Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
| Year: 1896 | PT id = 1383, Type = structure |
Discovery of Radioactivity
From The Nuclear Wallchart:
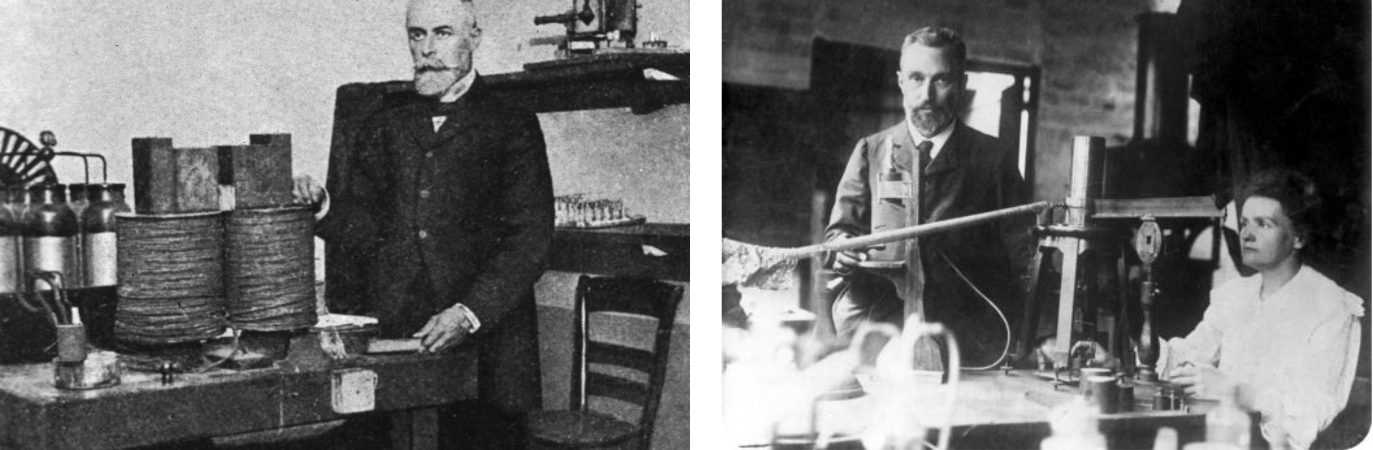
In 1896 Henri Becquerel was using naturally fluorescent minerals to study the properties of x-rays, which had been discovered in 1895 by Wilhelm Roentgen. He exposed potassium uranyl sulfate to sunlight and then placed it on photographic plates wrapped in black paper, believing that the uranium absorbed the sun’s energy and then emitted it as x-rays.
This hypothesis was disproved on the 26th-27th of February, when his experiment "failed" because it was overcast in Paris. For some reason, Becquerel decided to develop his photographic plates anyway. To his surprise, the images were strong and clear, proving that the uranium emitted radiation without an external source of energy such as the sun. Becquerel had discovered radioactivity.
Becquerel showed that the radiation he discovered could not be x-rays. X-rays are neutral and cannot be bent in a magnetic field. The new radiation was bent by the magnetic field so that the radiation must be charged and different than x-rays. When different radioactive substances were put in the magnetic field, they deflected in different directions or not at all, showing that there were three classes of radioactivity: negative, positive, and electrically neutral.
The term radioactivity was actually coined by Marie Curie, who together with her husband Pierre, began investigating the phenomenon recently discovered by Becquerel. The Curies extracted uranium from ore and to their surprise, found that the leftover ore showed more activity than the pure uranium. They concluded that the ore contained other radioactive elements. This led to the discoveries of the elements polonium and radium. It took four more years of processing tons of ore to isolate enough of each element to determine their chemical properties.
Ernest Rutherford, who did many experiments studying the properties of radioactive decay, named these alpha, beta, and gamma (α, β and γ) particles, and classified them by their ability to penetrate matter. Rutherford used an apparatus similar to Becquerel's. When the air from the chamber was removed, the alpha source made a spot on the photographic plate. When air was added, the spot disappeared. Thus, only a few centimeters of air were enough to stop the alpha radiation.
- α-particles carry more electric charge, are more massive, and move slowly compared to β and γ particles, they interact much more easily with matter.
- β-particles are much less massive and move faster, but are still electrically charged. A sheet of aluminum one millimeter thick or several meters of air will stop these electrons [and positrons].
- γ-rays carry no electric charge, they can penetrate large distances through materials before interacting–several centimeters of lead or a meter of concrete is needed to stop most γ-rays.
Henri Becquerel and Marie & Pierre Curie in their labs:
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.