Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables from the year 1939:
| 1939 | Periodic Chart in Spiral Form |
| 1939 | Francium, Discovery of |
| 1939 | XBL 769-10601, Periodic Table Before World War II |
| 1939 | Foster's Periodic Arrangement |
| Year: 1939 | PT id = 627, Type = formulation spiral |
Periodic Chart in Spiral Form
Periodic Chart in Spiral Form from K. Gordon Irwin, Periodicity Patterns of The Elements J.Chem.Ed. 1939 16 (7), 335 DOI: 10.1021/ed016p335
Thanks to René Vernon for the reference:

| Year: 1939 | PT id = 867, Type = element |
Discovery of Francium
Fr ![]()
Francium, atomic number 87, has a mass of 223 au.
Radioactive element.
Francium was first observed in 1939 by M. Perey.
| Year: 1939 | PT id = 943, Type = formulation |
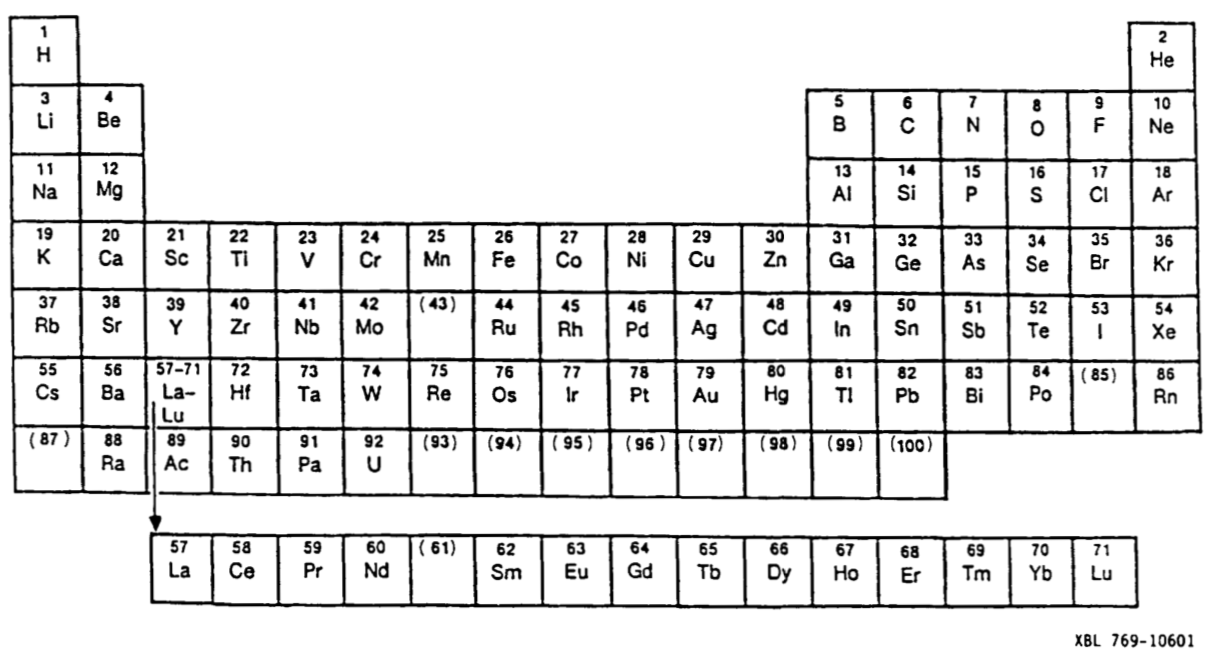
XBL 769-10601, Periodic Table Before World War II
An internal document of the Lawrence Berkeley Laboratory XBL 769-10601 shows a late 1930s (pre World War II) periodic table.
Note that this formulation erroneously predicts positions for transuranium elements:
- thorium is shown in the group below hafnium
- protactinium below tantalum
- uranium below tungsten
| Year: 1939 | PT id = 1056, Type = formulation |
Foster's Periodic Arrangement
L.S. Foster, "Why not modernise the textbooks also? I. The periodic table", Journal of Chemical Education, vol. 16, no. 9, pp. 409–412, https://pubs.acs.org/doi/10.1021/ed016p409
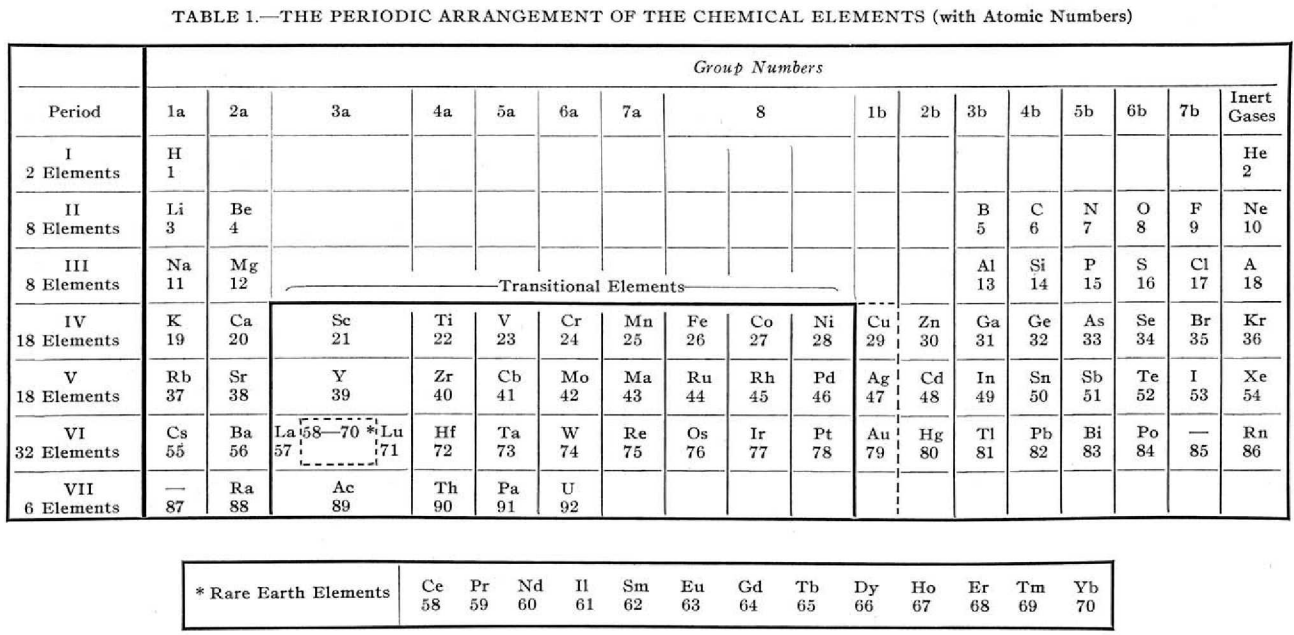
Foster writes:
"The [above] modern periodic table is simply an orderly array of the elements with all unnecessary ornamentation omitted, has been found highly satisfactory for instructional purposes.
"The transitional elements, with two unfilled electron shells, are separated from the non-metallic elements.
"The rare-earth elements, defined as those with three incomplete electron shells, are shown to be those of atomic numbers 58 to 70, while La and Lu, which have only two incomplete electron shells are classified as transitional elements.
"Copper, silver, and gold act as transitional elements except when the state of oxidation is one."
René Vernon writes:
Foster couldn't show the coinage metals – with their full d10 complements – as transitional elements, but by adding a broken line around them he was showing they had the capacity to act as if they were.
I tried to work out how he distinguished La & Lu from Ce to Yb. Foster seems to be saying that La 5d1 6s2, has incomplete 5th and 6th (ie. 6p) shells.
Same for Lu 4f14 5d1 6s2 having incomplete 5th and 6th shells. Whereas, for example, Ce 4f1 5d1 6s2 has incomplete 4th, 5th and 6th shells. Presumably this was in the years before the fact that the 4f shell became full at Yb was widely appreciated. So, strictly speaking, group 3a should have read:
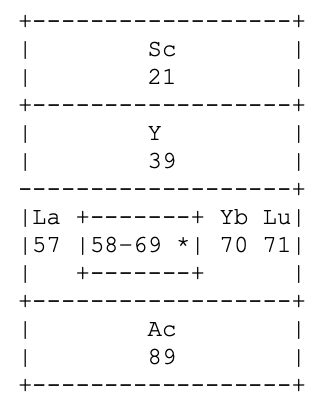
On the other hand, Yb3+ has an f13 configuration, so it does meet his three unfilled shells criterion. Had he known, he probably would've put a broken line around Yb to indicate its full f14 complement but that it normally acted as a rare earth, with an incomplete 4f shell; whereas neither La nor Lu have this capacity.
Good to see Foster put so much thought into organising his table, and his experience with using it for instructional purposes.
Van Spronsen does not mention Forster's table. Mazurs has a reference to Foster's table but lumps it in with the other medium-long tables, not appreciating its subtlety.
Mark Leach writes:
This formulation is very much like the XBL 769-10601, Periodic Table Before World War II used by Seaborg and the Manhattan project and is a precursor to the modern periodic table.
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.