Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
| Year: 1913 | PT id = 1370, Type = structure |
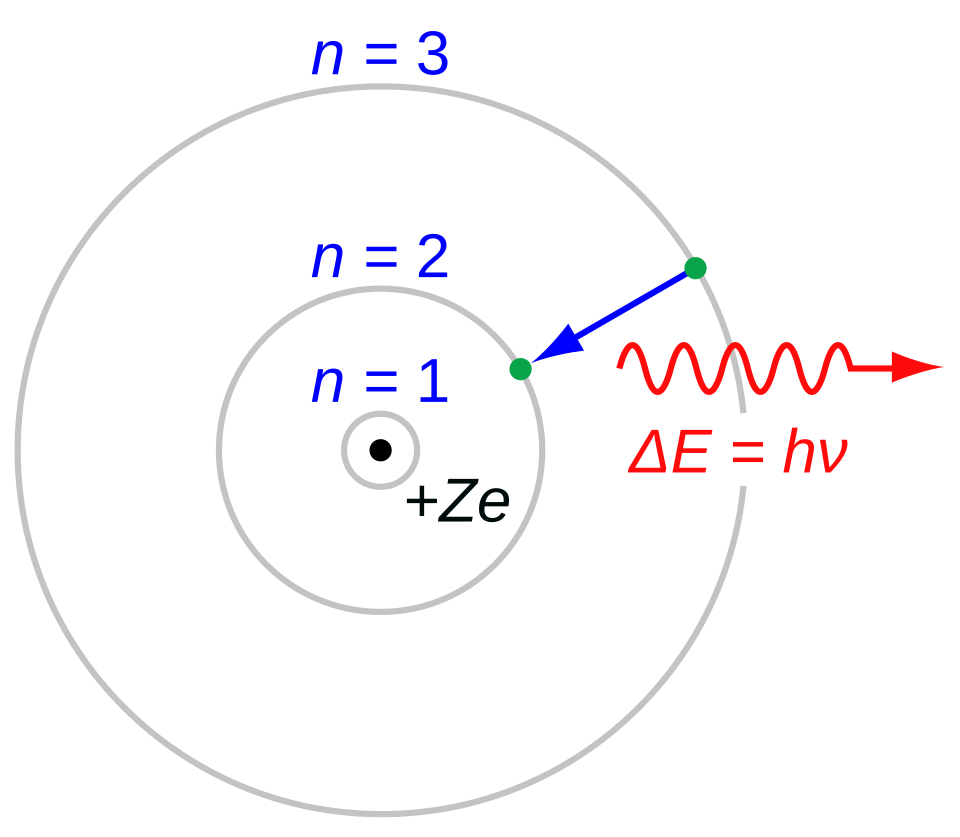
The Bohr Atom
Bohr, N. On the Constitution of Atoms and Molecules (Parts I–III). Philosophical Magazine, 26, 1–25; 476–502; 857–875 (1913).
"In the Bohr model (or the Rutherford–Bohr model) of the hydrogen atom (Z = 1), the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus. When an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy (where E = hν). The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 ? 2 transition produces the first line of the Balmer series, and for hydrogen (Z = 1) it results in a photon of wavelength 656 nm (red light).
"The Bohr atom consists of a small, dense atomic nucleus surrounded by orbiting electrons. It is analogous to the structure of the Solar System, but with attraction provided by electrostatic force rather than gravity, and with the electron energies quantised (assuming only discrete values). The Bohr model incorporated some early quantum concepts. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's discovery of the atom's nucleus, the model supplanted the plum pudding model of J. J. Thomson only to be replaced by the quantum atomic model in the 1920s.
"The Bohr model's key success lies in explaining the Rydberg formula for hydrogen's spectral emission lines. While the Rydberg formula had been known experimentally, it did not gain a theoretical basis until the Bohr model was introduced. Not only did the Bohr model explain the reasons for the structure of the Rydberg formula, it also provided a justification for the fundamental physical constants that make up the formula's empirical results."
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.