 | 1% |
| Zn | 41.00 | 65.596 | 65.380 | 0% |
| Hg | 125.50 | 200.787 | 200.590 | 0% |
| Pb | 129.50 | 207.187 | 207.980 | 0% |
| Ag | 135.00 | 215.987 | 107.870 | 50% |
- The elements hydrogen, nitrogen (azote) and chlorine have clearly been measured as the diatomic molecules, even if this was unknown to Wollaston in 1813.
- Phosphorus is out by 11%... [fair enough].
- Only silver is clearly wrong, but it is out by 50% so it looks like a simple stoichiometry error: Perhaps the oxide was assumed to be AgO was instead of the correct Ag2O.
Interestingly, Wollaston's analysis is far better than Daubeny's 1831 data seen in Oxford.
Read more in an entry concerning chemical slide rules.
Thanks to Nawa for the tip!
| Year: 1831 | PT id = 337, Type = formulation element weight |
Daubeny's Teaching Display Board & Wooden Cubes of Atomic Weights
The Museum of the History of Science, Oxford, has a display of Charles Daubeny's teaching materials, including a black painted wooden board with "SYMBOLS OF SIMPLE BODIES": showing symbols, atomic weights and names of elements in two columns, and a small pile of cubes with element symbols.
Charles Daubeny and Chemistry at the Old Ashmolean
Charles Daubeny (1795-1867) was appointed Aldrichian Professor of Chemistry at Oxford in 1822. In 1847 he moved from the original laboratory in this basement [in the museum] to a new one built at his own expense at the Botanic Garden. His apparatus went with him and was preserved there. Daubeny actively campaigned for the teaching of science in Oxford and held several professorships in addition to chemistry. He also conducted research on subjects such as photosynthesis.
From the HSM Database (Inventory no. 17504):
DAUBENY'S LIST OF ATOMIC WEIGHTS Wooden panel, black with white lettering, listing in two columns the symbols and names of twenty elements. This lecture board is identical to the table in the third edition (1831) of E. Turner, 'Elements of Chemistry', apart from the atomic weight for bromine. Daubeny wrote a useful 'Introduction to the Atomic Theory' (published in three versions: 1831, 1840, and 1850), the first edition of which also quotes Turner's table. Probably contemporary with this lecture board are the wooden cubes with the symbols for certain elements.
The period from 1810 to 1860 was crucial in the development of the periodic table. Most of the main group and transition elements had been discovered, but their atomic weights and stoichiometries (combining ratios) had not been fully deduced. Oxygen was assumed to have a weight of 6, and consequently carbon is assumed to have a mass of 6.
Daubeny's element symbols and weights – along with the modern mass data – are tabulated:
| Symbol | Daubeny's Weight | Modern Mass Data | % error | Stoichiometry Error |
| H | 1 | 1 | 0% | |
| C | 6 | 12 | -100% | factor of 2 |
| O | 8 | 16 | -100% | factor of 2 |
| Si | 8 | 28.1 | -251% | factor of 5 (?) |
| Al | 10 | 27 | -170% | factor of 3 |
| Mg | 12 | 24.3 | -103% | factor of 2 |
| N | 14 | 14 | 0% | |
| S | 16 | 32.1 | -101% | factor of 2 |
| P | 16 | 31 | -94% | factor of 2 |
| Fl | 19 | 19 | 0% | |
| Ca | 20 | 40.1 | -101% | factor of 2 |
| Na | 24 | 23 | 4% | |
| Fe | 28 | 55.8 | -99% | factor of 2 |
| Cl | 36 | 35.5 | 1% | |
| K | 40 | 39.1 | 2% | |
| Cu | 64 | 63.5 | 1% | |
| B | 80 | 79.9 | 0% | |
| Pb | 104 | 207 | -99% | factor of 2 |
| I | 124 | 127 | -2% | |
| Hg | 200 | 200.6 | 0% |
While quite a number of weights are close to the modern values, many are way out. However, the error is usually a stiotoimetric factor error.
From the HSM Database (Inventory no. 33732): SET OF WOODEN CUBES ILLUSTRATING ATOMIC WEIGHTS
Forty-two wooden cubes numbered 1-42, painted black with symbols for certain elements, compounds or radicals painted in white on the faces, together with the corresponding atomic, molecular or radical weights. The face markings appear in various combinations:
| H | C | P | Na | Ca° | S | N | K | Fe | K | Na° | Cy | K° |
| 1 | 6 | 16 | 24 | 28 | 16 | 14 | 40 | 28 | 48 | 32 | 26 | 48 |
A typical cube (no. 3) may be represented by the following figure. They present something of an enigma as their faces do not form an obvious pattern. The numbers indicate that there were 42 cubes. In style they are similar to the figures on the panel of atomic weights.
The cubes are listed in Daubeny's 1861 catalogue, p. 11 as: "Wooden cubes for illustrating atomic weight". [See D. R. Oldroyd, The Chemical Lectures at Oxford (1822-1854) of Charles Daubeny, M.D., F.R.S. Notes and Records of the Royal Society, vol. 33 (1979), pp. 217-259.]
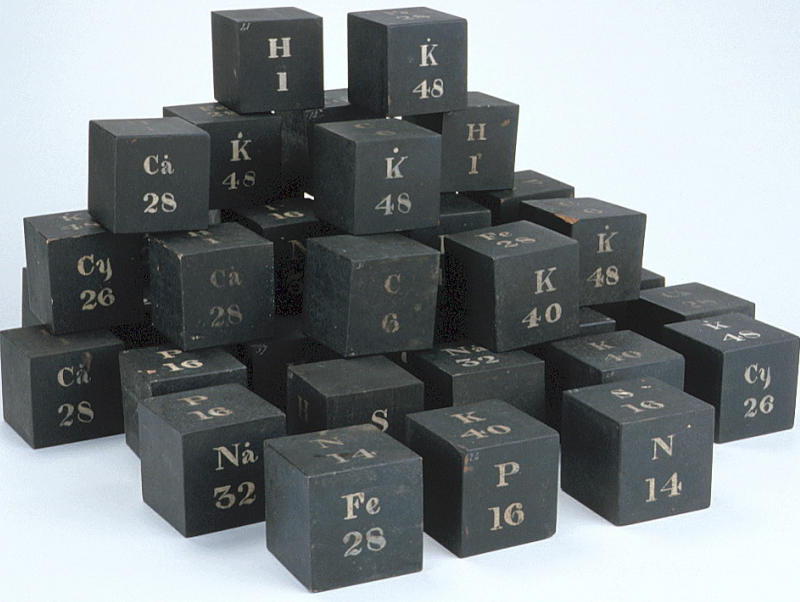
This display was spotted by Eric Scerri who was visiting the museum with Mark Leach in 2010.
There is a virtual tour on the museum, and the above display is in the basement.
| Year: 1850 | PT id = 474, Type = formulation element weight |
Elements Known in the Year 1850
Elements known in the year 1850, taken from this Wikipedia page:
| Year: 1858 | PT id = 1047, Type = formulation review element weight |
Cannizzaro's Letter
Letter of Professor Stanislao Cannizzaro to Professor S. De Luca: Sketch of a Course of Chemical Philosophy given in the Royal University of Genoa, Il Nuovo Cimento, vol. vii. (1858), pp. 321-366.

Many thanks to Carmen Giunta, Professor of Chemistry Emeritus, Le Moyne College who provided the information about, and link to, Cannizzaro's Letter. See a list of other classic chemistry papers.
Read the full letter/paper, in English translation, here. (The Italian version is here.)
"I believe that the progress of science made in these last years has confirmed the hypothesis of Avogadro, of Ampère, and of Dumas on the similar constitution of substances in the gaseous state; that is, that equal volumes of these substances, whether simple or compound, contain an equal number of molecules: not however an equal number of atoms, since the molecules of the different substances, or those of the same substance in its different states, may contain a different number of atoms, whether of the same or of diverse nature."
From the Science History of Science Institute:
"In 1858 Cannizzaro outlined a course in theoretical chemistry for students at the University of Genoa,where he had to teach without benefit of a laboratory. He used the hypothesis of a fellow Italian, Amedeo Avogadro, who had died just two years earlier, as a pathway out of the confusion rampant among chemists about atomic weights and the fundamental structure of chemical compounds."
Mark Leach writes:
"Before a periodic table of the chemical elements – which orders the elements by atomic weight and then groups them by property – could be developed it was necessary to know the atomic weight values. However, to deduce the atomic weights was a problem as it was necessary to know the ratios of how the elements combined, the stoichiometry.
"Tables of atomic weight data by Dalton (1808), Wollaston (1813), Daubeny (1831) and Kopp & Will (1858) show progress, but the 1858 Cannizzaro letter was the first where the atomic weight data is more or less both complete and accurate, thus removing stiochiometric errors.
"I have extracted the element atomic weight data from the paper, and given the % error with respect to modern atomic weight/mass data. Only titanium is significantly out! It is clear that Cannizzaron knew that hydrogen, nitrogen, oxygen, chlorine, bromine & iodine existed as diatomic molecules."
| Element | Symbol | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H | 1 | 1.008 | -0.8% |
| Boron | B | 11 | 10.81 | 1.7% |
| Carbon | C | 12 | 12.011 | -0.1% |
| Nitrogen | N | 14 | 14.007 | 0.0% |
| Oxygen | O | 16 | 15.999 | 0.0% |
| Sodium | Na | 23 | 22.99 | 0.0% |
| Magnesium | Mg | 24 | 24.305 | -1.3% |
| Aluminium | Al | 27 | 26.982 | 0.1% |
| Silicon | Si | 28 | 28.085 | -0.3% |
| Sulphur | S | 32 | 32.06 | -0.2% |
| Phosphorus | P | 32 | 30.974 | 3.2% |
| Chlorine | Cl | 35.5 | 35.45 | 0.1% |
| Potassium | K | 39 | 39.098 | -0.3% |
| Calcium | Ca | 40 | 40.078 | -0.2% |
| Chromium | Cr | 53 | 51.996 | 1.9% |
| Manganese | Mn | 55 | 54.938 | 0.1% |
| Iron | Fe | 56 | 55.845 | 0.3% |
| Titanium | Ti | 56 | 47.867 | 14.5% |
| Copper | Cu | 63 | 63.546 | -0.9% |
| Zinc | Zn | 66 | 65.38 | 0.9% |
| Arsenic | As | 75 | 74.922 | 0.1% |
| Bromine | Br | 80 | 79.904 | 0.1% |
| Zirconium | Zr | 89 | 91.224 | -2.5% |
| Silver | Ag | 108 | 107.87 | 0.1% |
| Tin | Sn | 117.6 | 118.71 | -0.9% |
| Iodine | I | 127 | 126.9 | 0.1% |
| Barium | Ba | 137 | 137.3 | -0.2% |
| Platinum | Pt | 197 | 195.08 | 1.0% |
| Mercury | Hg | 200 | 200.59 | -0.3% |
| Lead | Pb | 207 | 207.2 | -0.1% |
| Diatomic Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H2 | 2 | 2.016 | -0.8% |
| Oxygen | O2 | 32 | 31.998 | 0.0% |
| Sulphur | S2 | 64 | 64.12 | -0.2% |
| Chlorine | Cl2 | 71 | 70.9 | 0.1% |
| Bromine | Br2 | 160 | 159.808 | 0.1% |
| Iodine | I2 | 254 | 253.8 | 0.1% |
| Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Water | H2O | 18 | 18.015 | -0.1% |
| Hydrochloric Acid | HCl | 36.5 | 36.458 | 0.1% |
| Methane | CH4 | 16 | 16.043 | -0.3% |
| Hydrogen sulphide | H2S | 34 | 34.076 | -0.2% |
| Diethyl ether | CH3CH2OCH2CH3 | 74 | 74.123 | -0.2% |
| Carbon disulphide | CS2 | 76 | 76.131 | -0.2% |
| Chloroethane | CH3CH2Cl | 64.5 | 64.512 | 0.0% |
Below is a list of the elements showing which ones were included by Cannizzaro and which one were ommitted (because they had not been discovered) or are strangely missing. Odd ommissions (to the modern eye) include: Lithium, Beryllium, Cobalt, Nickel, Palladium, Tungsten and Gold.
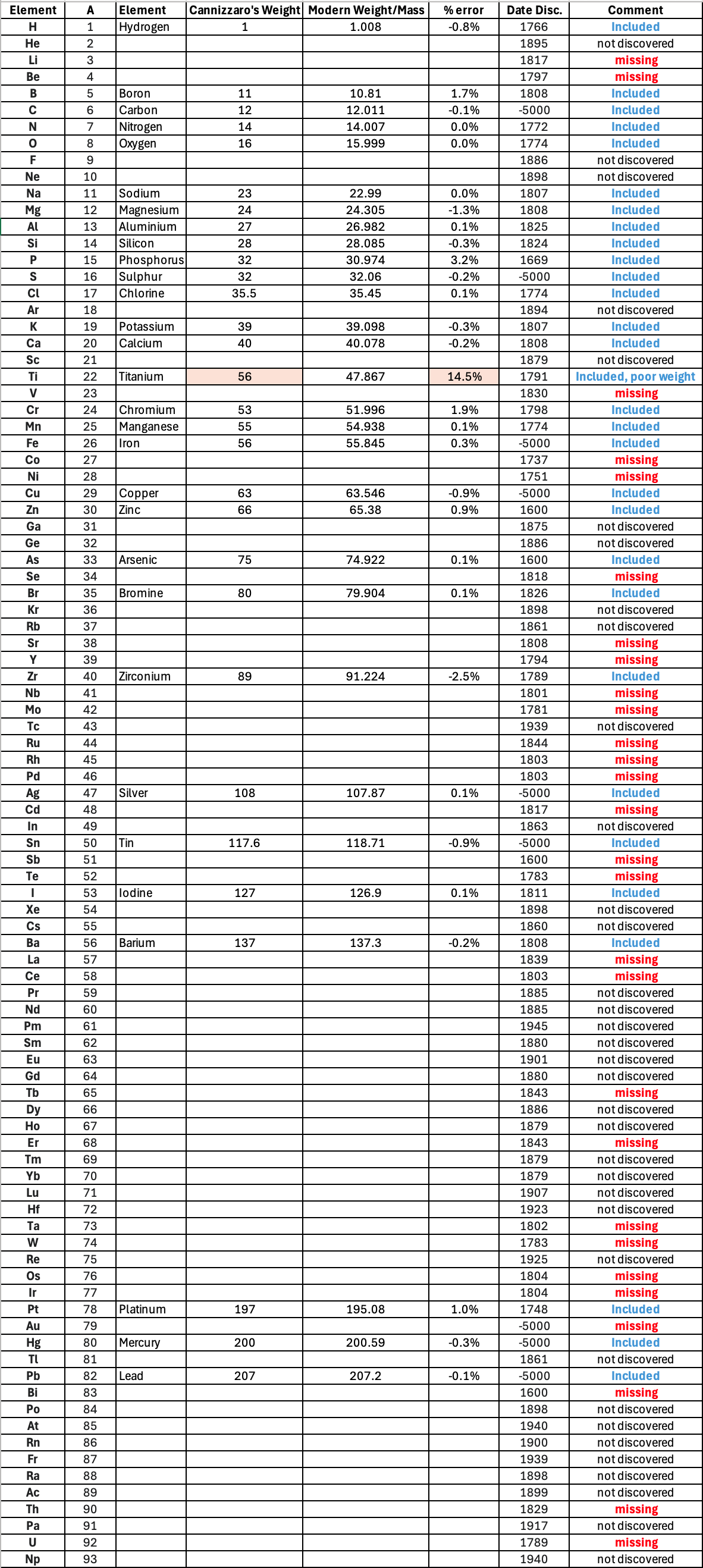
| Year: 1858 | PT id = 1348, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1858
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1858 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6
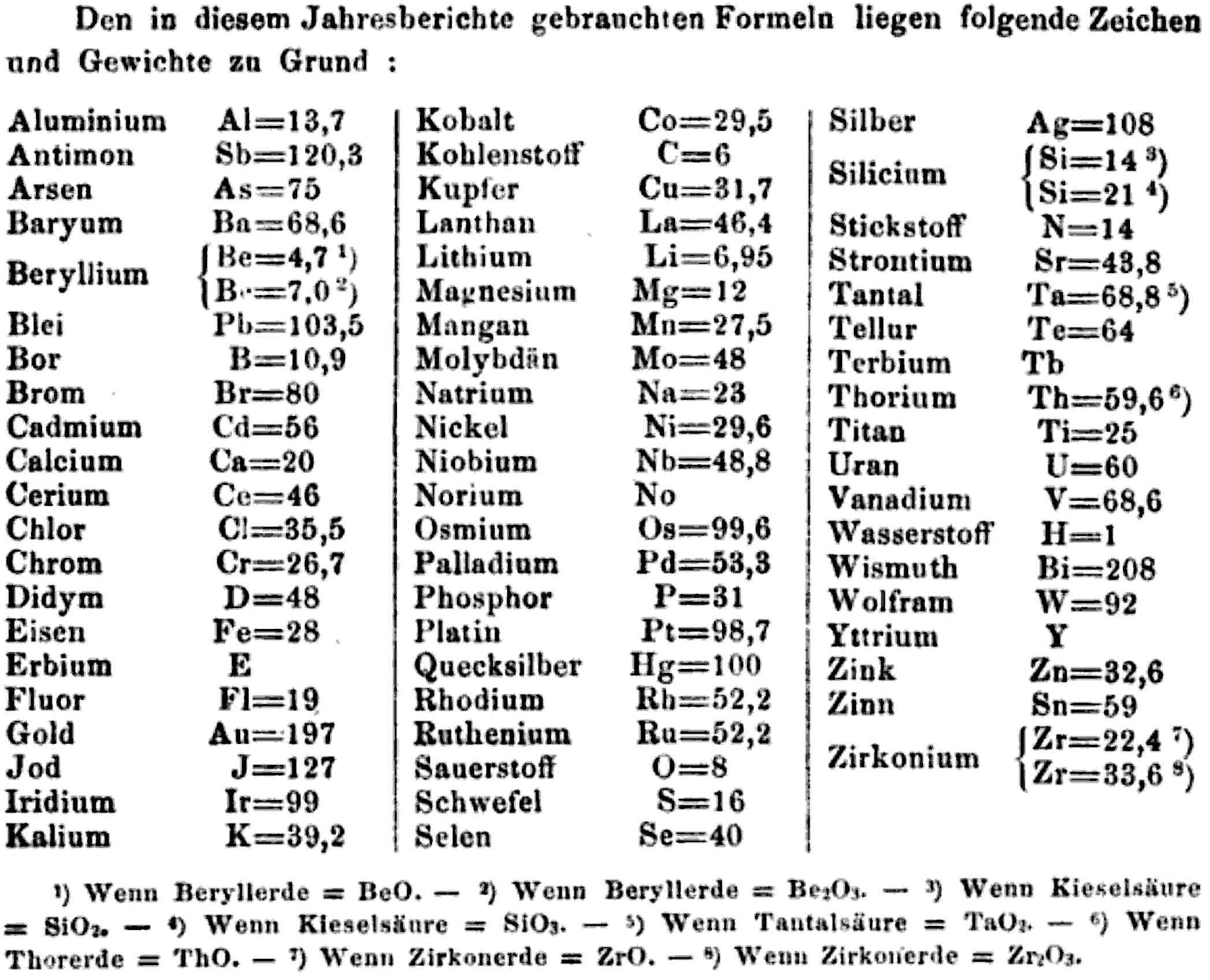
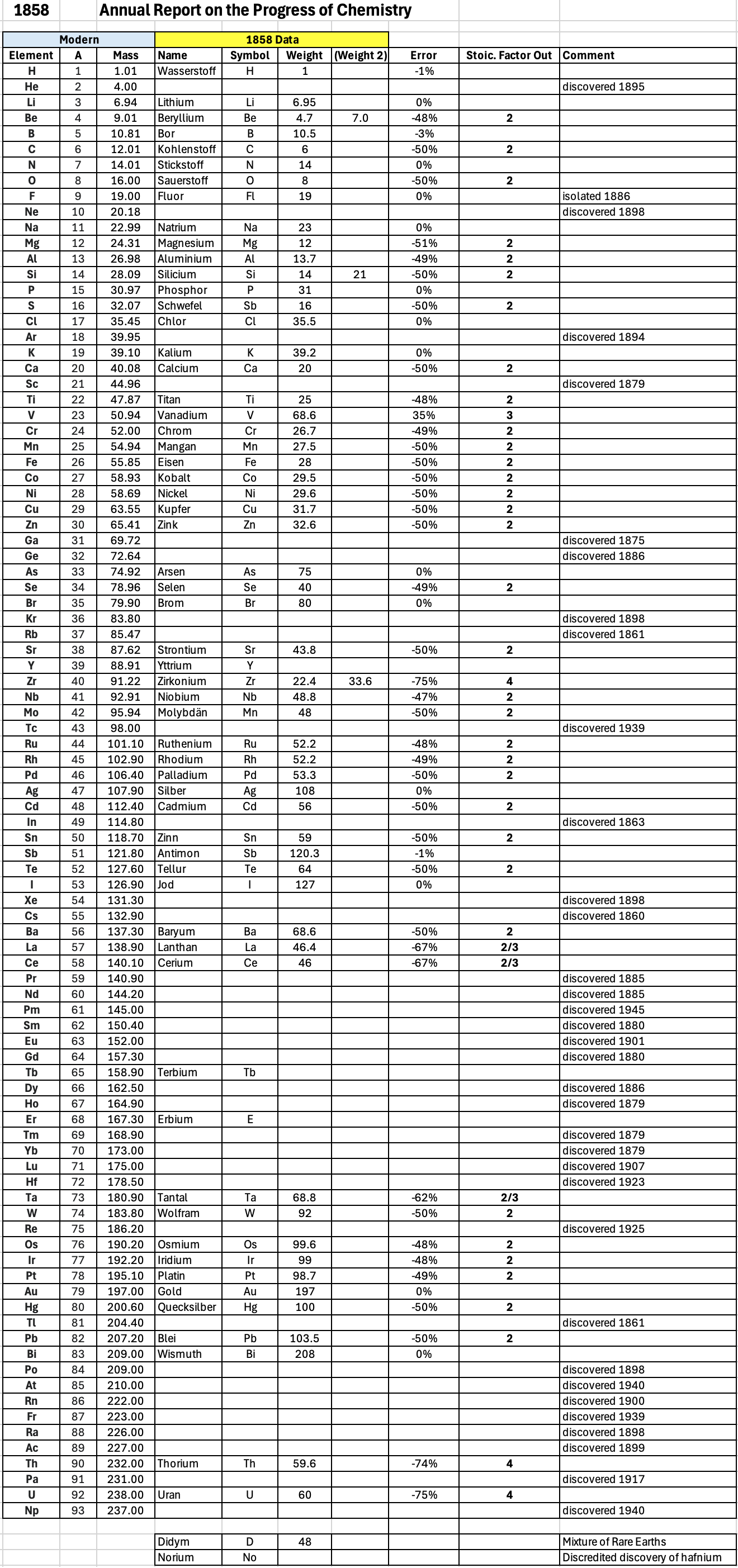
Thanks to René and Mario Rodriguez for the tip!
| Year: 1859 | PT id = 1349, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1859
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1859 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6

Thanks to René and Mario Rodriguez for the tip!
| Year: 1860 | PT id = 1350, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1860
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1860 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si =14 and 21
- Zr = 22.4, 33.6 and 44.8
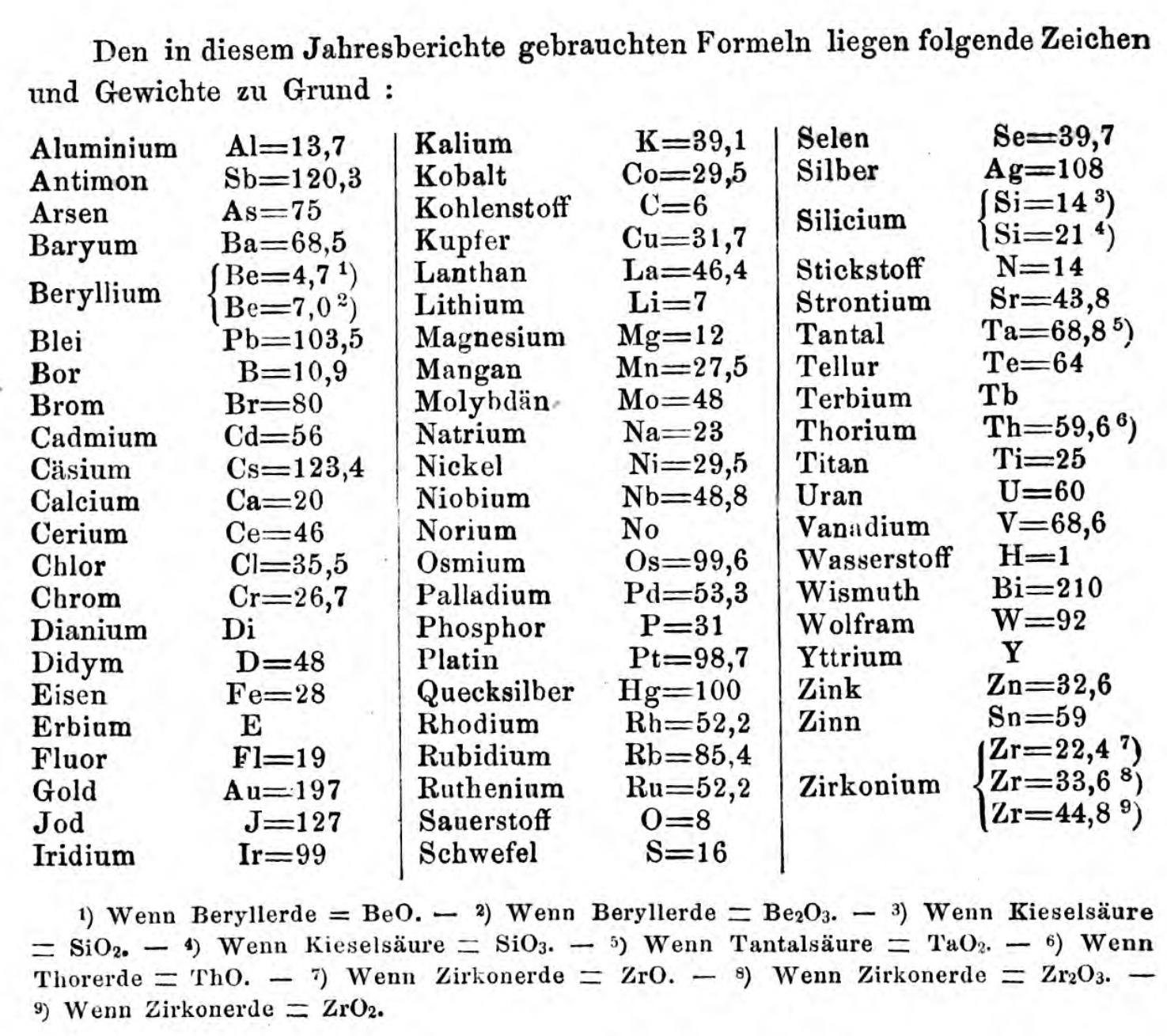
Thanks to René and Mario Rodriguez for the tip!
| Year: 1861 | PT id = 1351, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1861
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1861 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8
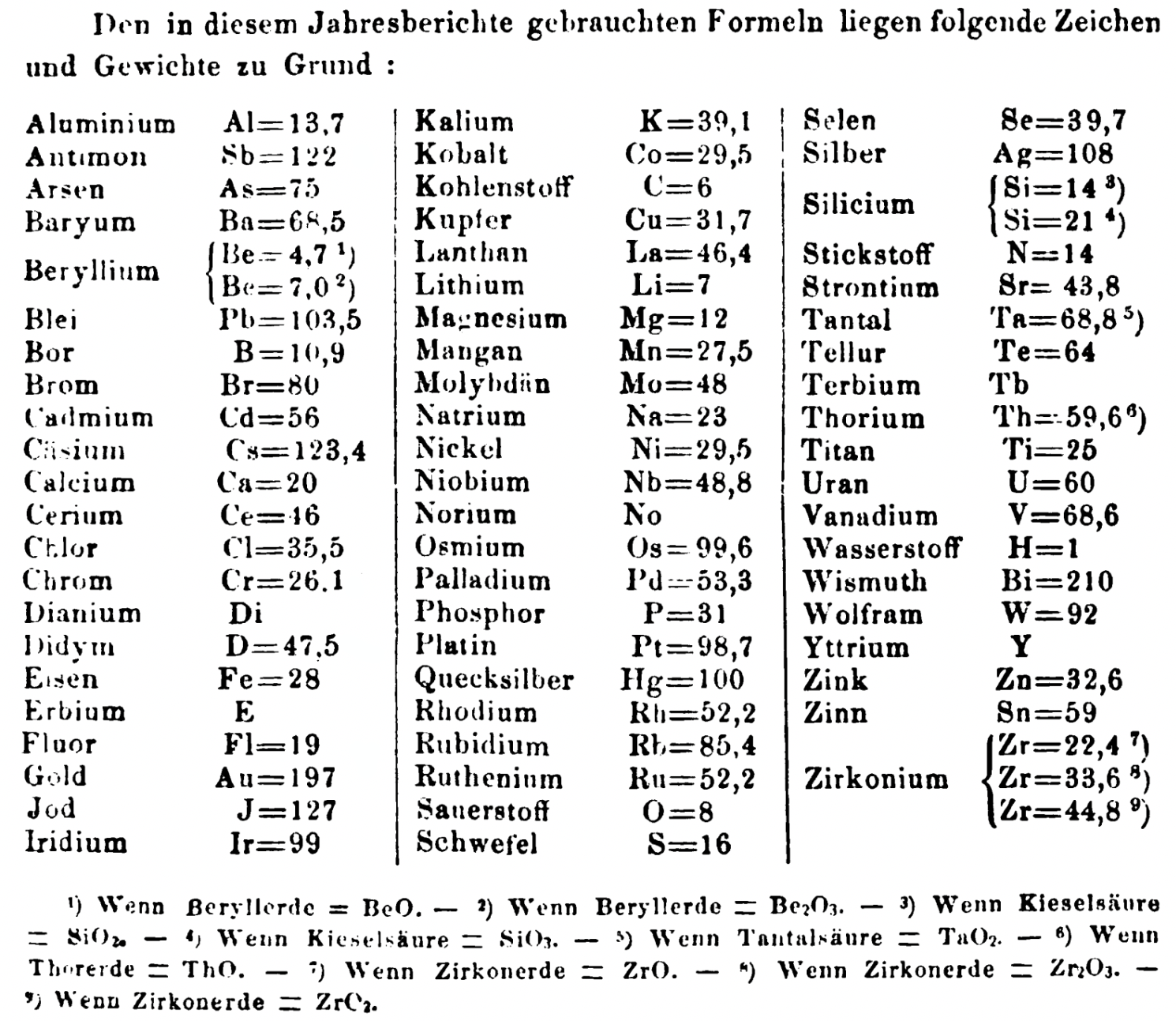
Thanks to René and Mario Rodriguez for the tip!
| Year: 1862 | PT id = 1352, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1862
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1862 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8

Thanks to René and Mario Rodriguez for the tip!
| Year: 1863 | PT id = 1353, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1863
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1863 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- C = 6 and 12
- Hg = 100 and 200
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Zr = 22.4 and 33.6 and 44.8
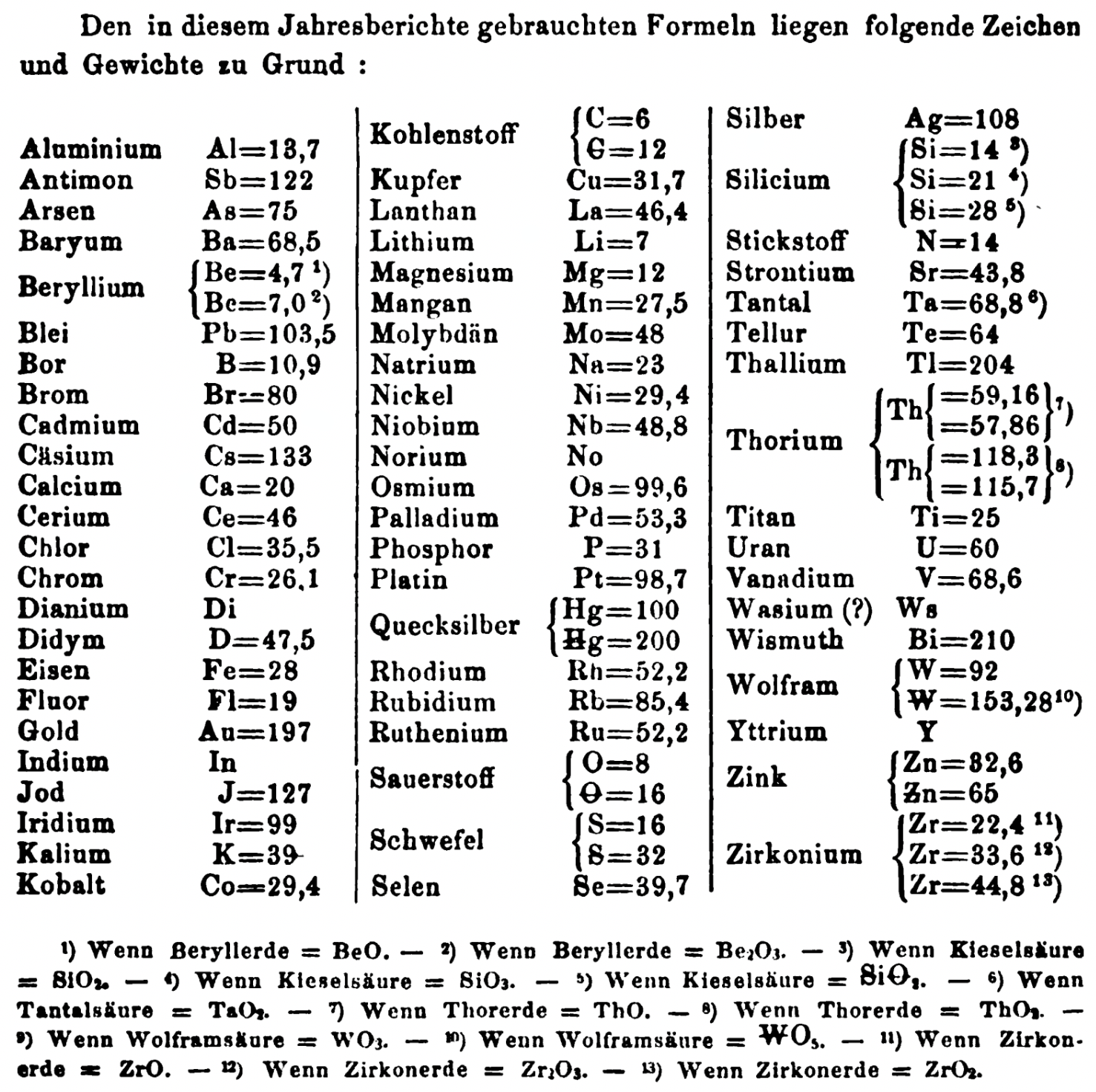
Thanks to René and Mario Rodriguez for the tip!
| Year: 1864 | PT id = 1354, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1864
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1864 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8
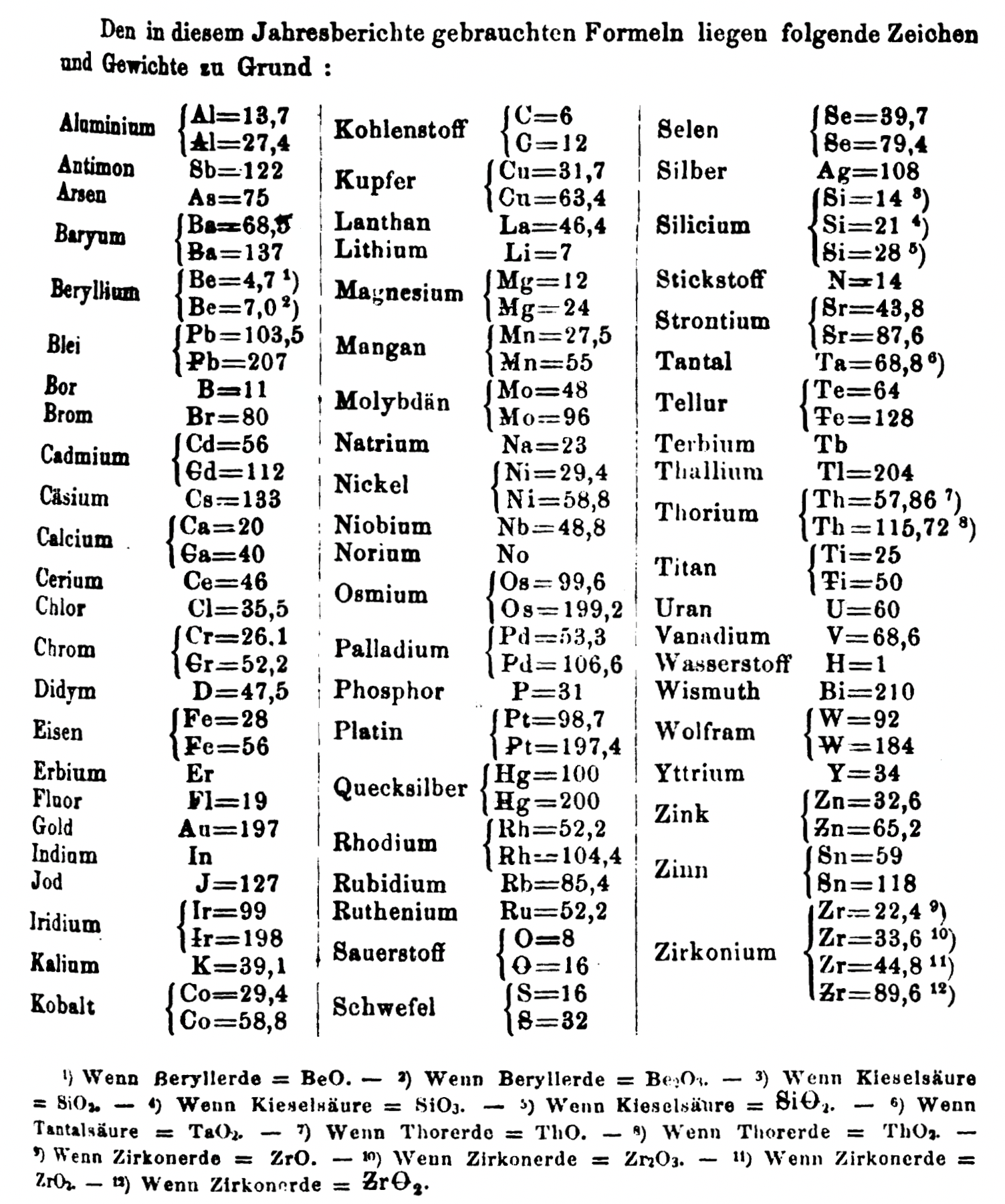
Thanks to René and Mario Rodriguez for the tip!
| Year: 1865 | PT id = 1355, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1865
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1865 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
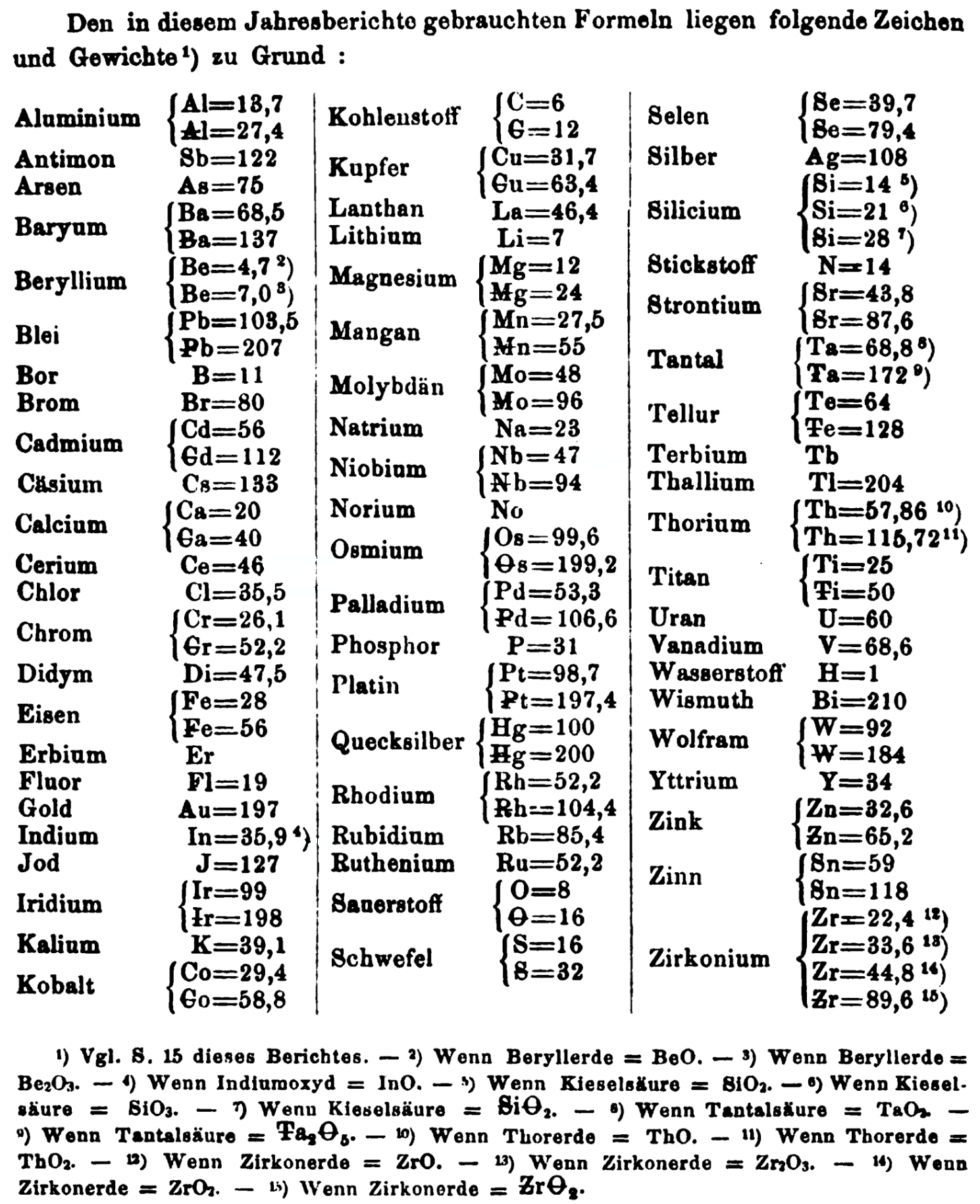
Thanks to René and Mario Rodriguez for the tip!
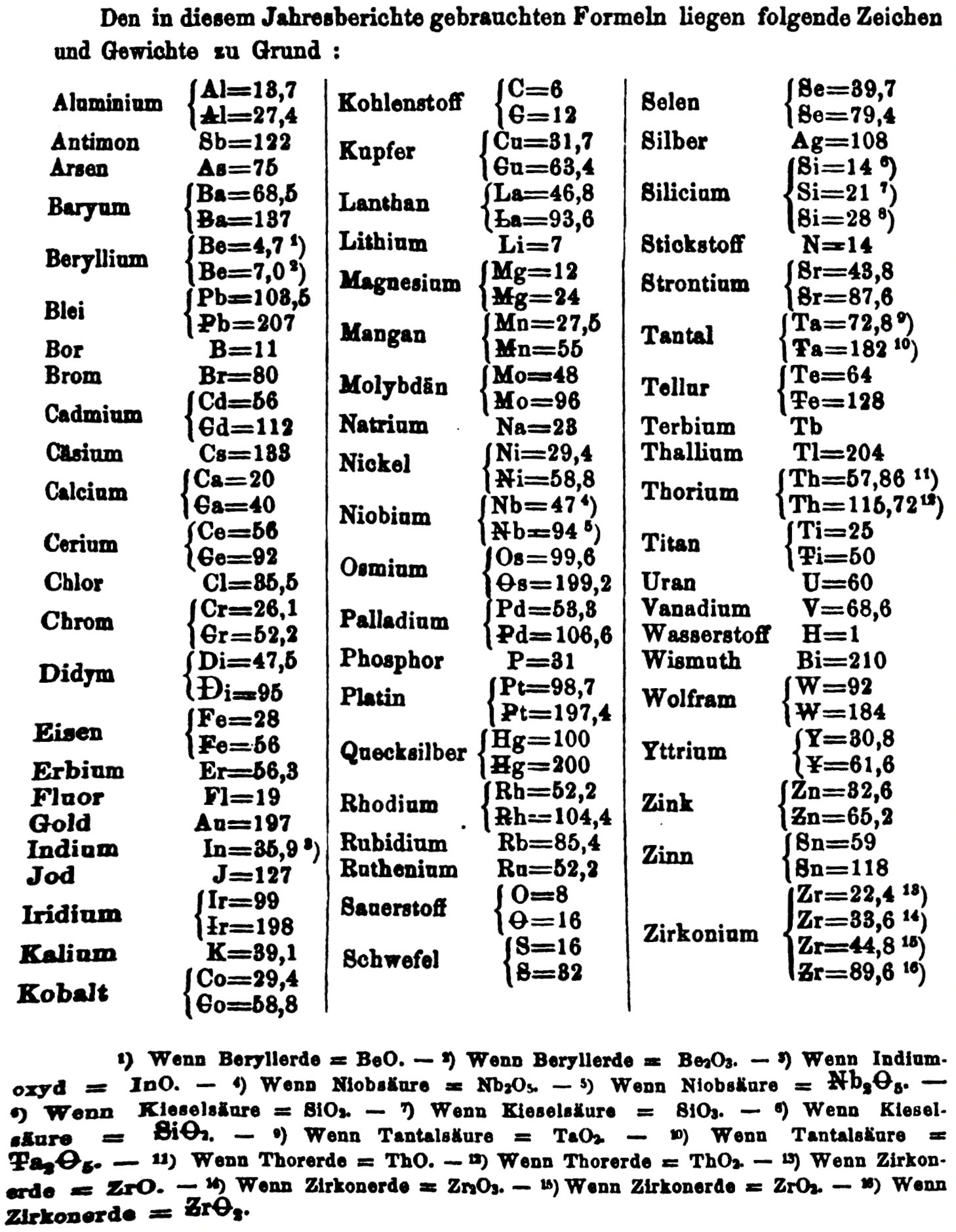
| Year: 1866 | PT id = 1356, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1866
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1866 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th = 57.86 and 115.72
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
Thanks to René and Mario Rodriguez for the tip!
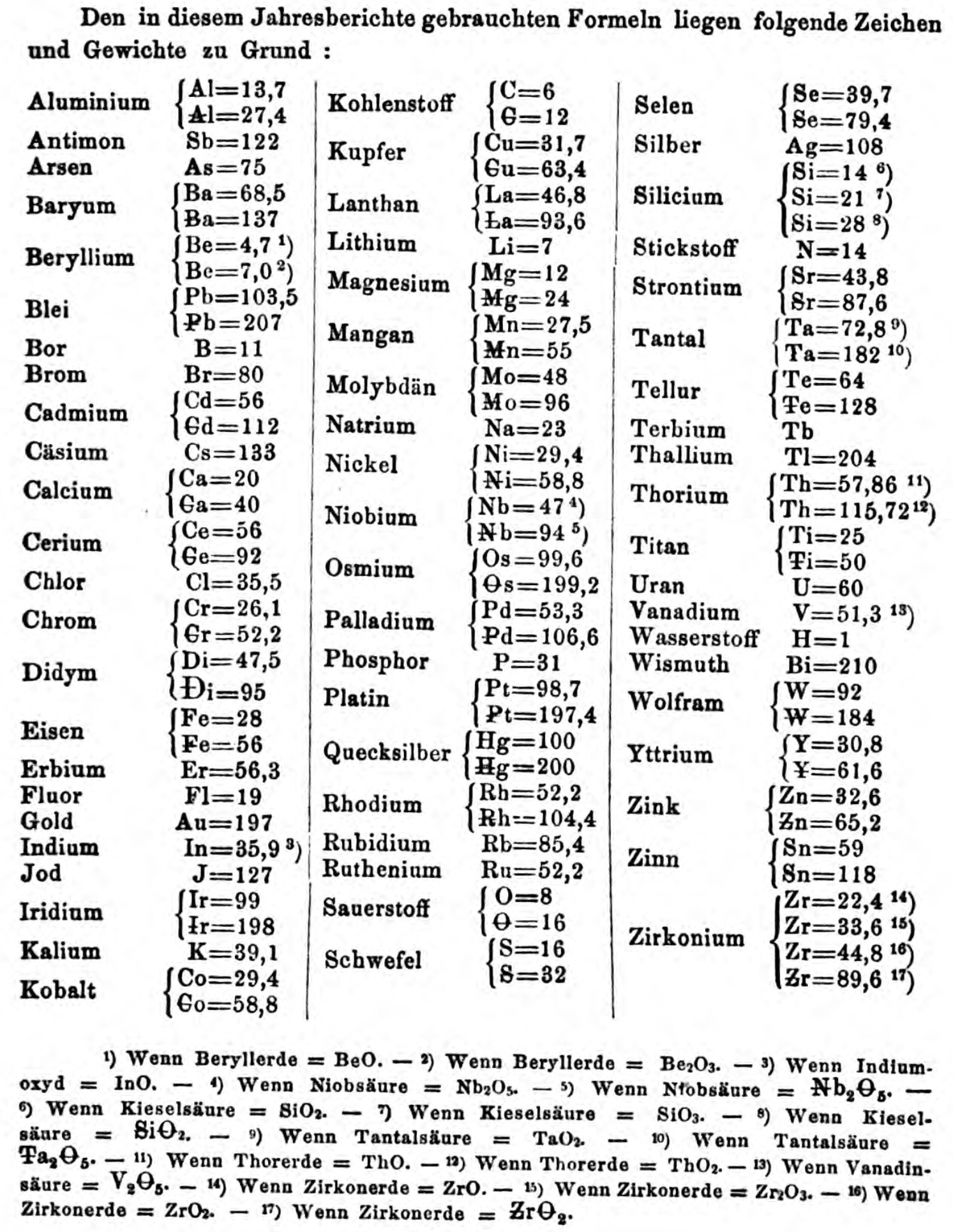
| Year: 1867 | PT id = 1357, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1867
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1867 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th =57.86 and 115.72
- W = 92 and 184
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
Thanks to René and Mario Rodriguez for the tip!
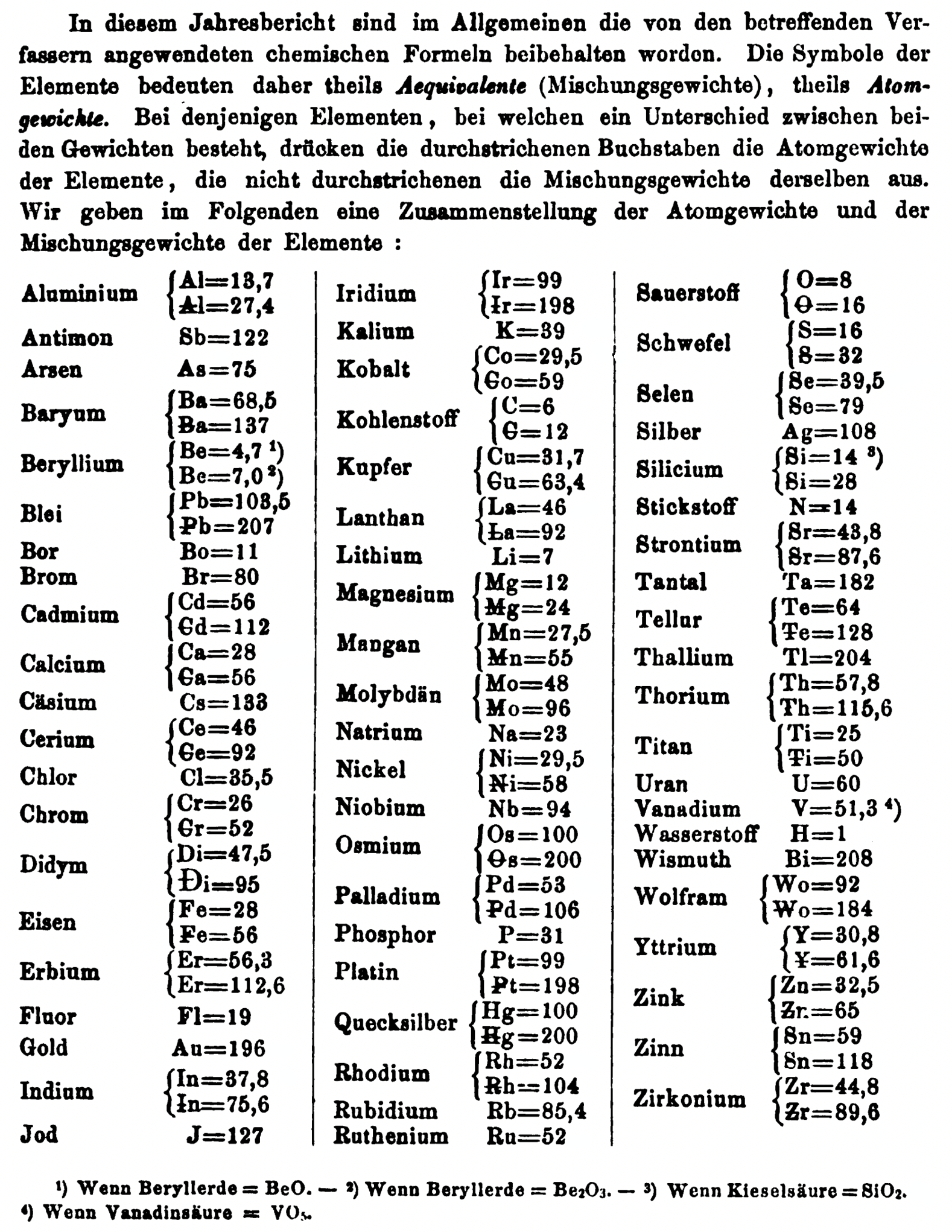
| Year: 1868 | PT id = 1358, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1868
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1868 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53.3 and 106.6
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 44.8 and 89.6
Thanks to René and Mario Rodriguez for the tip!
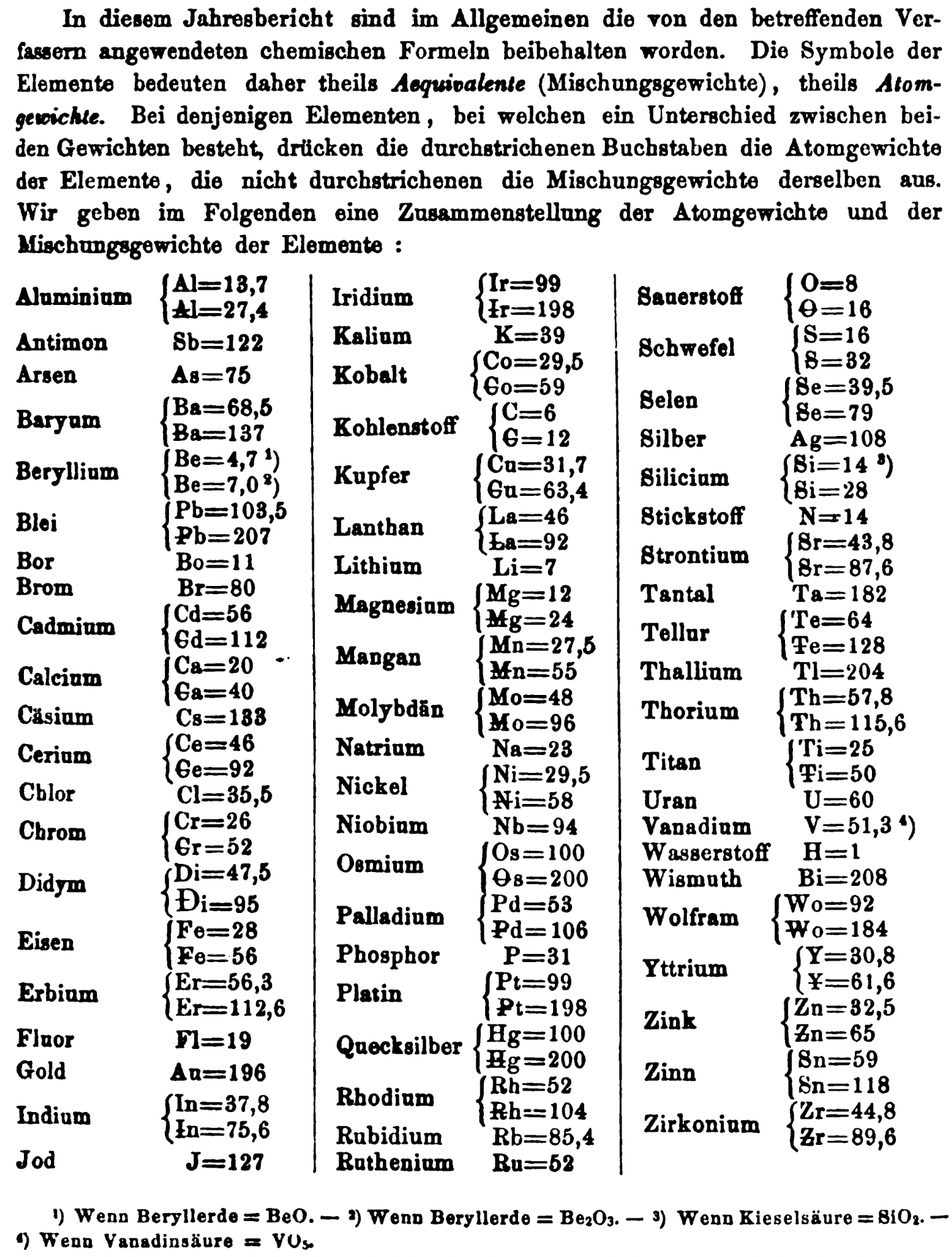
| Year: 1869 | PT id = 1359, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1869
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1869 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 44.8 and 89.6
Thanks to René and Mario Rodriguez for the tip!
| Year: 1870 | PT id = 1360, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1870
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1870 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
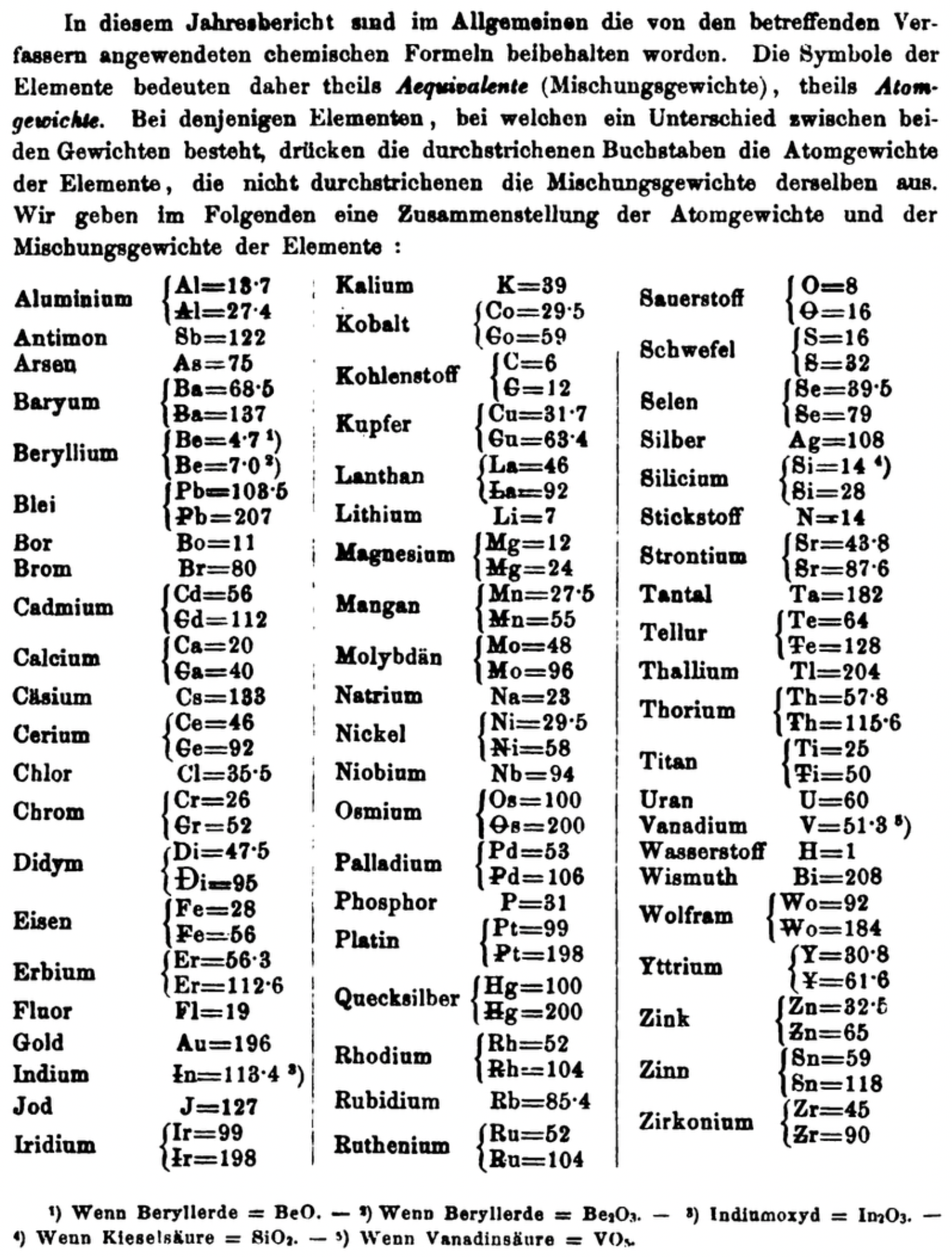
Thanks to René and Mario Rodriguez for the tip!
| Year: 1871 | PT id = 1361, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1871
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1871 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
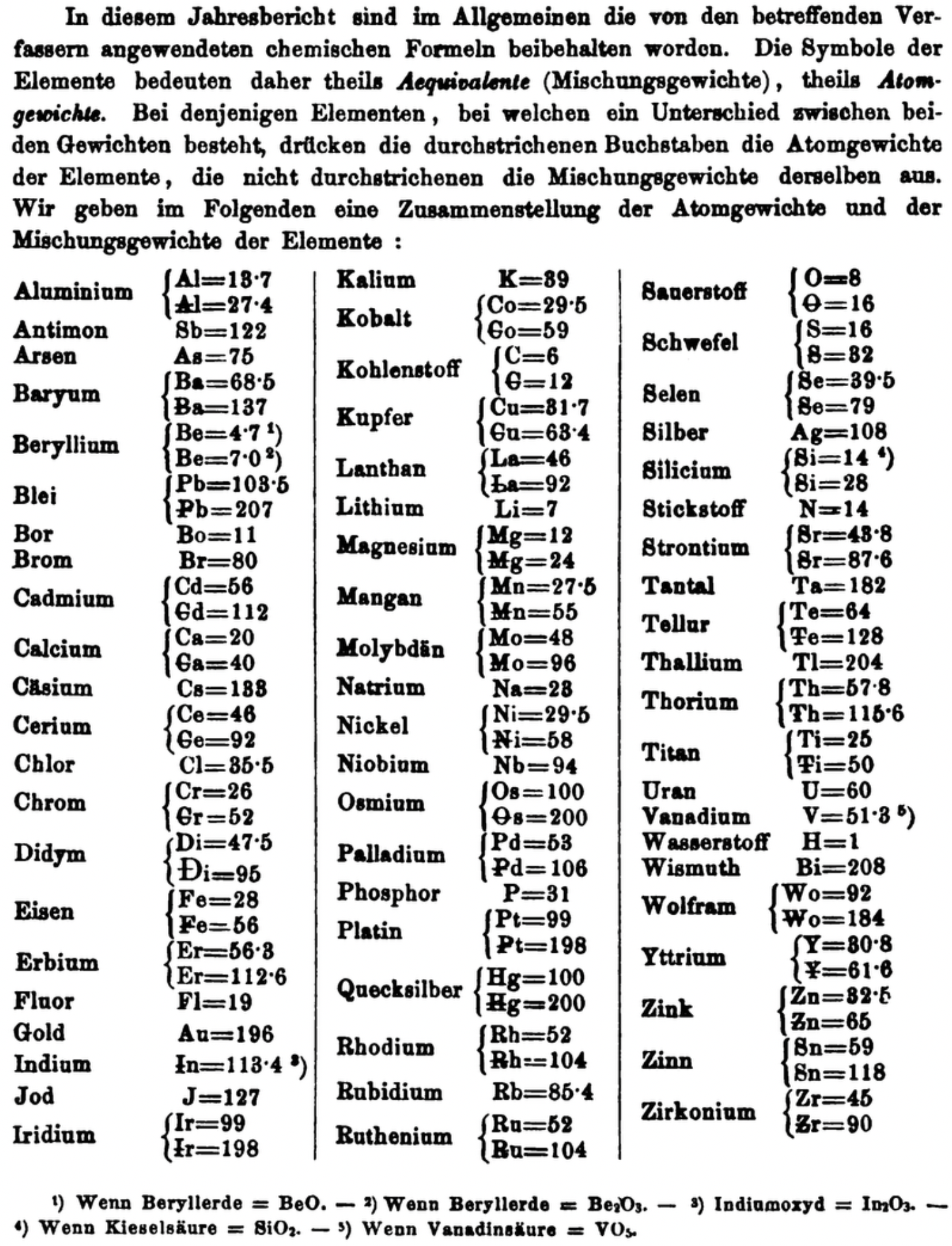
Thanks to René and Mario Rodriguez for the tip!
| Year: 1872 | PT id = 1362, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1872
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1872 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.5 and 59
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.8 and 115.6
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 29.8 and 59.7
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
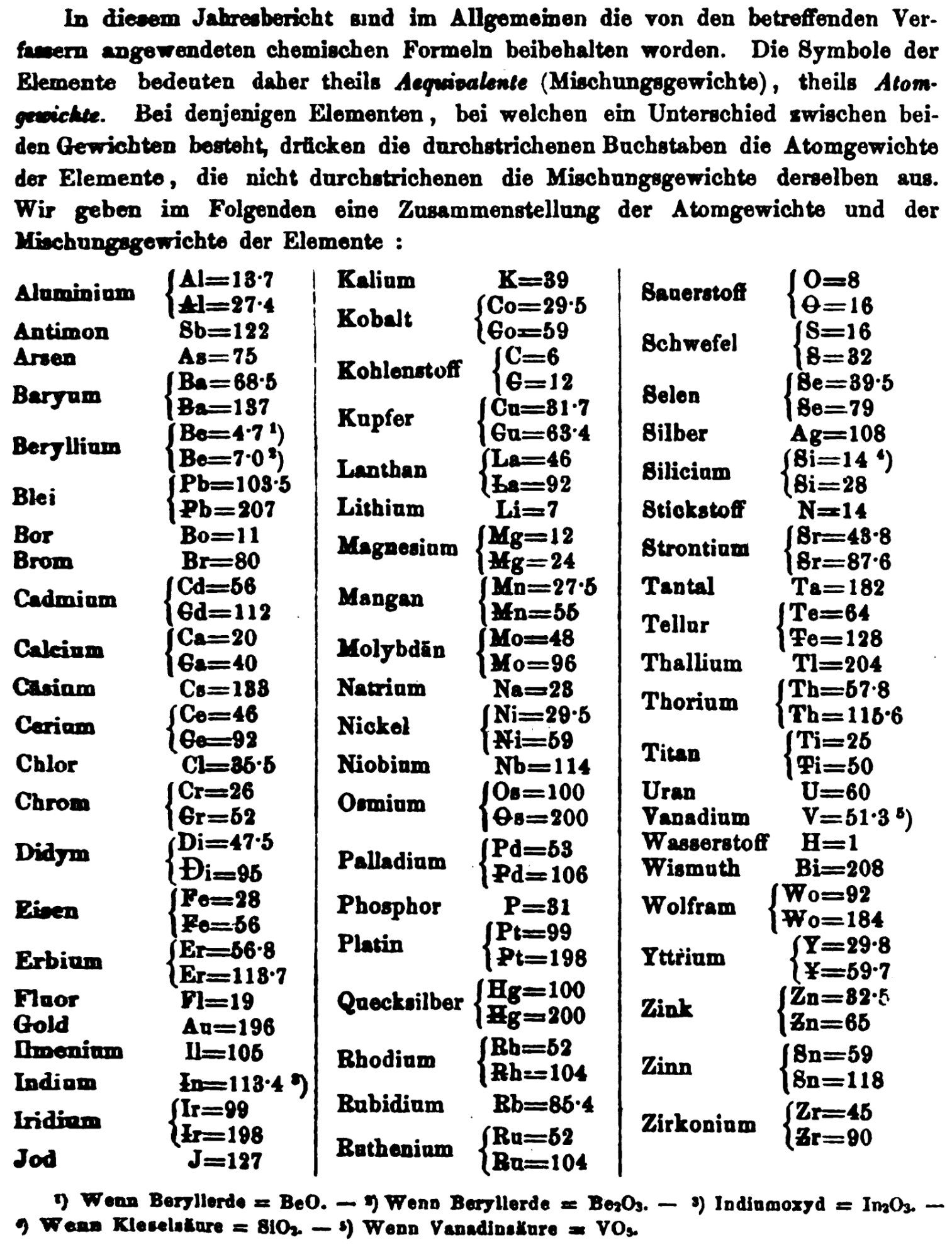
Thanks to René and Mario Rodriguez for the tip!
| Year: 1873 | PT id = 1363, Type = formulation review element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1873
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1873 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systematic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
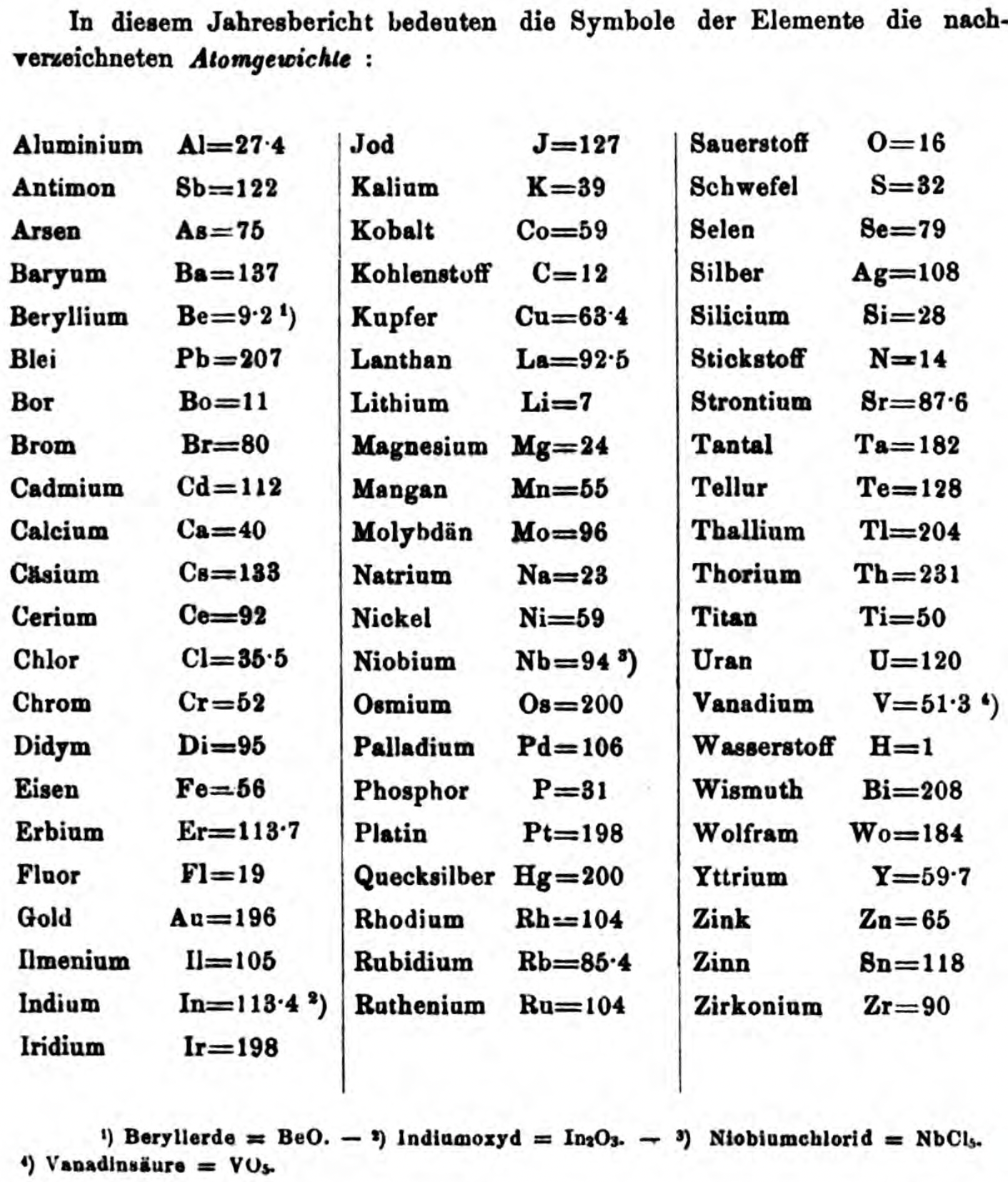
Notes:
- Didym D = 48 was actually a mixture of rare earth elements.
- Ilmenium, Il, was later found to be a mixture of niobium and tantalum.
- Generally, the elements missing had yet to be discovered (dates given below).
- The table below shows the progress from 1858 to 1873.
- By 1873 the only elements with incorrect atomic weights were the (at the time) somewhat obscure strontium, lanthanium, cerium and urananium.
- Previously, many elements were shown with two entries. Clearly, the stoichiometric and mass problems had largely been resolved (and the data agreed upon) by 1873.
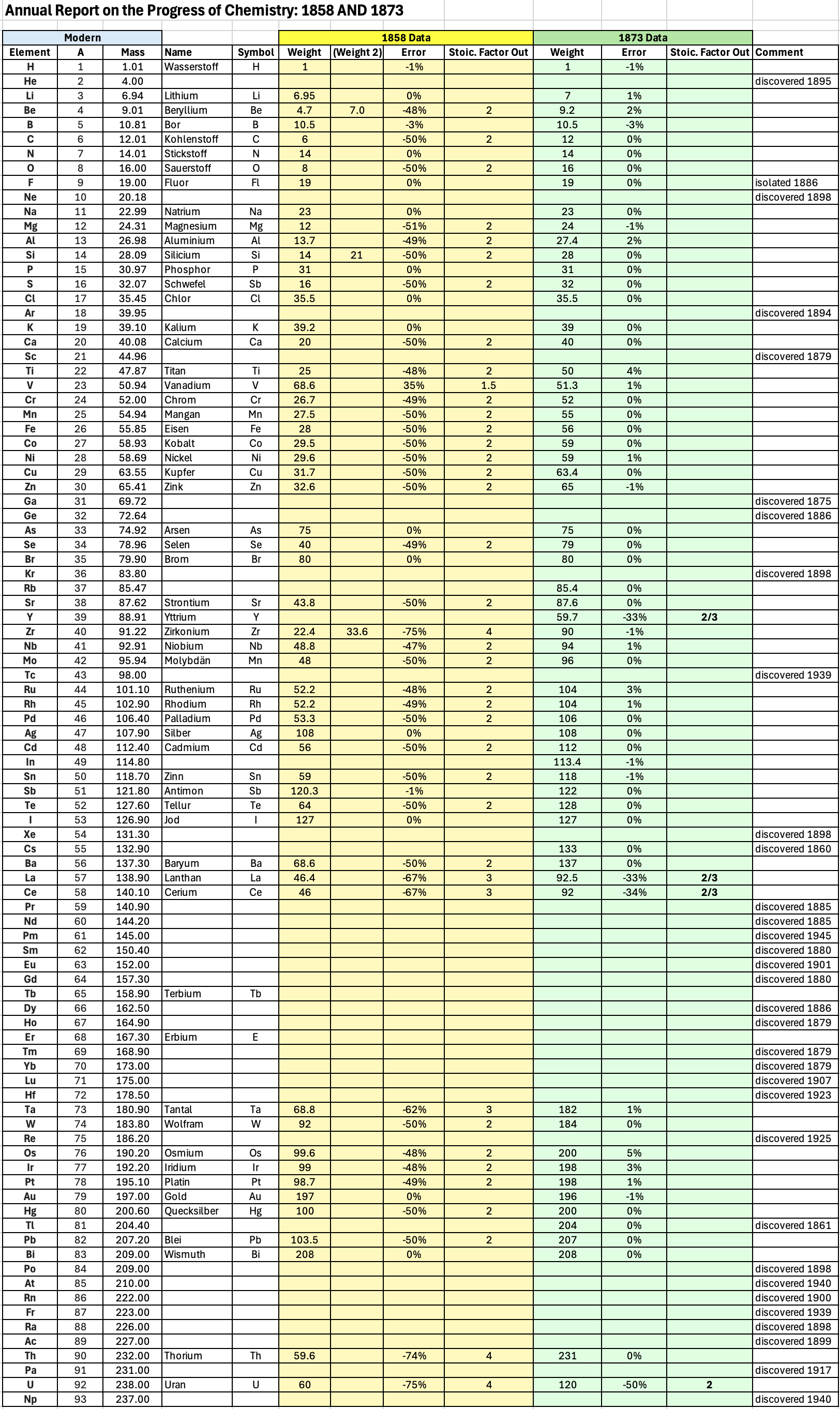
Thanks to René and Mario Rodriguez for the tip!
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.
