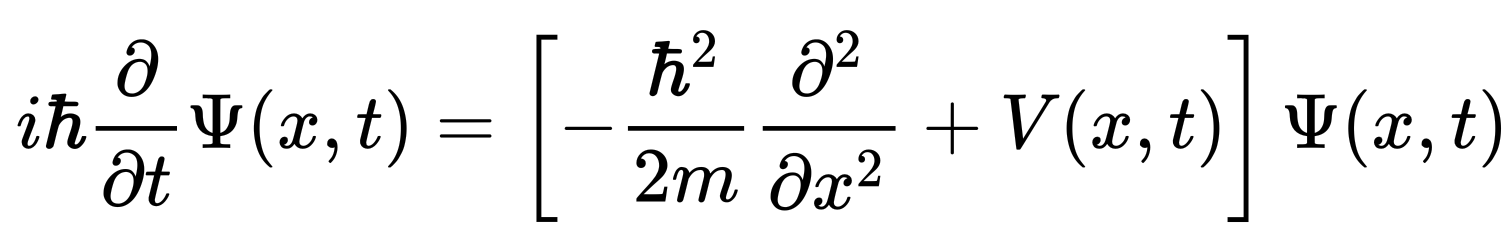Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
The 10 most recent entries to the database:
| Year: 1896 | PT id = 1383, Type = structure |
Discovery of Radioactivity
From The Nuclear Wallchart:
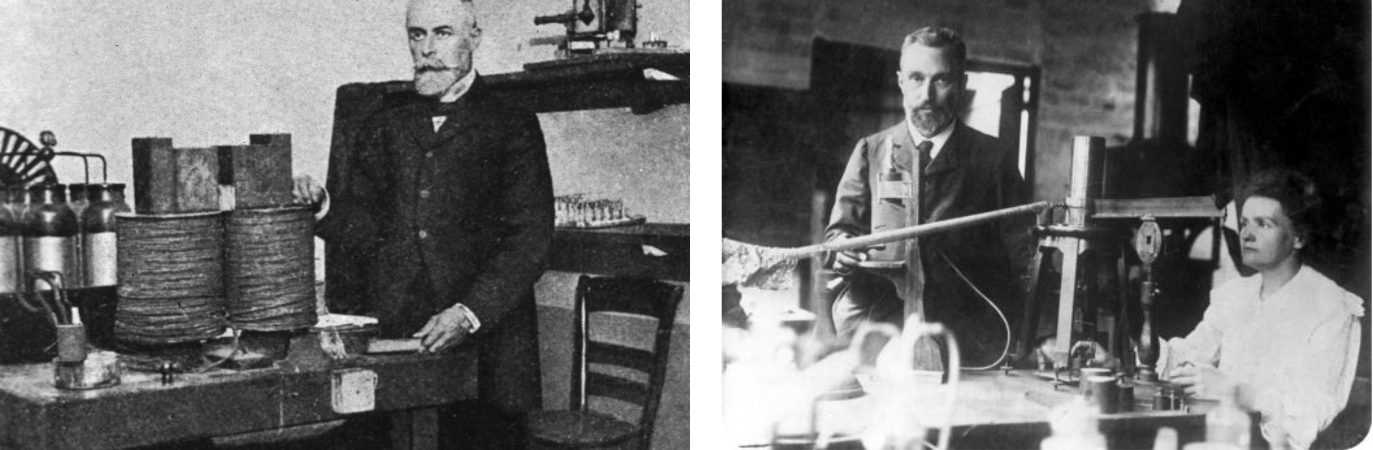
In 1896 Henri Becquerel was using naturally fluorescent minerals to study the properties of x-rays, which had been discovered in 1895 by Wilhelm Roentgen. He exposed potassium uranyl sulfate to sunlight and then placed it on photographic plates wrapped in black paper, believing that the uranium absorbed the sun’s energy and then emitted it as x-rays.
This hypothesis was disproved on the 26th-27th of February, when his experiment "failed" because it was overcast in Paris. For some reason, Becquerel decided to develop his photographic plates anyway. To his surprise, the images were strong and clear, proving that the uranium emitted radiation without an external source of energy such as the sun. Becquerel had discovered radioactivity.
Becquerel showed that the radiation he discovered could not be x-rays. X-rays are neutral and cannot be bent in a magnetic field. The new radiation was bent by the magnetic field so that the radiation must be charged and different than x-rays. When different radioactive substances were put in the magnetic field, they deflected in different directions or not at all, showing that there were three classes of radioactivity: negative, positive, and electrically neutral.
The term radioactivity was actually coined by Marie Curie, who together with her husband Pierre, began investigating the phenomenon recently discovered by Becquerel. The Curies extracted uranium from ore and to their surprise, found that the leftover ore showed more activity than the pure uranium. They concluded that the ore contained other radioactive elements. This led to the discoveries of the elements polonium and radium. It took four more years of processing tons of ore to isolate enough of each element to determine their chemical properties.
Ernest Rutherford, who did many experiments studying the properties of radioactive decay, named these alpha, beta, and gamma (α, β and γ) particles, and classified them by their ability to penetrate matter. Rutherford used an apparatus similar to Becquerel's. When the air from the chamber was removed, the alpha source made a spot on the photographic plate. When air was added, the spot disappeared. Thus, only a few centimeters of air were enough to stop the alpha radiation.
- α-particles carry more electric charge, are more massive, and move slowly compared to β and γ particles, they interact much more easily with matter.
- β-particles are much less massive and move faster, but are still electrically charged. A sheet of aluminum one millimeter thick or several meters of air will stop these electrons [and positrons].
- γ-rays carry no electric charge, they can penetrate large distances through materials before interacting–several centimeters of lead or a meter of concrete is needed to stop most γ-rays.
Henri Becquerel and Marie & Pierre Curie in their labs:
| Year: 2026 | PT id = 1382, Type = formulation spiral |
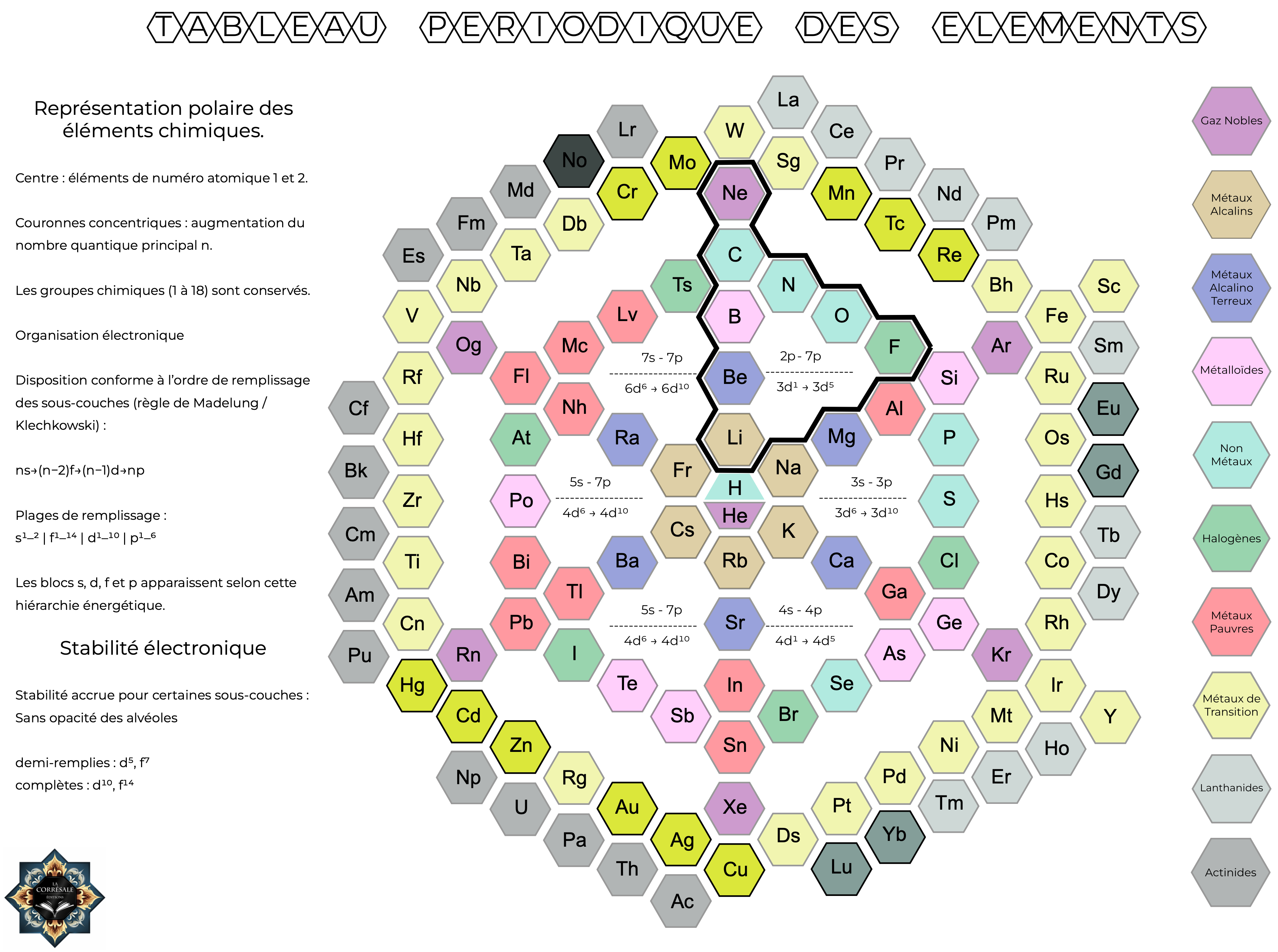
Tableau_Périodique des Elements
La Corrésale writes:
"I have been developing an alternative geometric representation of the periodic table, and I would greatly appreciate your perspective on it.
"The model is a radial (hexagonal) construction with hydrogen and helium positioned at the center, and successive concentric layers corresponding to principal quantum numbers (n = 1-7). The layout follows the Madelung (Klechkowski) filling order, so that the s, p, d, and f blocks arise naturally from the geometric structure.
"Lanthanides and actinides are fully integrated into the continuous sequence rather than separated.
"My intention is not to replace the conventional rectangular table, but to offer a complementary representation that makes the electronic layering and energetic hierarchy more visually explicit. The structure effectively behaves like a “macro-atom,” with shells accumulating outward in a way that mirrors electronic shell construction. Certain periodic trends, such as radial progression and diagonal relationships, appear to become more intuitive in this configuration.
"I am aware that many alternative periodic tables exist, and I have tried to avoid unnecessary aesthetic distortion while preserving structural coherence and regular tiling. I would be very interested to know whether you see any pedagogical or conceptual merit in such a representation, or any structural issues I may have overlooked."
| Year: 1930 | PT id = 1381, Type = structure review |
Quantum Atoms
Dirac, P. A. M. The Principles of Quantum Mechanics. Oxford University Press (1st ed. 1930; 2nd ed. 1935). Wikipeda entry on this work.
von Neumann, J., Mathematische Grundlagen der Quantenmechanik (Mathematical Foundations of Quantum Mechanics), 1932, Springer, Berlin, Germany. Wikepedia entry on this work.
By the 1930s, the mathematics of quantum mechanics was mature, as exemplified by these two text books. Dirac explicitly develops methods for atoms, molecules, radiation, and many-particle systems. Von Neumann formulates a fully general mathematical framework applicable to arbitrarily complex systems (though with few concrete examples).
"The Principles of Quantum Mechanics is an influential monograph written by Paul Dirac and first published by Oxford University Press in 1930. In this book, Dirac presents quantum mechanics in a formal, logically consistent, and axiomatic fashion, making the book the first of its kind. It is based on matrices and operators rather than wave–particle duality. Its 82 sections contain 785 equations with no diagrams. Nor does it have an index, a bibliography, or a list of suggestions for further reading. The first half of the book lays down the foundations of quantum mechanics while the second half focuses on its applications. Dirac did not pursue a historical approach to the subject. Nor did he discuss at length the philosophy of quantum mechanics."
"Von Neumann formalised quantum mechanics using the concept of Hilbert spaces and linear operators. He acknowledged the previous work by Paul Dirac on the mathematical formalisation of quantum mechanics, but was skeptical of Dirac's use of delta functions. He wrote the book in an attempt to be even more mathematically rigorous than Dirac. It was von Neumann's last book in German, afterwards he started publishing in English."
To read Dirac's The Principles of Quantum Mechanics click this link.

| Year: 1948 | PT id = 1380, Type = formulation structure data |
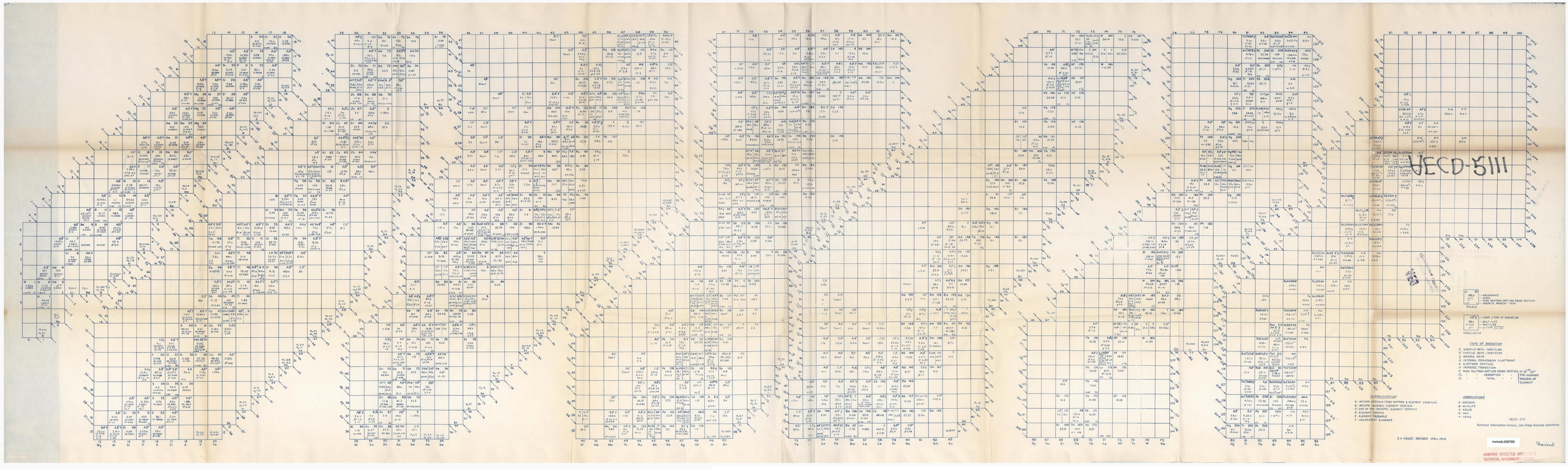
Segrè Chart (Original)
Segrè, Emilio. Segrè Chart [Isotope Chart], technical drawing, April 1948; Oak Ridge, Tennessee. (https://digital.library.unt.edu/ark:/67531/metadc100789/: accessed February 17, 2026), University of North Texas Libraries, UNT Digital Library, https://digital.library.unt.edu; crediting UNT Libraries Government Documents Department.
"A table or chart of nuclides is a two-dimensional graph of isotopes of the chemical elements, in which one axis represents the number of neutrons (symbol N) and the other represents the number of protons (atomic number, symbol Z) in the atomic nucleus. Each point plotted on the graph thus represents a nuclide of a known or hypothetical element. This system of ordering nuclides can offer a greater insight into the characteristics of isotopes than the better-known periodic table, which shows only elements and not their isotopes. The chart of the nuclides is also known as the Segrè chart, after Italian physicist Emilio Segrè."
Click Image to Enlarge:

| Year: 1932 | PT id = 1379, Type = structure |
Chadwick and the Discovery of the Neutron
Chadwick, J. Possible Existence of a Neutron. Nature, 129, 312 (1932).
"James Chadwick was a British experimental physicist who received the Nobel Prize in Physics in 1935 for his discovery of the neutron. In 1941, he wrote the final draft of the MAUD Report, which inspired the U.S. government to begin serious atomic bomb research efforts. He was the head of the British team that worked on the Manhattan Project during World War II.
"The discovery of the neutron and its properties was central to the extraordinary developments in atomic physics in the first half of the 20th century. Early in the century, Ernest Rutherford used alpha particle scattering to discover that an atom has its mass and electric charge concentrated in a tiny nucleus. By 1920, isotopes of chemical elements had been discovered, the atomic masses had been determined to be approximately integer multiples of the mass of the hydrogen atom, and the atomic number had been identified as the charge on the nucleus. Throughout the 1920s, the nucleus was viewed as composed of combinations of protons and electrons, the two elementary particles known at the time, but that model presented several experimental and theoretical contradictions.
"The essential nature of the atomic nucleus was established with the discovery of the neutron by James Chadwick in 1932 and the determination that it was a new elementary particle, distinct from the proton.
"The uncharged neutron was immediately exploited as a new means to probe nuclear structure, leading to such discoveries as the creation of new radioactive elements by neutron irradiation (1934) and the fission of uranium atoms by neutrons (1938). The discovery of fission led to the creation of both nuclear power and nuclear weapons by the end of World War II. Both the proton and the neutron were presumed to be elementary particles until the 1960s, when they were determined to be composite particles built from quarks. "
| Year: 1928 | PT id = 1378, Type = structure |
Dirac Equation
Dirac, P. A. M. (1928). "The Quantum Theory of the Electron". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 117 (778): 610–624. PDF of paper.
"The Dirac equation is a relativistic wave equation derived by British physicist Paul Dirac in 1928. In its free form, or including electromagnetic interactions, it describes all spin-?1/2? massive particles, called Dirac particles, such as electrons and quarks for which parity is a symmetry. It is consistent with both the principles of quantum mechanics and the theory of special relativity and was the first theory to fully account for special relativity in the context of quantum mechanics. The equation is validated by its rigorous accounting of the observed fine structure of the hydrogen spectrum and has become vital in the building of the Standard Model pf particle physics.

"The equation also implied the existence of a new form of matter, antimatter, previously unsuspected and unobserved. The existence of antimatter was experimentally confirmed several years later. It also provided a theoretical justification for the introduction of several component wave functions in Pauli's phenomenological theory of spin. The wave functions in the Dirac theory are vectors of four complex numbers (known as bispinors), two of which resemble the Pauli wavefunction in the non-relativistic limit, in contrast to the Schrödinger equation, which described wave functions of only one complex value."
If you require deeper dive into quantum spin and the Dirac equation, these two linked videos by Physics Explained will be of interest. The videos discuss the history and the mathematics of the equation. The videos show that the Dirac formulation of the relativistic electron inevitably has four answers or solutions which correspond to the:
- Spin-up electron
- Spin-down electron
- Spin-up positron (anti-electron)
- Spin-down positron
- The result also shows that the spin must be +1/2 or –1/2.
| Year: 1927 | PT id = 1377, Type = structure |
Fifth Solvay Conference on Physics
"The most famous conference was the fifth Solvay Conference on Physics, which was held from 24 to 29 October 1927. The subject was Electrons and Photons and the world's most notable physicists met to discuss the newly formulated quantum theory. The leading figures were Albert Einstein and Niels Bohr. Seventeen of the 29 attendees were or became Nobel Prize winners, including Marie Skłodowska-Curie who, alone among them, had won Nobel Prizes in two separate scientific disciplines. The anti-German prejudice that had prevented Einstein and others from attending the Solvay conferences held after the First World War had melted away. Essentially all of those names who had contributed to the recent development of the quantum theory were at this Solvay Conference, including Bohr, Born, de Broglie, Dirac, Heisenberg, Pauli, Planck, Lorentz, Compton, Ehrenfest, and Schrödinger. Heisenberg commented:
"Through the possibility of exchange between the representatives of different lines of research, this conference has contributed extraordinarily to the clarification of the physical foundations of the quantum theory. It forms, so to speak, the outward completion of the quantum theory."
"The photo taken of this conference's participants is sometimes entitled 'The Most Intelligent Photo Ever Taken', for its depiction of the world's leading physicists gathered together in one shot."
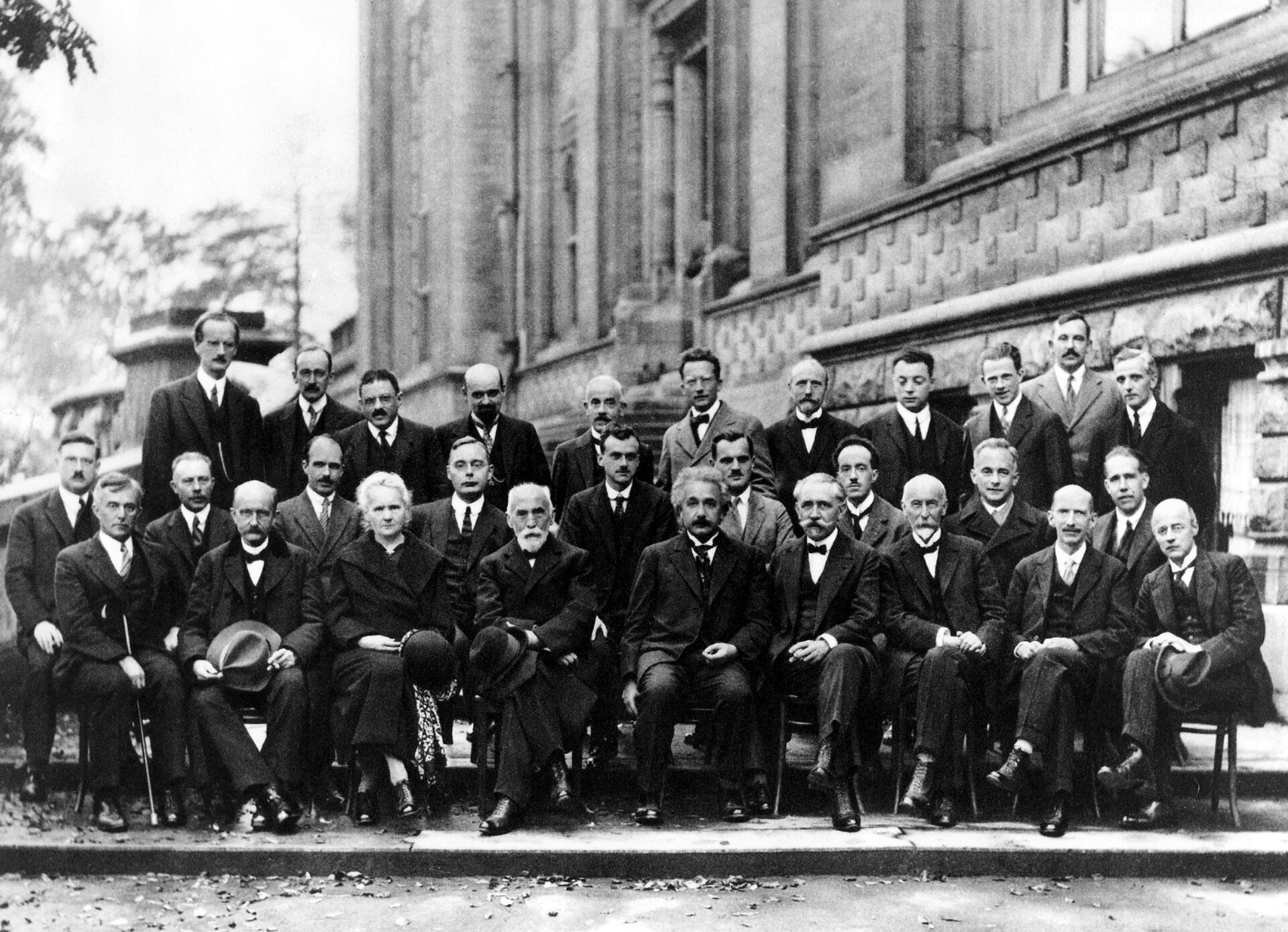
A. Piccard, E. Henriot, P. Ehrenfest, E. Herzen, Th. De Donder, E. Schrödinger, J.E. Verschaffelt, W. Pauli, W. Heisenberg, R.H. Fowler, L. Brillouin;
P. Debye, M. Knudsen, W.L. Bragg, H.A. Kramers, P.A.M. Dirac, A.H. Compton, L. de Broglie, M. Born, N. Bohr;
I. Langmuir, M. Planck, M. Skłodowska-Curie, H.A. Lorentz, A. Einstein, P. Langevin, Ch. E. Guye, C.T.R. Wilson, O.W. Richardson
Fifth conference participants, 1927. Institut International de Physique Solvay in Leopold Park.
| Year: 1926 | PT id = 1376, Type = structure |
Born Rule
Born, M. Zur Quantenmechanik der Stoßvorgänge. Zeitschrift für Physik, 37, 863–867 (1926).
"Max Born was a German–British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a number of notable physicists in the 1920s and 1930s. He shared the 1954 Nobel Prize in Physics with Walther Bothe 'for his fundamental research in quantum mechanics, especially for his statistical interpretation of the wavefunction.'
"The Born rule is a postulate of quantum mechanics that gives the probability that a measurement of a quantum system will yield a given result. In one commonly used application, it states that the probability density for finding a particle at a given position is proportional to the square of the amplitude of the system's wavefunction at that position. So is the wave function is ψ, the probability of finding the electron is |ψ|2."
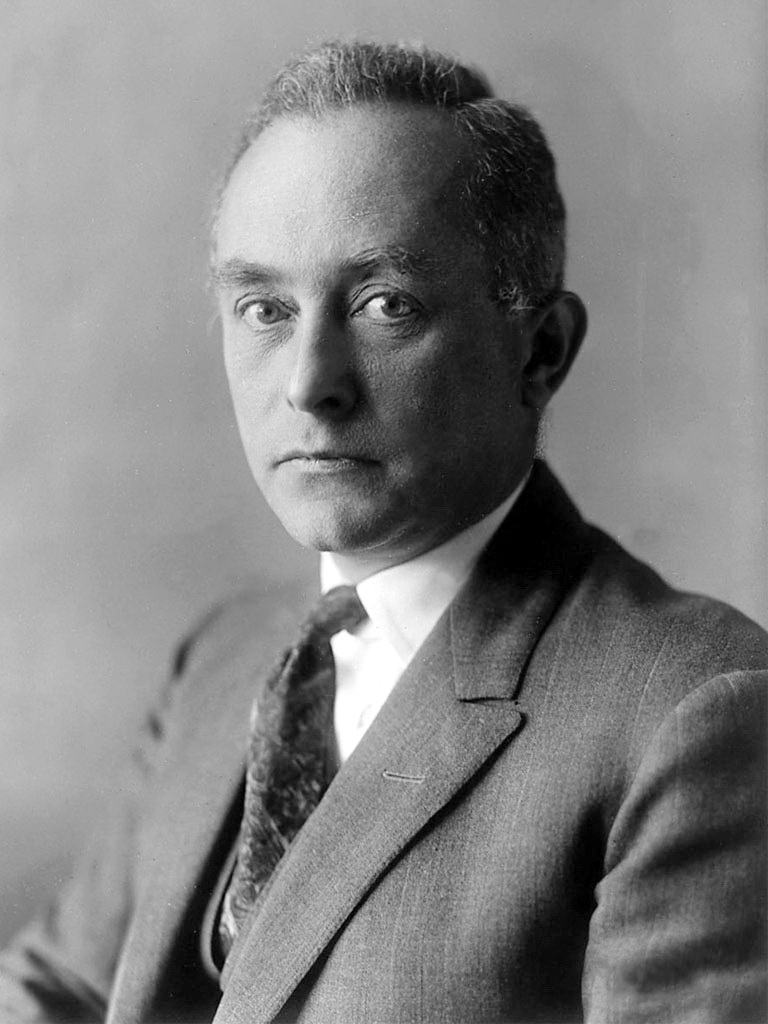
| Year: 1926 | PT id = 1375, Type = structure |
Schrödinger Wave Equation
Schrödinger, E. Quantisierung als Eigenwertproblem (Quantization as an eigenvalue problem) (Parts I–IV). Annalen der Physik, 79, 361–376; 489–527; 734–756; 80, 437–490 (1926).
"Erwin Schrödinger was an Austrian–Irish theoretical physicist who developed fundamental results in quantum theory. In particular, he is recognised for devising the Schrödinger equation, an equation that provides a way to calculate the wave function of a system and how it changes dynamically in time."
"A special case of the Schrödinger equation is the position-space Schrödinger equation for a single nonrelativistic particle in one dimension:
"The ψ is a wave function, a function that assigns a complex number to each point x at each time t. The parameter m is the mass of the particle, and V(x,t) is the potential energy function that represents the environment in which the particle exists. The constant i is the imaginary unit, and ħ is the reduced Planck constant, which has units of action (energy multiplied by time).
"Schrödinger coined the term 'quantum entanglement' in 1935. Schrödinger shared the 1933 Nobel Prize in Physics with Paul Dirac 'for the discovery of new productive forms of atomic theory.''
"The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. Conceptually, the Schrödinger equation is the quantum counterpart of Newton's second law in classical mechanics. Given a set of known initial conditions, Newton's second law makes a mathematical prediction as to what path a given physical system will take over time. The Schrödinger equation gives the evolution over time of the wave function, the quantum-mechanical characterisation of an isolated physical system. The equation was postulated by Schrödinger based on a postulate of Louis de Broglie that all matter has an associated matter wave. The equation predicted bound states of the atom in agreement with experimental observations."

| Year: 1925 | PT id = 1374, Type = structure |
Heisenberg’s Matrix Mechanics and the Uncertainty Principle
Heisenberg, W. Über quantentheoretische Umdeutung kinematischer und mechanischer Beziehungen. (On the quantum-theoretical reinterpretation of kinematic and mechanical relationships.) Zeitschrift für Physik, 33, 879–893 (1925); Heisenberg, W. Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik. (On the intuitive content of quantum-theoretical kinematics and mechanics. "The Uncertainty Principle") Zeitschrift für Physik, 43, 172–198 (1927).
"Werner Karl Heisenberg was a German theoretical physicist, one of the main pioneers of the theory of quantum mechanics. Heisenberg published his Umdeutung paper in 1925, a major reinterpretation of old quantum theory. In the subsequent series of papers with Max Born and Pascual Jordan, during the same year, his matrix formulation of quantum mechanics was substantially elaborated. He is also known for the uncertainty principle, which he published in 1927. He received the Nobel Prize in Physics in 1932 'for the creation of quantum mechanics'."

 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.