Periodic Table |
 |
 |
 |
 |
 |
 |
 |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
The 10 Periodic Tables most recently added to the database:
| Year: 2020 | PT id = 1316 |
Ziggurat Formulation
Thanks to René Vernon for finding this "Ziggurat" formulation (with a dash of Segrè Chart, upper left) on the RSC page for Oganesson:

| Year: 2024 | PT id = 1315 |
Dufour’s Elementree in 2D by Vernon
A 2 dimension (flat) drawing of Dufour’s 3 dimensional Elementree by René Vernon.
René Vernon writes:
"I was surprised by its lack of symmetry in Dufour’s Elementree, caused by the awkward placement of He, and the assignment of H as floating above Li and Be. Hydrogen is as much subject to the periodic law as any other element. Without aligning H over Li, and He over Be, I am not sure that Elementree can be made symmetrical."

| Year: 1966 | PT id = 1314 |
Tottle's Periodic Table
Tottle CR 1974, The Science of Engineering Materials, reprint of 1966 ed., Heinemann Educational Books, London, p. 20
René Vernon writes:
I was drawn to the attached periodic table by the strange-looking arrangement of dividing lines, one "full" and one dashed, in the p-block.
Semimetals
Ge, As, Se, Sn, Sb, Te, Bi and Po are shown as semi-metals. Tottle does not explain the basis for this division.
Showing Sn as a semi-metal or metalloid is dubious. Sure, white-Sn becomes gray-Sn at a temperature of below 13.2 °C but even here it has the electronic band structure of a semi-metal.
The same can be said for Po which has electronic band structure of a true metal, unlike the situation in As, Sb and Bi, all of which have electronic band structures of semi-metals.
Metals & Nonmetals
Starting with H, note the left to right path of the full dividing line between metals and nonmetals is continuous, except for the unique break above Be, presumably to show that there is no element above Be. This is actually not well thought-out since the metallic or nonmetallic status of the IIA elements is not then clarified.
Tottle is further interesting since, as well as referring to metals and nonmetals in the periodic table sense he later includes a chapter on Metals and alloys, and a chapter on Non-metallic materials. Some examples given by him of non-metallic materials are alumina, magnesia, graphite, beryllia, titanium carbide, glass, rubber, nylon and wood. So, he here is mixing nonmetallic elements and nonmetallic materials (which is fine).
Tottle gets into trouble in his chapter on Metals and alloys, since he includes some discussion on interstitial solid solutions, such as cementite Fe3C, which is an insulator, and intermetallic compounds, which appears fine on the surface, until one realises that some intermetallic compounds are semiconductors, such as FeGa3, RuGa3, and IrGa3. I have never heard of semiconducting or insulating metals or alloys.

| Year: 2024 | PT id = 1313 |
Dynamic, Formulation Morphing, 3-Dimensional, Web App Periodic Table
A really nice, data filled, dynamic, 3D web-app periodic table by Morishita, a Japanese software engineer living in Vietnam: https://periodic-table-3d.vercel.app
All of the information here: https://periodic-table-3d.vercel.app/posts/shapes-of-periodic-table
| Year: 2024 | PT id = 1312 |
Rectangular Array Model of Chemical Elements
Dr. Laith H . M . Al-ossmi, Rectangular Array Model of Chemical Elements https://www.academia.edu/119516234/Rectangular_Array_Model_of_Chemical_Elements
Abstract: This paper introduces a novel graphical representation of the periodic table, termed the "Rectangular Array Model". This innovative representation adheres to the group numbering recommendations provided by the International Union of Pure and Applied Chemistry (IUPAC). The rectangular array model arranges the known chemical elements (118 elements) according to their similarities in chemical properties, within 7 horizontal periods and 14 vertical groups. Hydrogen has been relocated to join oxygen within the elements of the first period and group 12. The rectangular array model reattached the position of 30 elements of lanthanides and actinidines into the table body. We respectfully encourage educators across various academic institutions to adopt this new periodic table format in their teaching practices.
Mark Leach the PT database administrator & curator writes:
WARNING: This formulation is NOT contiguous with respect to atomic number (surely, a prerequisite for any periodic table formulation?); it does NOT use IUPAC Group Numbers correctly, even though the abstract states that it does; it does not present electron or orbital structure and neither does it show periodicity in any way.

| Year: 2024 | PT id = 1311 |
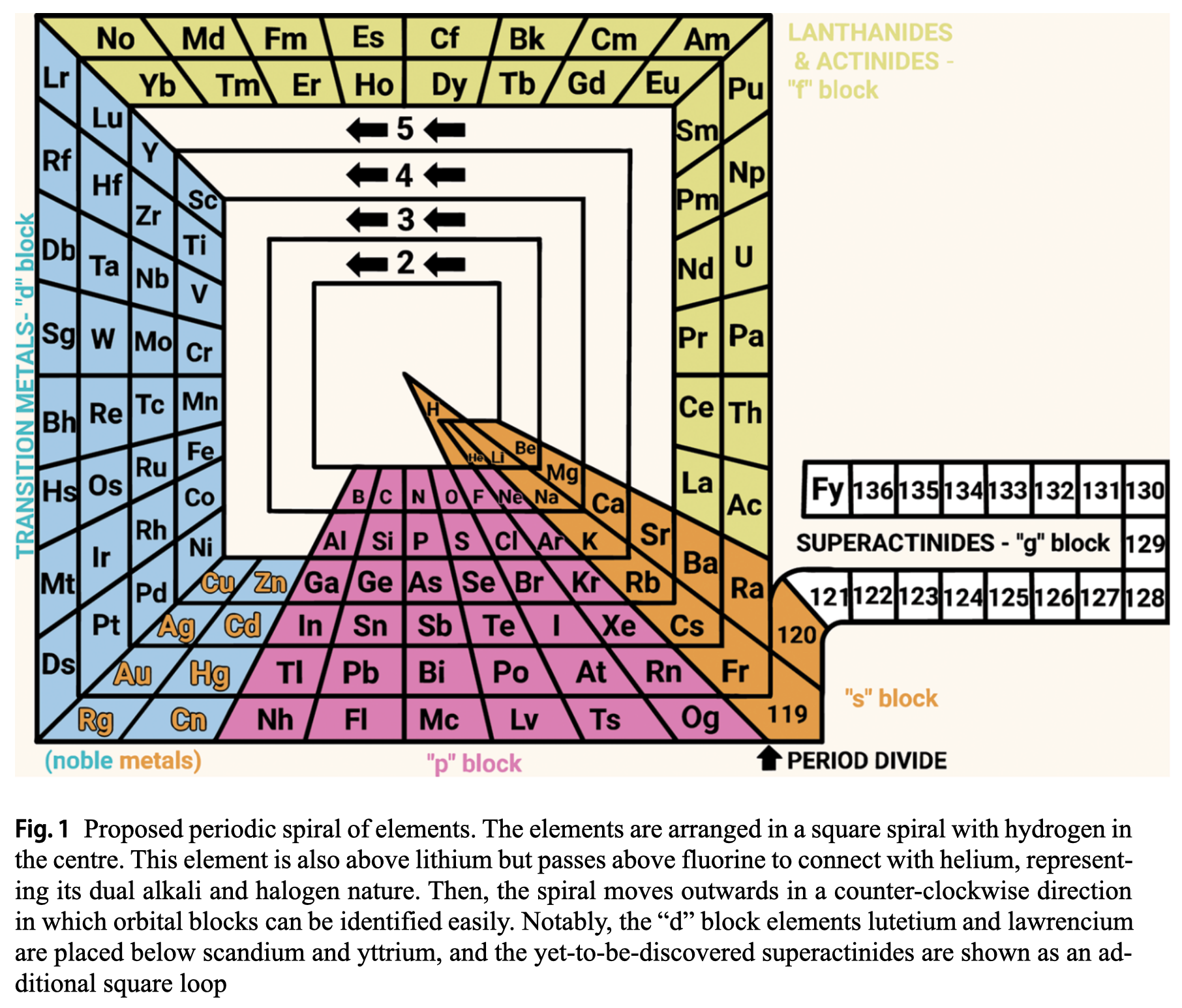
Rodríguez Peña & García Guerra's Periodic Spiral of The Elements
Rodríguez Peña, M., García Guerra, J.Á. The periodic spiral of elements. Found Chem (2024). https://doi.org/10.1007/s10698-024-09510-4
Abstract There are 2 main problems with the current periodic table: artificial breaks from a given noble gas to the next alkali metal (along with the common protrusion of the "f" block) and hydrogen placed in the alkali group, although this gas also exhibits halogen properties. This paper proposes arranging chemical elements in a square spiral with hydrogen at the centre. This element is also above lithium but passes above fluorine to connect with helium, representing its dual alkali and halogen nature effectively. Then the spiral moves outwards in a counter-clockwise direction, avoiding artificial breaks and following the natural direction of reading for the "s" and "p" blocks elements placed at the bottom of the spiral. Furthermore, this proposed square spiral improves upon previous Janet's and Benfey's representations with a more regular shape to draw, an effective depiction of the dual nature of hydrogen, and easily identifiable orbital blocks without the need for protrusions.
| Year: 2010 | PT id = 1310 |
Epicylindrical Periodic Table
An Epicylindrical Periodic Table by Steven Fowkes, who writes: "All the twist is confined to the s orbitals, 1/2 slant x 2 elements = one period lower."
Published in the Reed College Alumni Magazine March 2010.



| Year: 2018 | PT id = 1309 |
Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements
Robinson, Ann E., "Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements" (2018). Doctoral Dissertations. 1385.
https://doi.org/10.7275/12706048 https://scholarworks.umass.edu/dissertations_2/1385. Download and view the PDF.
See Ann E. Robinson's ORCID page.
Mark Leach writes:
"An excellent, comprehensive study that is full of details and references."

| Year: 1969 | PT id = 1308 |
100 Years of the Periodic Law of Chemical Elements
A Soviet Union publication in Russian celebrating Medeleeve's seminal work of 1869: 100 Years of the Periodic Law of Chemical Elements, X Centennial (Jubilee) Mendeleev Congress. The work is the product of 23 Authors. (Thanks to Ann E. Robinson, René Vernon & Valery Tsimmerman for the info.)




| Year: 2024 | PT id = 1307 |
Cylindrical Periodic Table with Seven Vertical Columns
The Cylindrical Periodic Table with Seven Vertical Columns by Laith H. M. Al-ossmi, College of Engineering, University of Thi-Qar, Iraq; Thi-Qar University Pres. Read the full paper here.
Abstract: In this article, a new model of the periodic table in cylindrical form wrapped around its outer circumference is presented, departing from the traditional periodic table of elements adopted by the International Union of Pure and Applied Chemistry (IUPAC). The cylinder is designed to encompass seven periodic periods, with elements distributed throughout based on their atomic order. This design allows for six vertical columns on the surface of the cylinder to represent the distribution of elements.


 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.