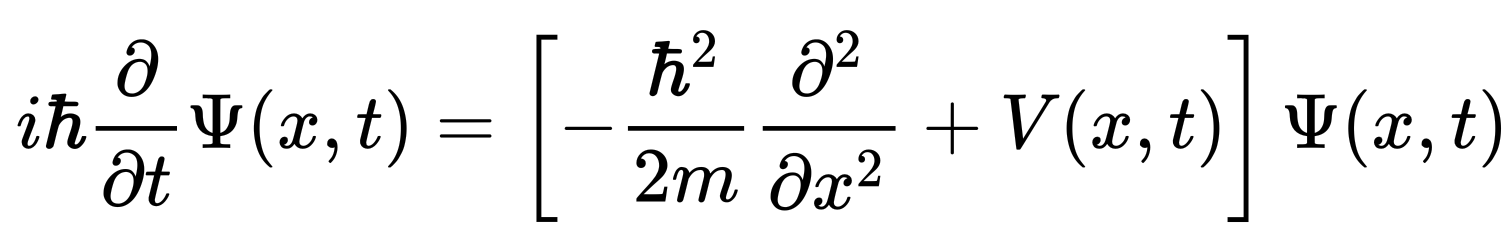Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
| Year: 1926 | PT id = 1375, Type = structure |
Schrödinger Wave Equation
Schrödinger, E. Quantisierung als Eigenwertproblem (Quantization as an eigenvalue problem) (Parts I–IV). Annalen der Physik, 79, 361–376; 489–527; 734–756; 80, 437–490 (1926).
"Erwin Schrödinger was an Austrian–Irish theoretical physicist who developed fundamental results in quantum theory. In particular, he is recognised for devising the Schrödinger equation, an equation that provides a way to calculate the wave function of a system and how it changes dynamically in time."
"A special case of the Schrödinger equation is the position-space Schrödinger equation for a single nonrelativistic particle in one dimension:
"The ψ is a wave function, a function that assigns a complex number to each point x at each time t. The parameter m is the mass of the particle, and V(x,t) is the potential energy function that represents the environment in which the particle exists. The constant i is the imaginary unit, and ħ is the reduced Planck constant, which has units of action (energy multiplied by time).
"Schrödinger coined the term 'quantum entanglement' in 1935. Schrödinger shared the 1933 Nobel Prize in Physics with Paul Dirac 'for the discovery of new productive forms of atomic theory.''
"The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. Conceptually, the Schrödinger equation is the quantum counterpart of Newton's second law in classical mechanics. Given a set of known initial conditions, Newton's second law makes a mathematical prediction as to what path a given physical system will take over time. The Schrödinger equation gives the evolution over time of the wave function, the quantum-mechanical characterisation of an isolated physical system. The equation was postulated by Schrödinger based on a postulate of Louis de Broglie that all matter has an associated matter wave. The equation predicted bound states of the atom in agreement with experimental observations."

 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.