Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables from the year 1969:
| Year: 1969 | PT id = 15, Type = formulation |
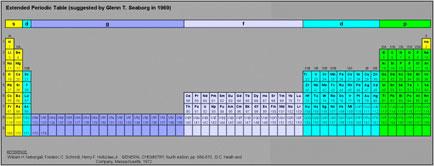
Glen T. Seaborg's g-Block Formulation
An long periodic table – developed by Glenn T. Seaborg in 1969 – containing the yet-to-be-discovered g-block elements can be constructed. For the full version and discussion, go to Jeries Rihani's pages, here and here.
| Year: 1969 | PT id = 16, Type = formulation |
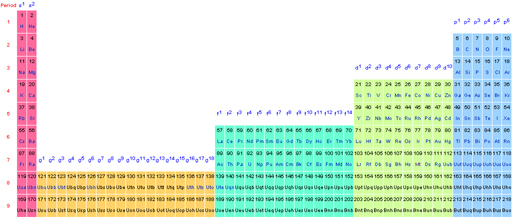
Wikipedia Extended Periodic Table
There is an extended Seaborg periodic Table on Wikipedia, here:
| Year: 1969 | PT id = 109, Type = review formulation |
J. W. van Spronsen, The Periodic System of Chemical Elements: A History of the First Hundred Years, Elsevier 1969
This book gives a good review and discussion of periodic table formulations. Anybody who is seriously interested in periodic table formulations will want to see/read/own this book.
| Year: 1969 | PT id = 520, Type = formulation 3D data |
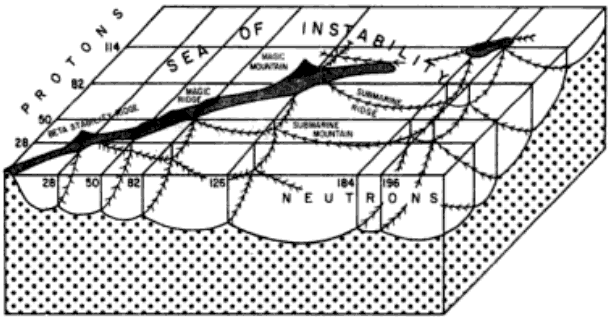
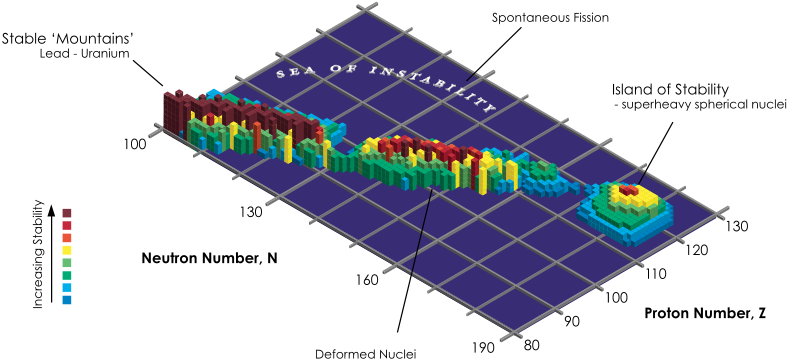
Island of Stability
From Wikipedia: The island of stability in nuclear physics describes a set of as-yet undiscovered isotopes of transuranium elements which are theorized to be much more stable than others. The possibility was proposed by Glenn T. Seaborg in the late 1960s: Prospectd for Further Considerable Extension of the Periodic Table, J.Chem.Educ., 46, 626-633 (1969) and reprinted in Modern Alchemy: Selected Papers of Glenn T. Seaborg (1994).
The hypothesis is that the atomic nucleus is built up in "shells" in a manner similar to the structure of the much larger electron shells in atoms. In both cases, shells are just groups of quantum energy levels that are relatively close to each other.
| Year: 1969 | PT id = 624, Type = formulation |
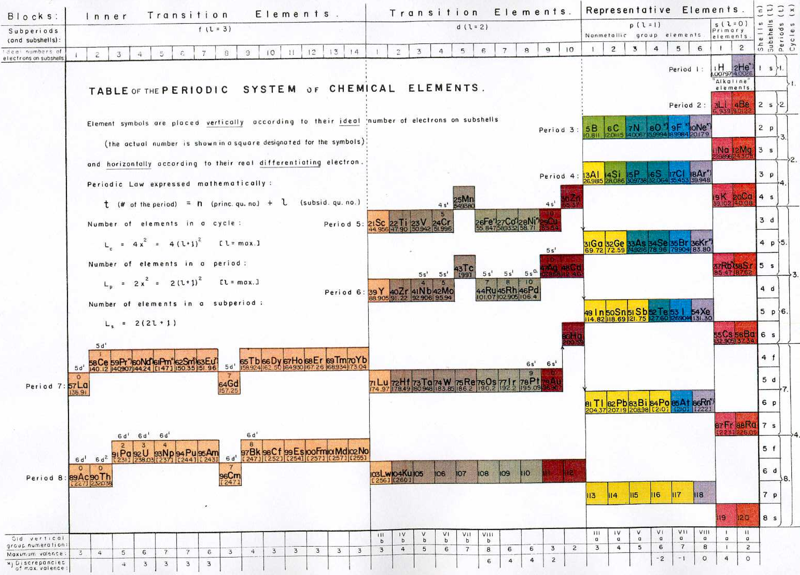
Mazurs Periodic System of Chemical Elements
A foldout from the Mazurs book, Graphical Representations of The Periodic System During 100 Years.
Mazurs said he drew it in 1967 and published it in 1969: ref. E Mazurs, A new numeration of periods in the periodic system and the Kessler Principle for the construction of the periodic table, Canad. Chem. Edu. 4(3), 21-23, 1969.
It is a Janet's modified system to show the irregularities – Lu, Cr, Pd etc. Click here for a larger version:
Thanks to Philip Stewart for the tip!
| Year: 1969 | PT id = 625, Type = formulation |
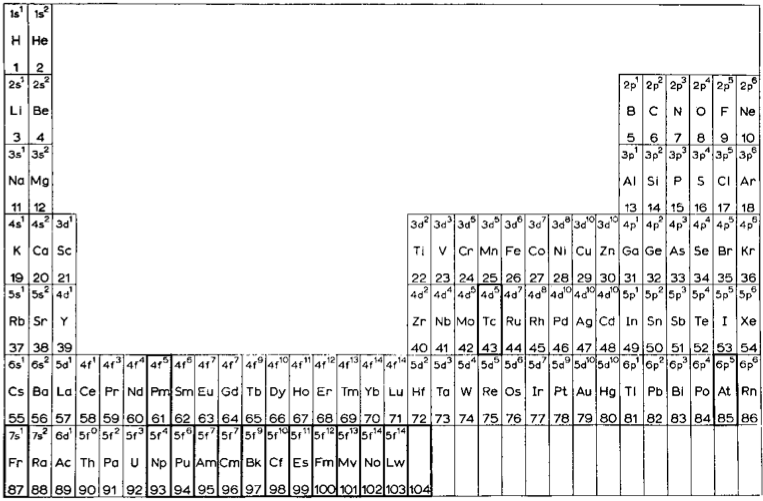
van Spronsen Periodic Table
From the van Spronsen book, The Periodic System of Chemical Elements: A History of the First Hundred Years:
Thanks to Philip Stewart for the tip!
| Year: 1969 | PT id = 884, Type = element |
Discovery of Rutherfordium
Rf ![]()
Rutherfordium, atomic number 104, has a mass of 267 au.
Synthetic radioactive element.
Rutherfordium was first observed in 1969 by A. Ghiorso et al. and I. Zvara et al.
| Year: 1969 | PT id = 1010, Type = formulation |
Dash's Quantum Table of the Periodic System of Elements
Harriman H. Dash, A quantum table of the periodic system of elements, International Journal of Quantum Chemistry, vol. 3, no. S3A, supplement: Proceedings of the International Symposium on Atomic, Molecular, and Solid?state Theory and Quantum Biology, 13/18 January 1969, pp. 335–340.
The abstract reads:
"The shortcomings of the long form of the periodic table of the chemical elements and the evident need for updating this format are briefly reviewed. To the question 'what format?' quantum physics provides an unequivocal answer. The foundations for the design of a quantum table are outlined. These are based on the principal quantum number as derived from the Schroedinger wave equation, the law of second order constant energy differences, and the coulomb–momentum interaction. These concepts are all combined into a single format which optimally and explicitly relates periodicity to atomic structure and the physical, chemical, and biological properties of the elements. This relationship emphasizes the unity and universality of all sciences."
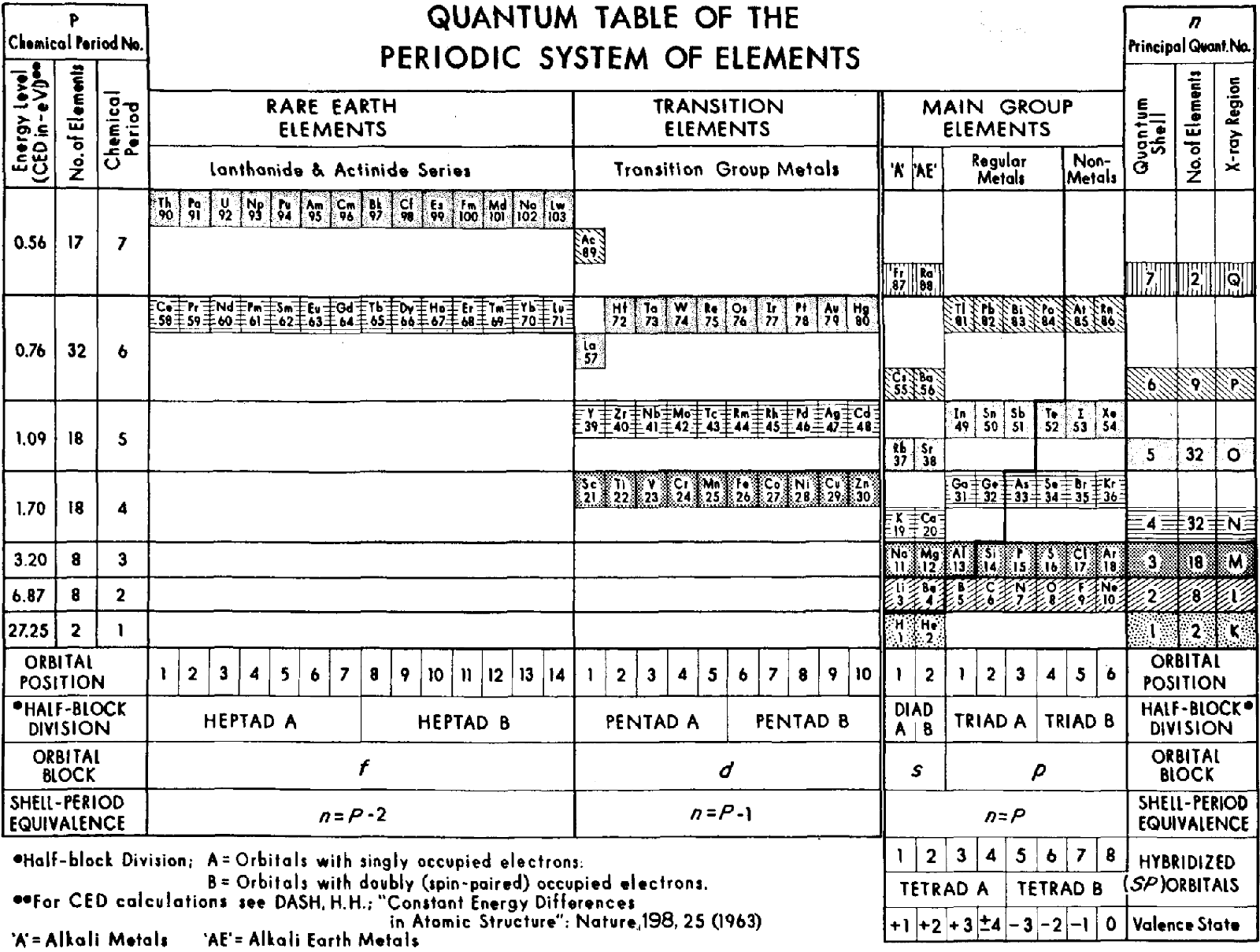
Thanks to René for the tip!
| Year: 1969 | PT id = 1126, Type = formulation |
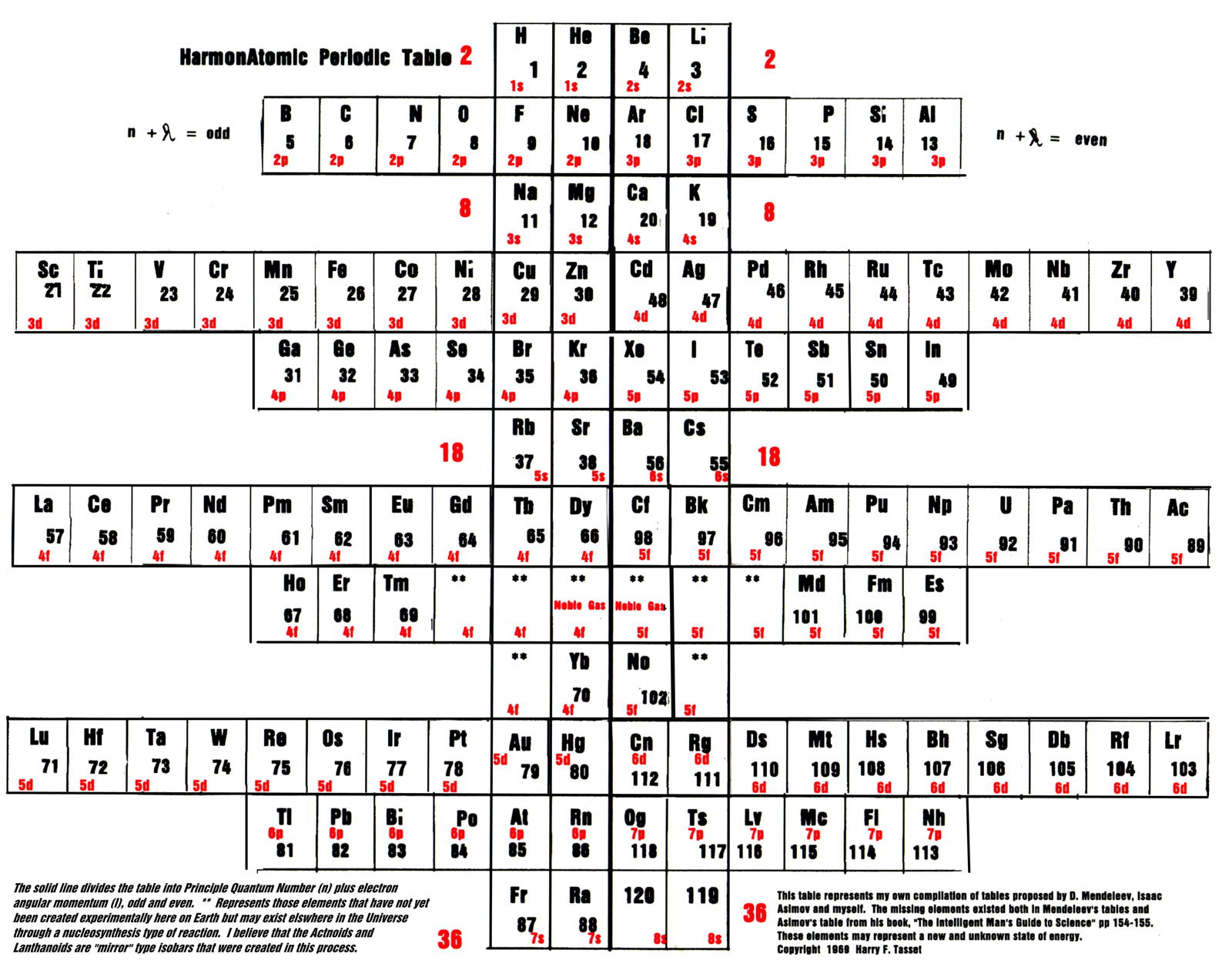
Tasset's HarmonAtomic Periodic Table
Harry F. Tasset writes:
"The periodic table (below) was originally copyrighted by me in 1969 and represents those missing elements that were lost due to the influence of physicists who were trying to mold the table into a more 'suitable' form. While they did succeed for many years, innovators like Charles Janet (left step table) and Isaac Asimov (missing elements table) had other ideas.
"Below you will see that the laws of chemistry, first discovered by Mendeleev, triumphed."
Click the image to enlarge
| Year: 1969 | PT id = 1146, Type = review |
Mendeleevian Conference, Periodicity and Symmetries in the Elementary Structure of Matter
Atti del Convegno mendeleeviano : periodicità e simmetrie nella struttura elementare della materia : Torino-Roma, 15-21 settembre 1969 / [editor M. Verde] Torino : Accademia delle Scienze di Torino ; Roma : Accademia Nazionale dei Lincei, 1971 VIII, 460 p.
Google Translate: Proceedings of the Mendeleevian Conference: periodicity and symmetries in the elementary structure of matter: Turin-Rome, 15-21 September 1969 / [editor M. Verde] Turin: Turin Academy of Sciences; Rome: National Academy of the Lincei, 1971 VIII, 460 p.
From the Internet Archive, the scanned book. Papers are in Italian & English.
For the 100th Anniversary of Mendeleev's iconic periodic table, a conference was held to look at (review) the elementary structure of matter. The 1960s saw huge developments in particle physics, including the theory of quarks. Papers were presented by many notable scientists including John Archibald Wheeler and the Nobel laureates: Emilio Segrè & Murray Gell-Mann.Thanks to René for the tip!
| Year: 1969 | PT id = 1270, Type = formulation data misc |
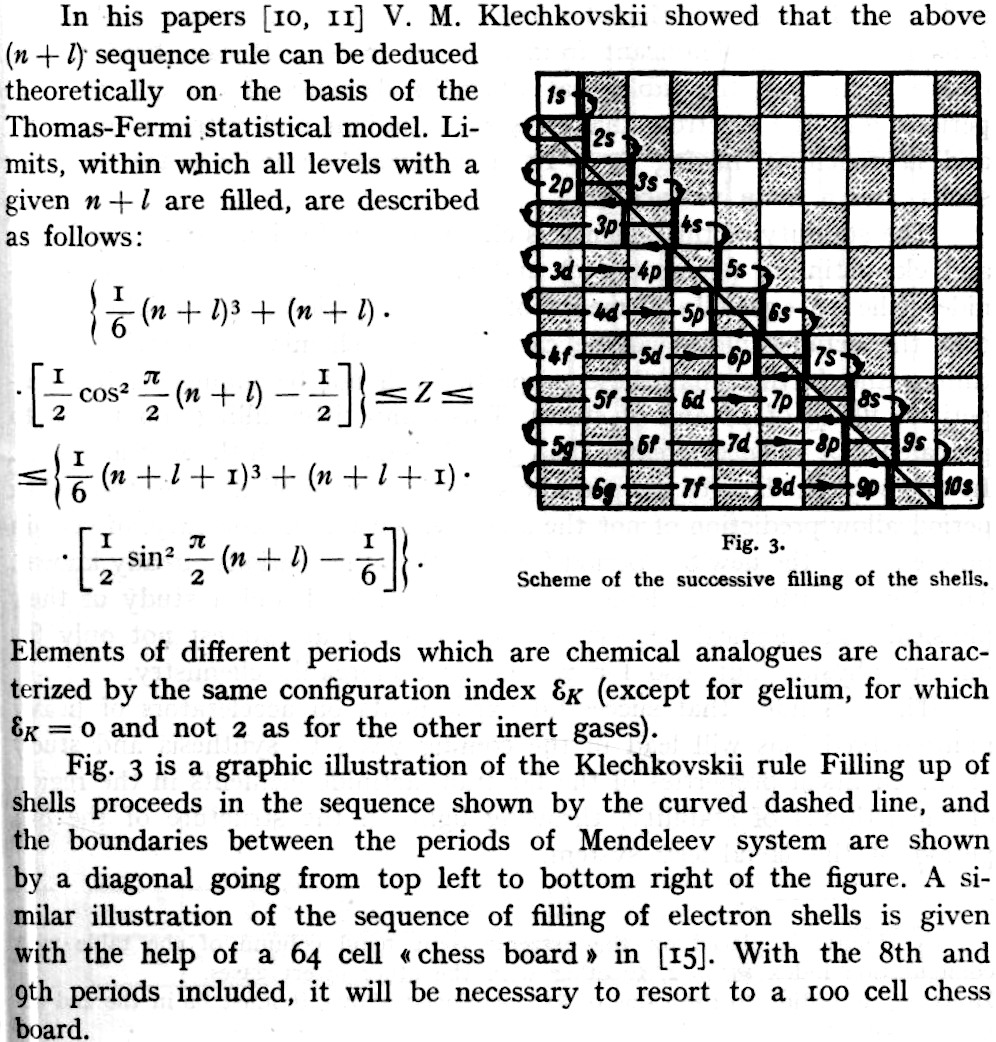
Seel-Klechkovskii Version of Madelung's Rule for Orbital Filling
Seel F., Bild der Wissenschaft, 6, 44 (1969), a monthly popular scientific journal.
- Klechkovskii VM, Dokl. Akad. Nauk. SSSR, 135. 855 (1980). [In Russian]
- Klechkovskii VM, Zh. Eksperim. i. Teoret. Fiz., 41. 465 (1961). [In Russian]
Thanks to René for the tip!
| Year: 1969 | PT id = 1273, Type = formulation data |
Martin's Crystal Structure Periodic Table
Ref: Martin JW 1969, Elementary Science of Metals, Wykeham Publications, London
René Vernon writes:
Note the unusual placement of La-Ac in two places, under Y and before Ce-Th. On another aspect, Martin writes:
"The non-metals, which occupy the top right-hand corner of the Periodic Table... form about one-sixth of all elements, and they are characterized by having melting-points and boiling points below about 500°C, and by having their solid and liquid phases not conducting electricity. About two-thirds of all elements are metals, and a further one sixth have properties intermediate between those of metals and non-metals."
His approach to the question of which elements are metals and non-metals, and which are intermediate may be the most useful "rough-and-ready" rubric I've seen. It is remarkable for its use of four criteria.
Perhaps we can then parse the elements as follows
Non-metals (16) = 15.5%
Fluids: H, N, O, F, Cl, Br; He, Ne, Ar, Kr, Xe, Rn 2
Solids: P, S, Se*, IIntermediate (16) = 15.5%
Metalloids: B, Si, Ge, As, Sb, Te
Near metalloids: C, At 3
Sub-metalloids: Al, Ga, In, Tl; Sn, Pb; Bi; PoMetals (71) = 68.9%
Be,^ Zn^
All the rest^ Borderline intermediate
Dingle (2017, The Elements: An Encyclopedic Tour of the Periodic Table, Quad Books, Brighton, p. 101) puts the situation this way:
"...the gap between the two extremes [of metals and nonmetals] is bridged... by the poor metals, and... the metalloids – which, perhaps by the same token, might collectively be renamed the poor non-metals.”
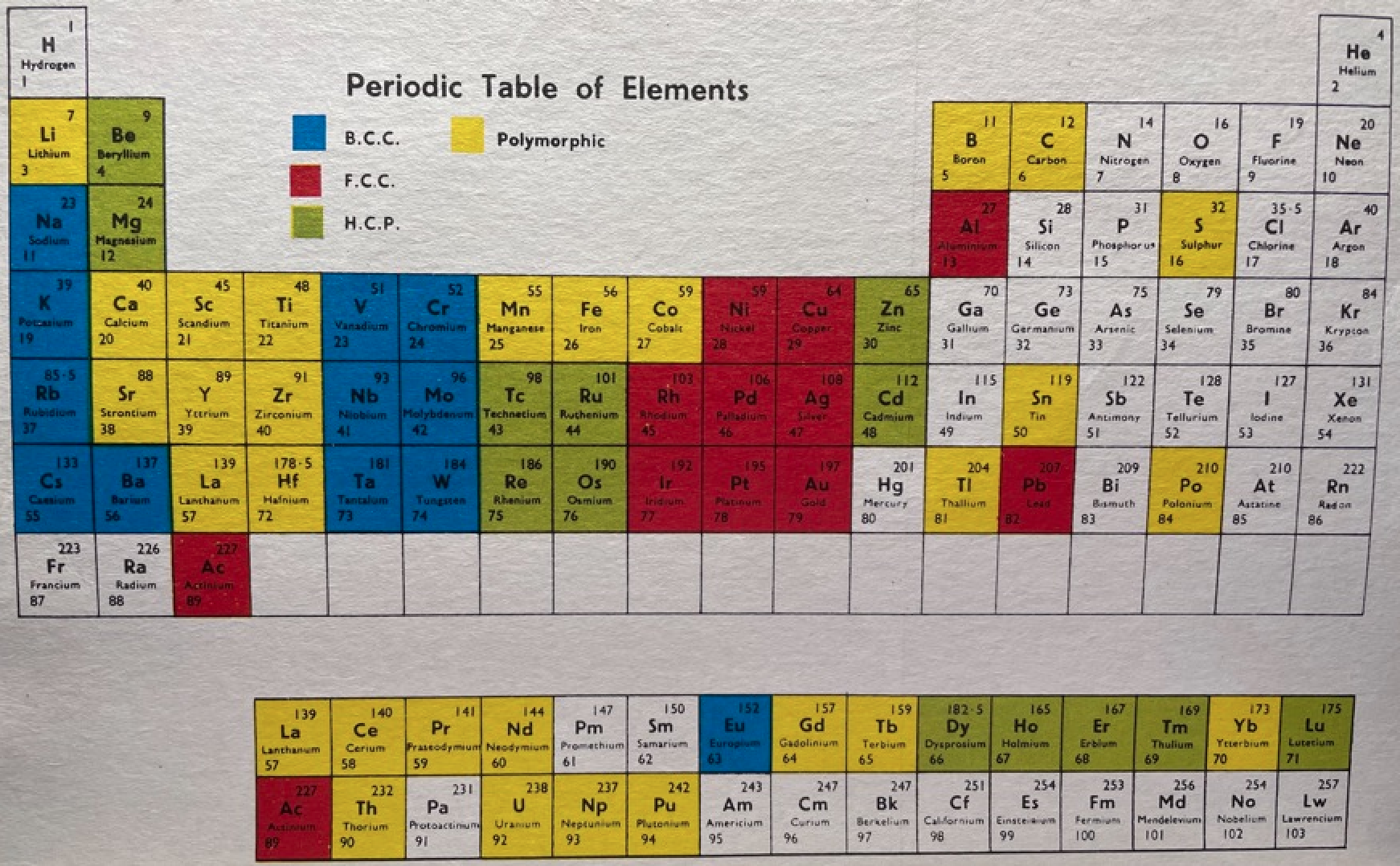
Redrawn by Vernon:
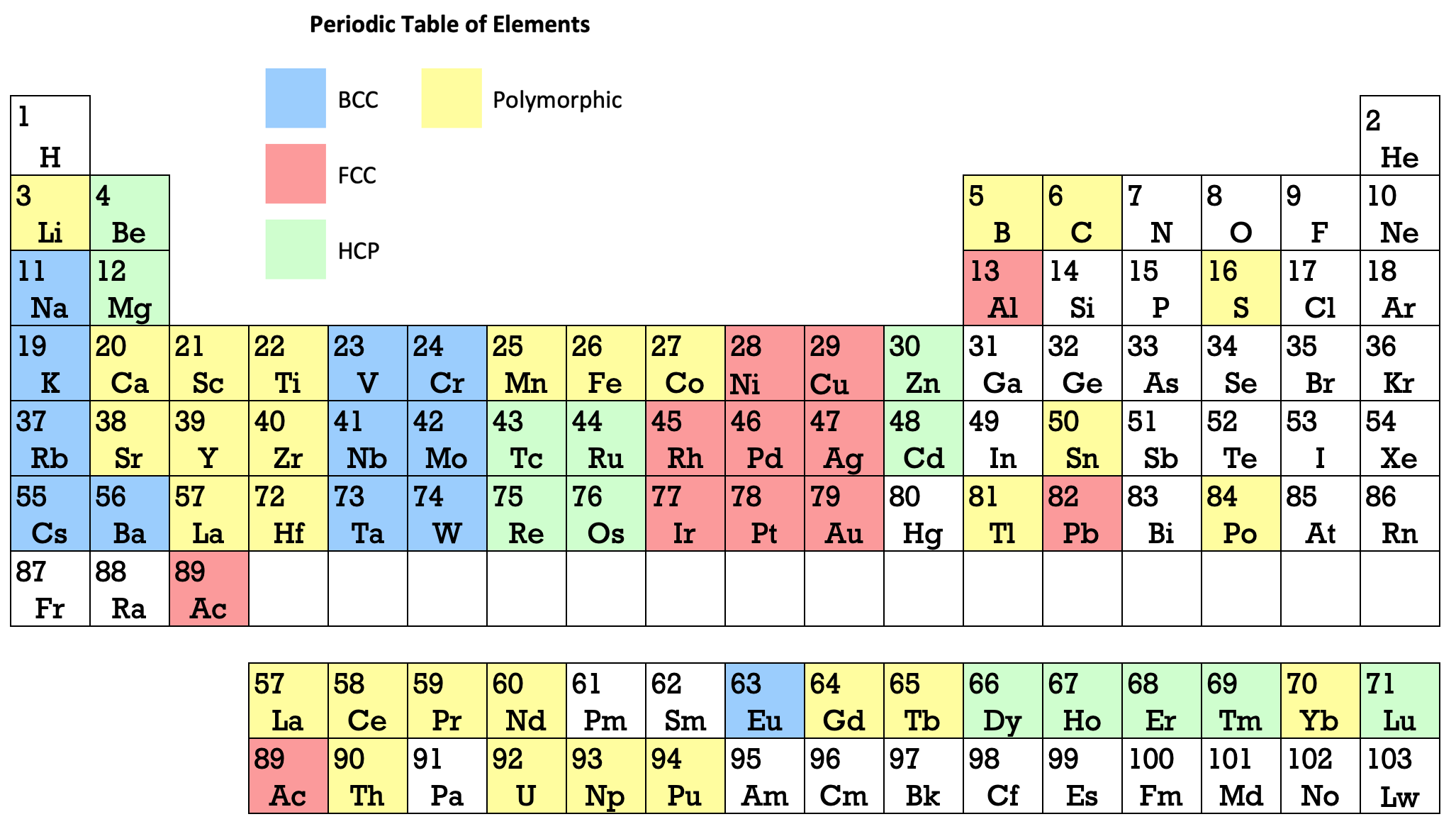
Thanks to René for the tip!
| Year: 1969 | PT id = 1308, Type = review formulation |
100 Years of the Periodic Law of Chemical Elements
A Soviet Union publication in Russian celebrating Medeleeve's seminal work of 1869: 100 Years of the Periodic Law of Chemical Elements, X Centennial (Jubilee) Mendeleev Congress. The work is the product of 23 Authors. (Thanks to Ann E. Robinson, René Vernon & Valery Tsimmerman for the info.)




 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.