Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH the Database:
-
By Year
Formulations 2020 to present day Formulations 2010 – 2019 Formulations 2000 – 2009 Formulations 1950 – 1999 Formulations 1900 – 1949 Formulations 1850 – 1899 Formulations 1800 – 1849 Formulations Before 1800 =================== All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added =================== 2026 2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
- By Type
- Pre-Selected
| Year: 2019 | PT id = 1099, Type = formulation |
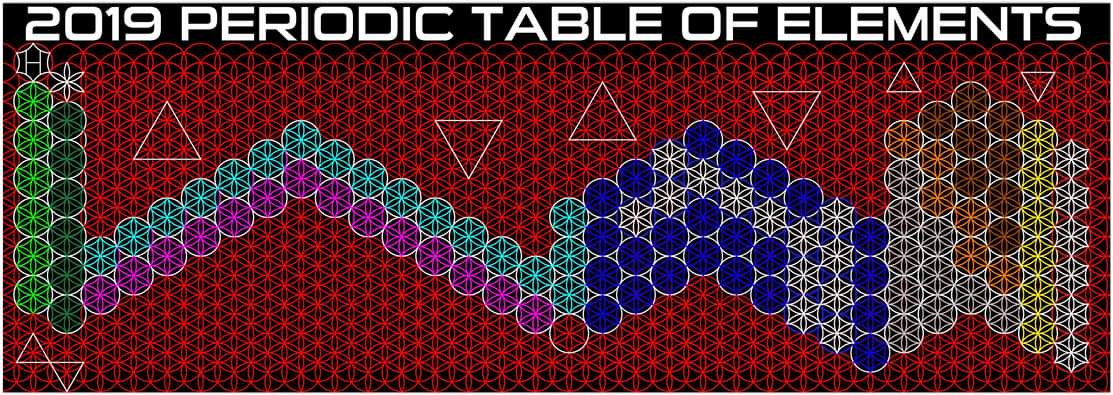
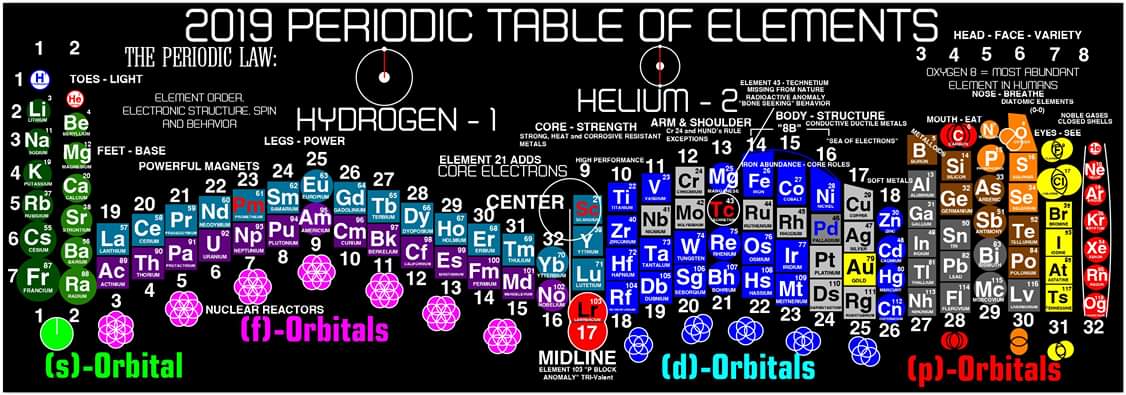
Colburn's 2019 Periodic Table of The Elements
Justin Lee Colburn writes:
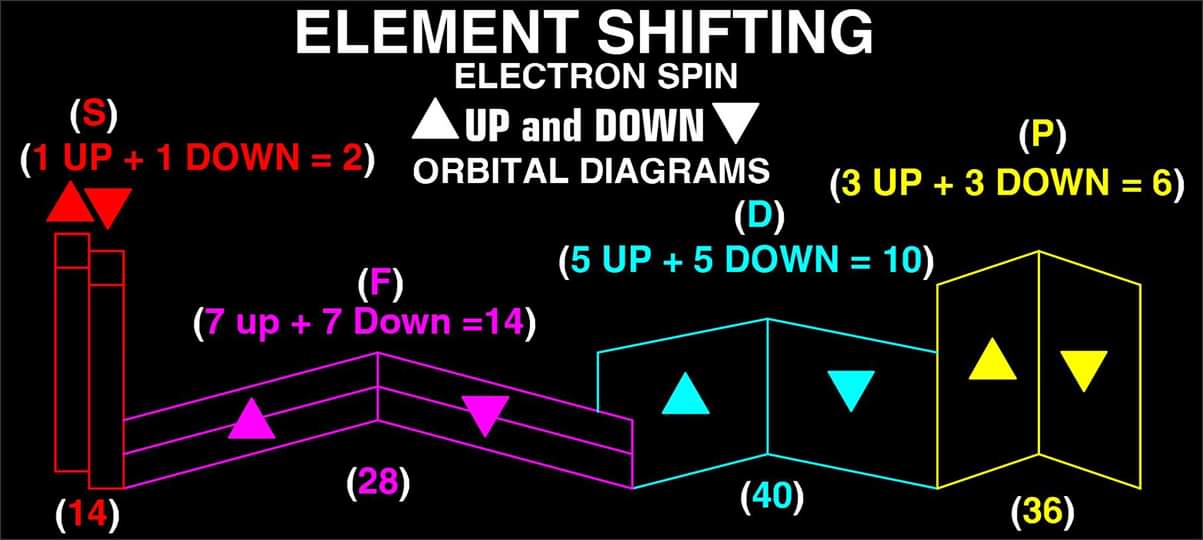
"What is unique to my Periodic Table is the fact that any Elements Electron Spin can be identified for Orbital Diagrams using a technique I have called 'Element Shifting'.
"Elements with an Up spin Valence Electron are shifted up and Elements with a down spin are shifted down. Also, the Hund's rule Exceptions are Highlighted in the Transition Metals so their orbital diagrams can also be identified easily.
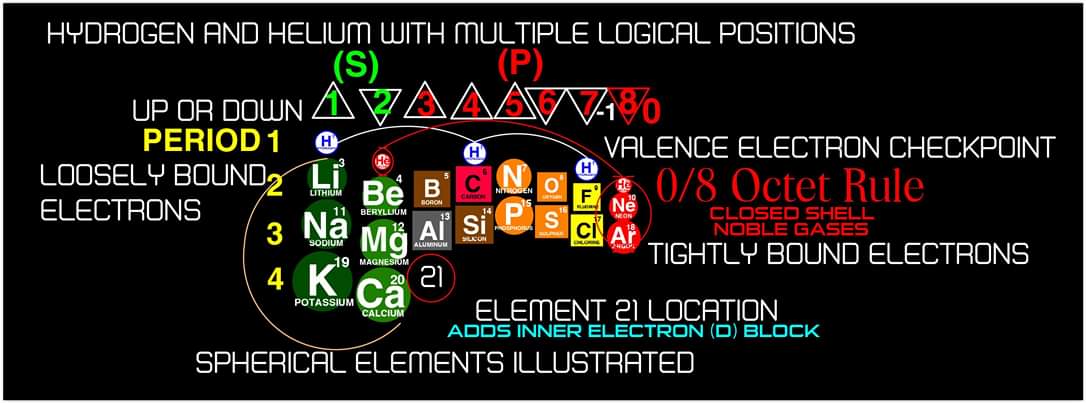
"In addition, an accurate numbering system can be applied to all the elements with Helium placed in Group 2 instead of Group 18. I believe that quantitative data should take priority when giving elements their position, but this system is meant to be dynamic rather than static. In my Periodic Table System, (1-8) corresponds to Valence Electrons in the s and p orbitals and then the 9-18 and 19-32 corresponds to core electron in d and f orbitals .
I believe that it is important to begin by showing students the first 20 Elements FIRST because they all add Outer Valence Electrons which makes the Periodic Table logic easy to follow. Also explaining that Hydrogen and Helium are anomalies with more than one logical position, can really help clear up confusion for new students.
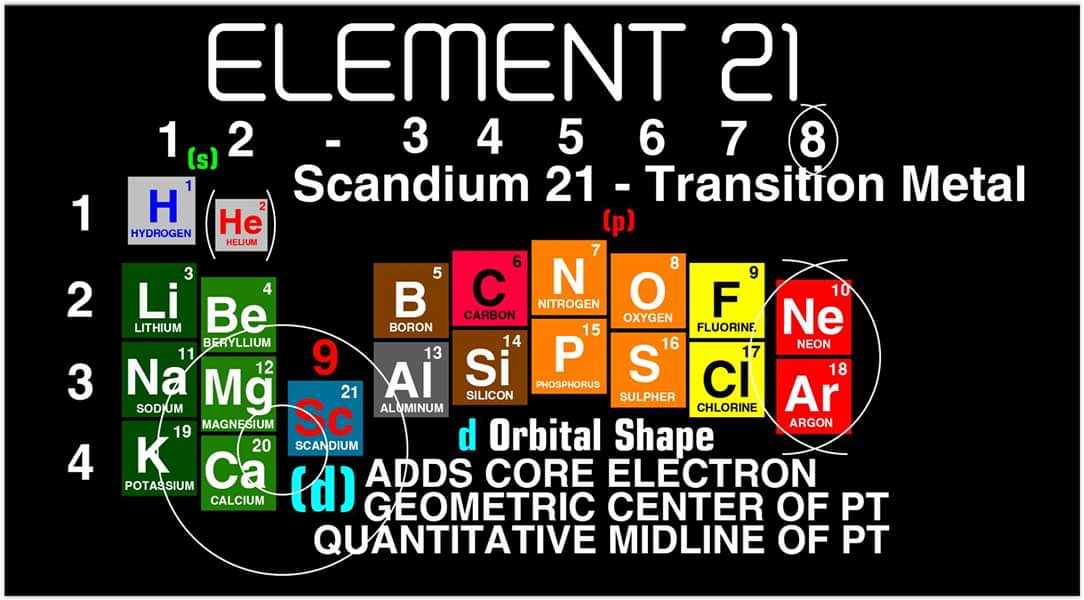
"After element 20, the Transition Elements such as scandium 21 begin adding core electrons in the d orbital the current standard (1-18) numbering system does not reflect this. One of the reasons why I prefer keeping the s and p block elements on the outside of the table and the f and d block elements on the inside is because of how they add electrons to their orbitals.
"I have been developing a curriculum based on this system that I believe will help students learn and understand the logic and trends of The Periodic Table more efficiently than the standard. Rather than memorizing Element information, Students will truly be able to follow the logic based on the location of the Elements, simple counting and using the numbering system."
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.