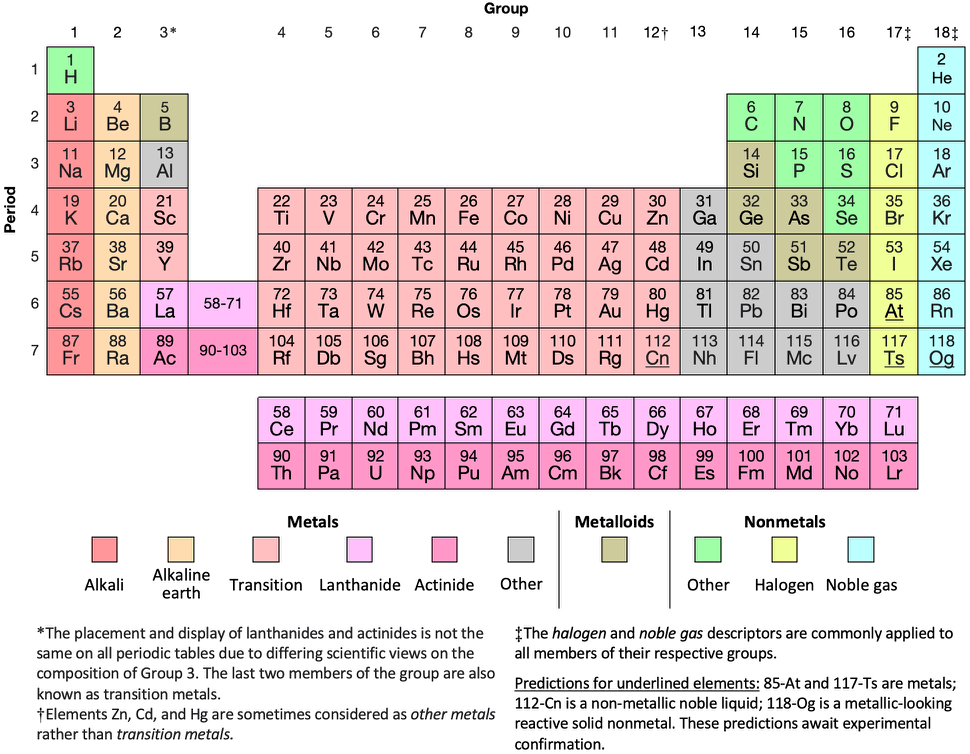
Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH the Database:
-
By Year
Formulations 2020 to present day Formulations 2010 – 2019 Formulations 2000 – 2009 Formulations 1950 – 1999 Formulations 1900 – 1949 Formulations 1850 – 1899 Formulations 1800 – 1849 Formulations Before 1800 =================== All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added =================== 2026 2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
- By Type
- Pre-Selected
Periodic Tables referencing the text string "Pauling", listed by date:
| Year: 1836 | PT id = 453, Type = formulation data |
Berzelius' Electronegativity Table
Berzelius' electronegativity table of 1836.
The most electronegative element (oxygen or Sauerstoff) is listed at the top left and the least electronegative (potassium or Kalium) lower right. The line between hydrogen (Wasserstoff) and gold seperates the predomently electronegative elements from the electropositive elements. Page 17 and ref. 32 from Bill Jensen's Electronegativity from Avogadro to Pauling Part I: Origins of the Electronegativity Concept, J. Chem. Educ., 73, 11-20 (1996):

| Year: 1870 | PT id = 454, Type = formulation data |
Baker's Electronegativity Table
Baker's electronegativity table of 1870 differs from Berzelius' listing of 1836 only by the addition of the newly discovered elements. Page 280 and ref. 5 from Bill Jensen's: Electronegativity from Avogadro to Pauling Part II: Late Nineteenth- and Early Twentieth-Century Developments, J. Chem. Educ., 80, 279-287 (2003):

| Year: 1895 | PT id = 368, Type = formulation |
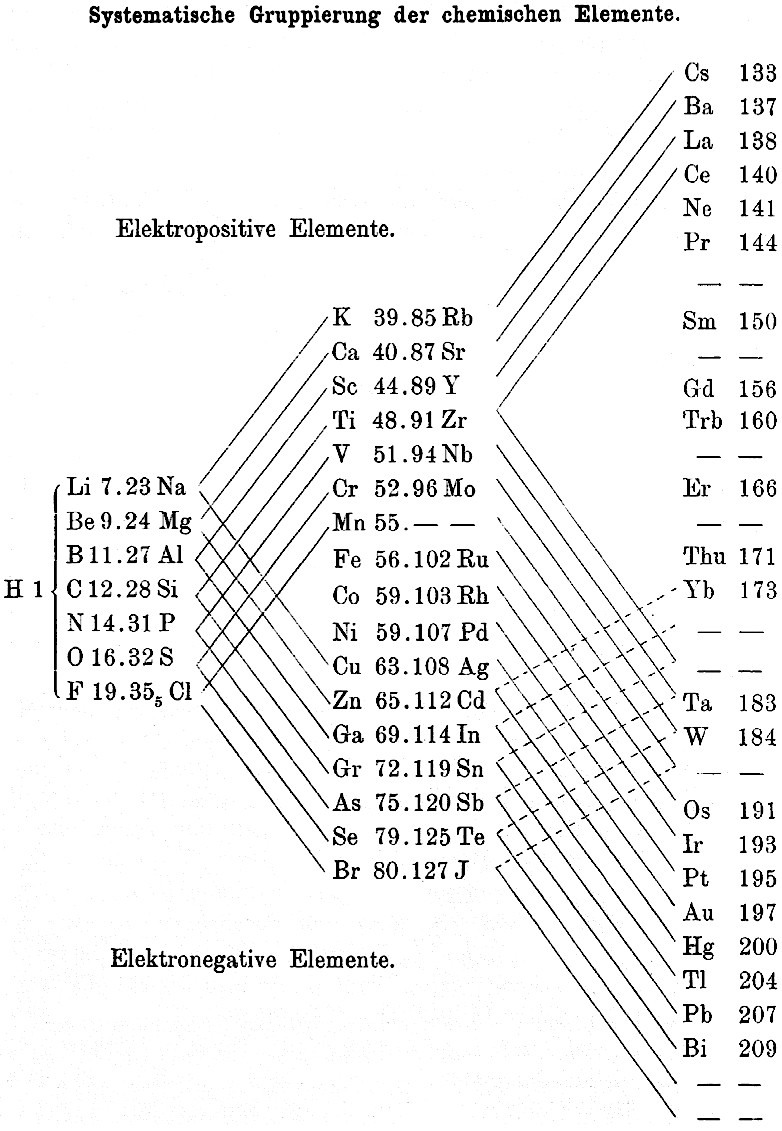
Thomsen's Systematic Arrangement of the Chemical Elements
In 1895 the Danish thermochemist Hans Peter Jørgen Julius Thomsen proposed (Thomsen, J., 1895. Z. Anorg. Chem. 9, 190 & Chemical News, 72, 89–91, p. 90) a pyramidal/ladder representation.
Notice how this formulation identifies the electropositive & electronegative elements with respect to the periodic table, thirty years before Linus Pauling.
| Year: 1926 | PT id = 26, Type = formulation |
Antropoff's Periodic Table
The Andreas von Antropoff periodic table, restored by Philip Stewart on the basis of the article 'Eine neue Form des periodischen Systems der Elementen'. Zeitschrift für angewandte Chemie 39, pp. 722-725, 1926:
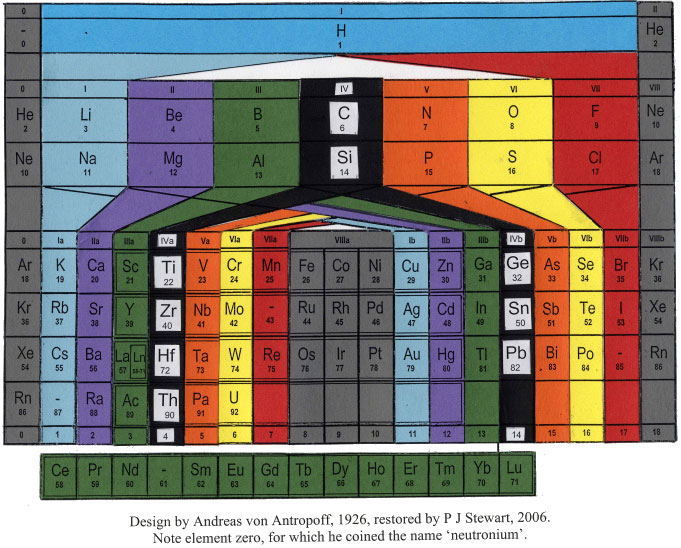
This formulation has a satisfying balance compared to most other tables and was the most popular wall-chart in German schools for many years but quickly disappeared after von Antropoff was disgraced in 1945 for his Nazi activities: he presided over the raising of the swastika over Bonn University in 1933. But he put science above politics and was a stout defender of Einstein's theories.
A recently restored wall version of the von Antropoff formulation from the University of Barcelona, origionally painted in 1934 (thanks to Philip Stewart & Claudi Mans):

Perhaps it was the disgrace of von Antropoff which led Linus Pauling to borrow his design, without acknowledgement, for his 1949 book, General Chemistry (and subsequently in later editions of The Chemical Bond).
The PT below is scanned in from Pauling's The Nature of The Chemical Bond, 3rd ed., 1960:
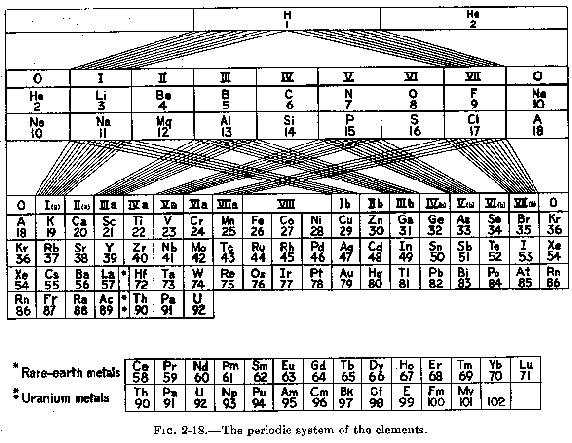
| Year: 1949 | PT id = 27, Type = formulation |
Pauling's Formulation
Linus Pauling borrowed von Antropoff 1926 design, without acknowledgement, for his 1949 book, General Chemistry (and subsequently in later editions of The Chemical Bond).
The periodic table below is scanned in from Pauling's The Nature of The Chemical Bond, 3rd ed., 1960:

| Year: 1958 | PT id = 1263, Type = formulation |
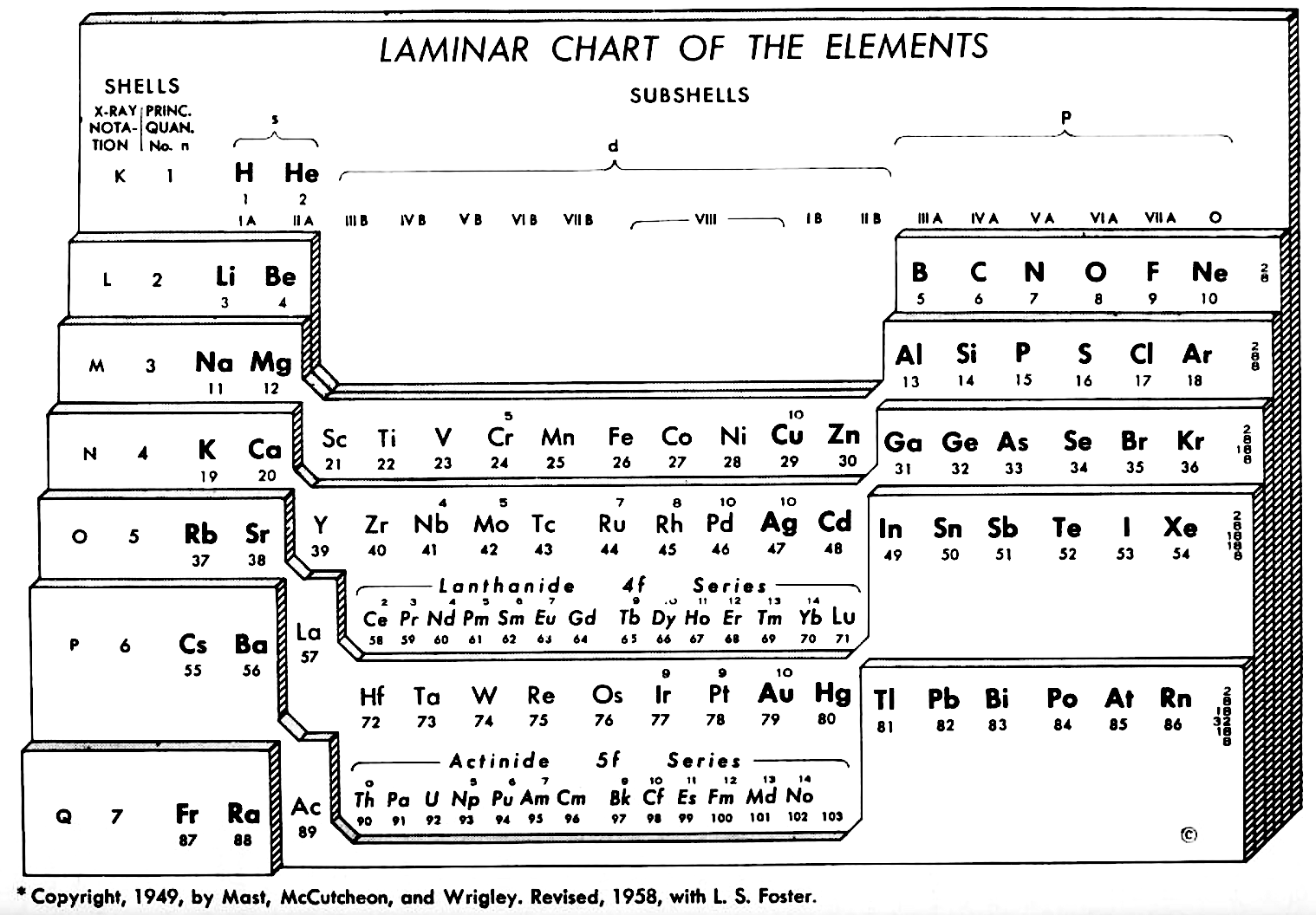
Weaver & Foster's Laminar Chart of the Elements
Weaver EC & Foster LS 1960, Chemistry For Our Times. 3rd ed., McGraw-Hill, New York, p. 382
René Vernon writes:
An earlier version of this table appeared in JChemEd in 1949. The authors then wrote:
"It is apparently difficult to give a proper idea of electronic configuration in two dimensions without spreading out vertically or horizontally, and thereby sacrificing the order of atomic number, or compactness, or both. In three dimensions it is entirely feasible, but the first reaction is to discard three dimensions as too awkward. The laminar chart here proposed seems to the authors to possess the advantages of both the two dimensional and three dimensional charts and to have none of their disadvantages.
"A minor feature of the table, introduced for reasons of expediency, is the artificial break between the first and the second main shell. Use is made of this space to print the traditional group headings, I A, III A, IVB, etc., which are firmly entrenched in the literature, and still find active use as classifying labels. Other objects in making the artificial break were to minimize the resemblance between hydrogen and the alkali metals and to emphasize helium's character as an inert gas (completed 1s subshell), rather than, as might otherwise be supposed, a member of the alkaline earth family.
"CONTOUR LAMINAR TABLE
"By another modification, constructing the Periodic Chart in the form of contour laminae, it is possible to represent actual energy levels without the necessity of referring to auxiliary tables. This is done by proportioning the rises between each subshell to correspond to the Pauling energy diagram. Thus, although the subshells having the same principal quantum number will be on the same contour lamina, they will not be on the same planar level. The recognition of these contour laminae is facilitated by the use of a different color for each one. A table of this type will then be more physically correct than the previous laminar models, and it is a question as to which form has the most practical utility.
"We believe that the laminar periodic tables, in either the original or a modified form, will greatly facilitate systematic teaching of the properties of the chemical elements. Students indoctrinated with the new system cannot fail to obtain a clearer and more lasting conception of the fundamental principles of inorganic chemistry."
Note the 4f and 5f series have been split into dyads of seven apiece. This is consistent with Shchukarev (1974, p. 118) who wrote that the filling sequence among the 4f metals is periodic, with two periods. Thus, after the occurrence of a half-full 4f subshell at europium and gadolinium, the filling sequence repeats with the occurrence of a full subshell at ytterbium and lutetium (Rokhlin 2003, pp. 4–5). A similar, but weaker, periodicity (Wiberg 2001, pp. 1643–1645) is seen in the actinoids, with a half-full 5f subshell at americium and curium, and a full subshell at nobelium and lawrencium.
Note that Zn, Cd, Lu and Hg have no electron numbers above them since the underlying shells were filled at Cu, Cd, Yb, and Au respectively.
- Rokhlin LL 2002, Magnesium Alloys Containing Rare Earth Metals: Structure and Properties, Taylor & Francis, London
- Shchukarev SA 1974, Neorganicheskaya khimiya, vol. 2. Vysshaya Shkola, Moscow (in Russian)
- Weaver EC & Foster LS 1960, Chemistry For Our Times. 3rd ed., McGraw-Hill, New York, p. 382
- Wiberg N 2001, Inorganic Chemistry, Academic Press, San Diego
- Wrigley AN, Mast WC & McCutcheon TP 1949, A laminar form of the periodic table, Part I, Journal of Chemical Education, 26(4), 216
- —— A laminar form of the periodic table, Part II, Journal of Chemical Education, 26(5), 248
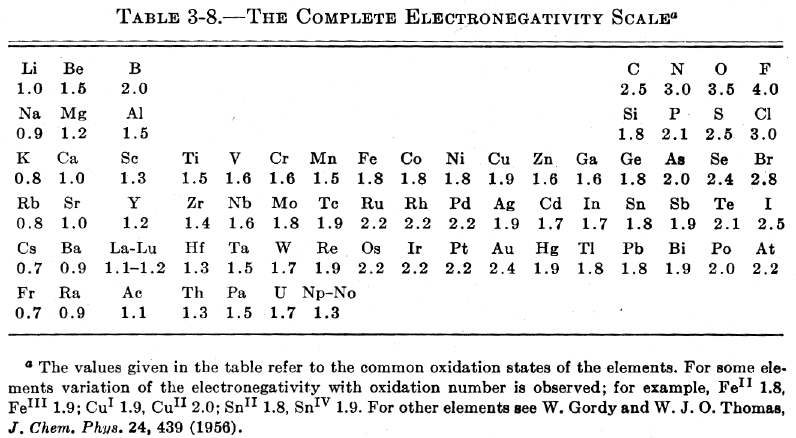
| Year: 1960 | PT id = 444, Type = formulation data |
Pauling's Complete Electronegativity Scale
From The Nature of The Chemical Bond, 3rd Ed, pp 93, Pauling gives a periodic table showing the electronegativity of the elements.
Notice how the d block appears between groups 3 and 4 (13 & 14), rather than between groups 2 and 3 (2 & 13):
| Year: 1964 | PT id = 1006, Type = formulation |
Haward's Periodic Table
Roger Hayward created this periodic table for the book: Pauling & Hayward, p4, The Architecture of Molecules, W H Freeman and Company, San Francisco (1964).
From The Pauling Blog:
"By the end of the 1950s, Roger Hayward had retired from his professional work as an architect at the same time that his career as an illustrator was reaching its peak. Hayward signed a contract in the early 1960s that helped to solidify his position as a technical artist. The contract that Hayward signed was with W.H. Freeman & Company, a San Francisco-based publishing house that rose out of relative obscurity primarily by publishing Linus Pauling's hugely popular textbook, General Chemistry."
Thanks to René for the tip!
| Year: 1970 | PT id = 1188, Type = formulation data |
Energy Level Diagram of Electron Shells & Subshells of the Elements
Figure 5-11 from page 128 of Linus Pauling's General Chemistry, W.H. Freeman, San Francisco 1970 (Dover Edition 1988):
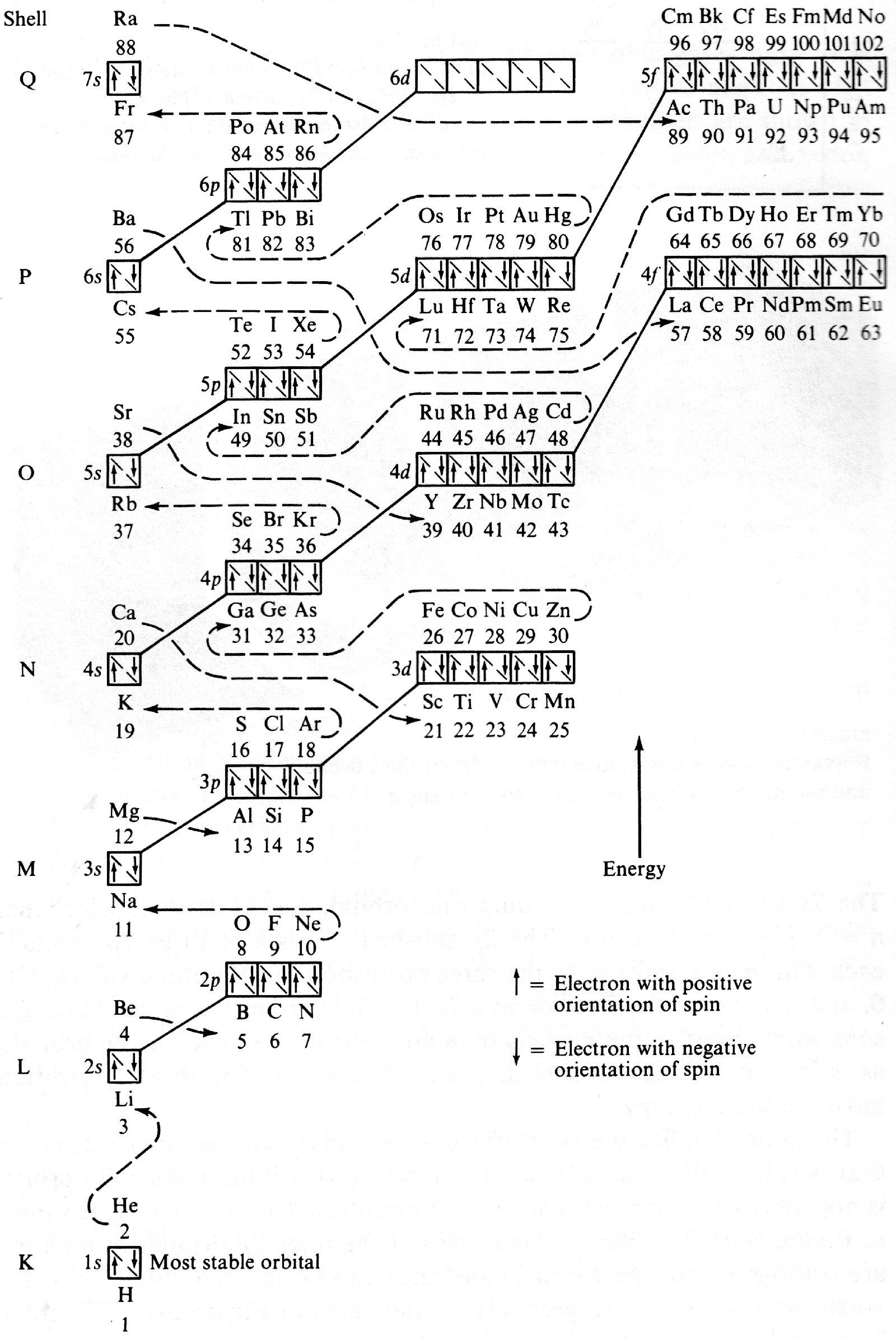
| Year: 1970 | PT id = 1007, Type = formulation |
Pauling's "General Chemistry" Periodic Table
From Linus Pauling's General Chemistry (3rd Ed.). Notice that the noble gases apear twice, at the beginning and the end of each period.
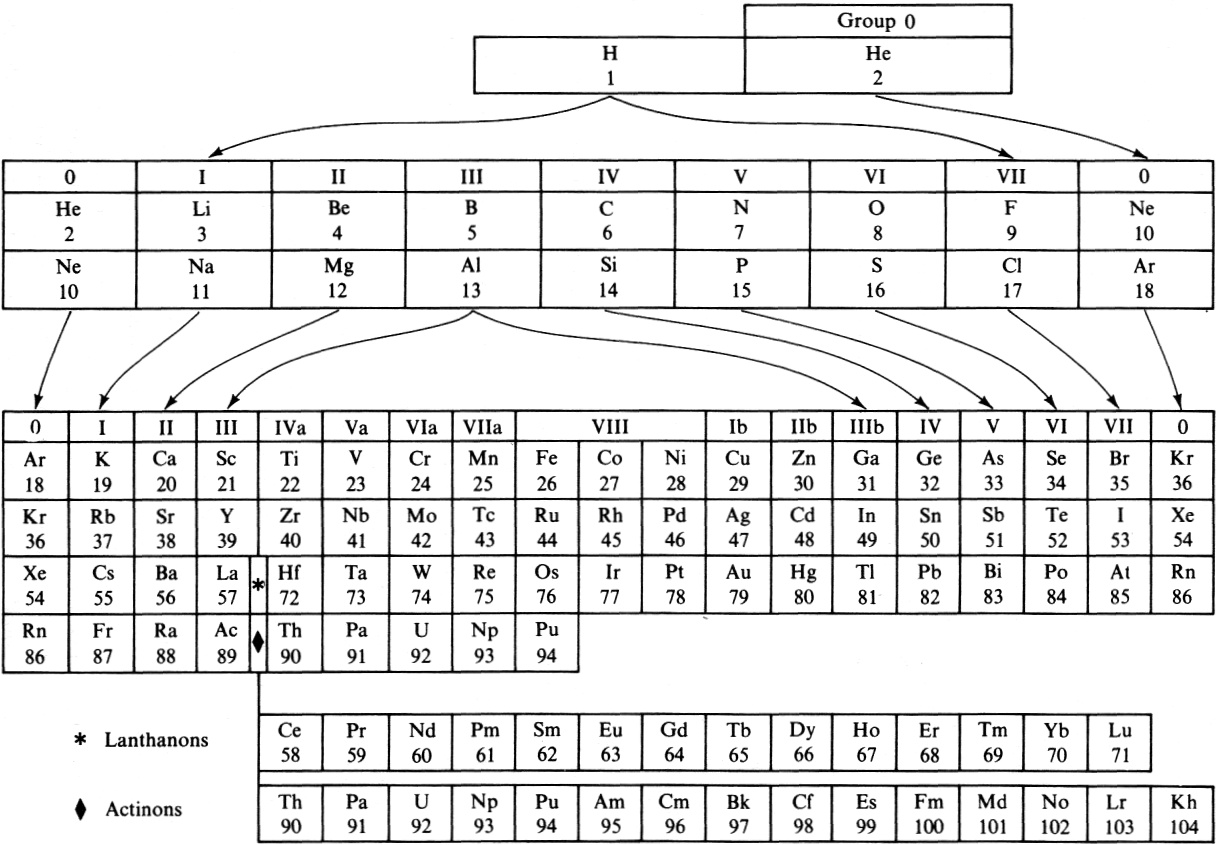
Thanks to René for the tip!
| Year: 1987 | PT id = 743, Type = data |
Elsevier's Periodic Table of the Elements
Prepared by P. Lof is Elsevier's Periodic Table of the Elements.
This educational wall chart features the periodic table of the elements supported by a wealth of chemical, physical, thermodynamical, geochemical and radiochemical data laid down in numerous colourful graphs, plots, figures and tables. The most important chemical and physical properties of the elements can be found - without turning a page.
All properties are presented in the form of tables or graphs. More than 40 properties are given, ranging from melting point and heat capacity to atomic radius, nuclear spin, electrical resistivity and abundance in the solar system. Sixteen of the most important properties are colour coded, so that they may be followed through the periodic system at a glance. Twelve properties have been selected to illustrate periodicity, while separate plots illustrate the relation between properties. In addition, there are special sections dealing with units, fundamental constants and particles, radioisotopes, the Aufbau principle, etc. All data on the chart are fully referenced, and S.I. units are used throughout.
Designed specifically for university and college undergraduates and high school students, "Elsevier's Periodic Table of the Elements" will also be of practical value to professionals in the fields of fundamental and applied physical sciences and technology. The wall chart is ideally suited for self-study and may be used as a complementary reference for textbook study and exam preparation.
- atomic number
- standard atomic weight
- ground-state electronic configuration
- element symbol
- element name
- discoverer and year of discovery
- melting point; boiling point
- critical temperature
- molar enthalpy of atomization
- molar enthalpy of fusion
- molar enthalpy of vaporization
- atomic energy levels of the outermost three orbitals
- formal oxidation states
- selection of standard reduction potentials
- first, second & third molar ionization energies
- Pauling electronegativity
- Allred-Rochow electronegativity
- molar electron affinity
- molar volume
- crystal structures
- polymorphic transition temperatures
- atomic radius
- effective ionic radii
- volumic mass (density)
- electrical resistivity
- thermal conductivity
- abundance in the solar system
- abundance in the Orgueil meteorite
- abundance in the solar photosphere
- abundance in the continental crust
- abundance in the primitive mantle
- abundance in the oceanic crust
- naturally occurring isotopes
- mass number and representative isotopic composition
- molar heat capacity
- Debye temperature
- coefficient of linear thermal expansion
- price; annual mining production
- world reserve base
- nuclear spin and NMR receptivity
- Mossbauer active nuclides
- physical (standard) state
- metallic character
- abundance in food (human daily intake)
- principal hazardous property
- Other information: Aufbau principle, quantum numbers, orbitals and sequence of orbital filling; trivial group names; drawings of crystal lattice structures; 12 plots of a chemical/physical property against atomic number; 9 plots of a property against another property; list of SI units and SI prefixes; list of other units and their conversion to SI; list of fundamental physical constants; scheme of fundamental particles; list of radioisotopes with half-life longer than 5 days, presenting half-life and mode(s) of decay, indicating cosmogenic isotopes and isotopes produced by U-235 fission, as well as radioisotopes used in geochronology, pharmacology and nuclear medicine.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 2001 | PT id = 1322, Type = review misc formulation |
Oliver Sacks, Uncle Tungsten: Memories of Tungsten of a Chemical Beyond
René Vernon writes:
On the paperback cover of Oliver Sack's Uncle Tungsten (below) the periodic table shows a 16–wide set of elements at its base. This is quite unusual since this set is normally shown as being 15— or 14— elements wide. See, for example, the table found on the site of the International Union of Pure & Applied Chemistry which shows a 15–wide set of elements at its base.
It looks like the second pair are La and Ac, but what then are two immediately preceding elements?
I suspect they are probably the alkaline earth metals, Ba and Ra. This may be an homage to Mr Rare Earth^ aka Karl A. Gschneidner Jr (1930–2016), who wrote that:
...since Ba has a 4f06s2 configuration, these three elements are the first (Ba), mid (Eu), and end (Yb) members of the divalent 4f transition series.
The notion of 4f0 is not unprecedented; the IUPAC periodic table, with its 15-wide f-block presumably implies La as 4f0 5d1 6s2.
There is some good chemistry going on here, given the pronounced similarities between Ba and the lanthanides, and the alkaline earth metals generally with about 20 properties involved:
- Most of the physical properties of Eu and Yb, "such as the atomic volumes, metallic radii, melting and boiling points, heats of sublimation, compressibilities, and coefficients of expansion are more like those of the alkaline-earth metals, Ca, Sr, and Ba, than those of the rare-earth metals" (Pauling 1960, p. 418; Gschneidner 1964, p. 286).
- Liquid ammonia dissolves certain alkali, alkaline earth, and Ln metals, and... combines with them to form solid compounds. Those metals whose compound-forming ability has been confirmed are Li, Ca, Sr, Ba, Eu and Yb. (Mammano (1970, p. 367)
- The lanthanides are sometimes regarded as trivalent versions of the alkaline earth metals (Evans 1982).
- The electron configurations of lanthanide cations are similar to those of alkaline earth metal cations, as the inner f- orbitals are largely or completely unavailable for bond formation; (Choppin & Rizkalla 1994)
- The lanthanide trivalent cations are essentially spherical and present an environment very similar to alkali and alkaline earth ions towards complex formation... the standard electrode potentials for the lanthanides have similar values and are comparable with the redox potentials of alkaline earth metals (Sastri et al. 2003)
- Ba-Eu-Yb have cubic crystalline structures whereas the rest of the Ln are hexagonal, or rhombohedral in the case of Sm (Russell & Lee 2005)
- There is a close alloying similarity between the lanthanides and Ca, Sr and Ba (Artini 2007)
- Lanthanides are effective mimics of calcium and can stimulate or inhibit the function of calcium-binding proteins (Brayshaw 2019)
- Lanthanide cations can substitute for Ca2+ and Sr2+ cations in host materials for solid state lasers (Ikesue 2013)
- There is a knight’s move relationship between Ca and La:
- The ionic radius of Ca2+ is 114 pm; that of La3+ is 117 pm
- The similarity in sizes means La3+ will compete with Ca2+ in the human body, and usually win on account of having a higher valence for roughly the same hydrated radius
- The basicity of La2O3 is almost on par with CaO2 Freshly prepared La2O3 added to water reacts with such vigour that it can be quenched like burnt lime (CaO)
- The electronegativity of Ca is 1.0; that of La is 1.1.
Kudos to Oliver.
^Pecharsky 2016
Sources
- Artini C (ed.) 2017, Alloys and Intermetallic Compounds: From Modeling to Engineering, CRC Press, Boca Raton, p. 92
- Brayshaw et al. 2019, Lanthanides compete with calcium for binding to cadherins and inhibit cadherin-mediated cell adhesion, Metallomics, vol. 11, no. 5, 2019, pp. 914–924
- Choppin GR & Rizkalla EN 1994, Solution chemistry of actinides and lanthanides, Handbook on the Physics and Chemistry of Rare Earths, pp. 559–590(560)
- Evans WJ 1982, Recent advances in the low valent approach to f-element organometallic chemistry, in McCarthy GJ, Silber HB and Rhyne JJ (eds), The Rare Earths in Modern Science and Technology, vol. 3, Plenum Press, New York, pp. 61–70(62)
- Gschneidner KA 1965, in Seitz F & Turnbull D (eds), Solid State Physics, vol. 16, Academic Press, New York, p. 286
- Ikesue A, Aung YL, Lupei V 2013, Ceramic Lasers, Cambridge University Press, Cambridge, pp. 26, 28
- Mammano N 1970, Solid metal ammonia compounds, in Metal–Ammonia Solutions, Proceedings of an International Conference on the Nature of Metal–Ammonia Solutions: Colloque Weyl II, pp. 367-393 (367), https://doi.org/10.1016/B978-0-408-70122-8.50030-4
- Pauling L 1960, The Nature of the Chemical Bond, 3rd ed., Cornell University Press, Ithaca, p. 418
- Pecharsky V 2016, Karl A. Gschneidner Jr (1930–2016), Nature Materials, vol. 15, no. 1059, https://doi.org/10.1038/nmat4751
- Russell AM & Lee KL 2005, Structure-property relations in nonferrous metals, John Wiley & Sons, Hoboken, inside cover
- Sastri et al. 2003, Modern Aspects of Rare Earths and their Complexes, Elsevier, Amsterdam, pp. 377, 878

| Year: 2003 | PT id = 123, Type = data |
Electronegativity Periodic Table
A periodic table showing electronegativity, "The ability of an atom to attract electron density from a covalent bond" (Linus Pauling). Blue elements are electronegative, red elements are electropositive, and purple elements are intermediate. Notice how hydrogen is intermediate in electronegativity between carbon and boron and is positioned above and between these elements:
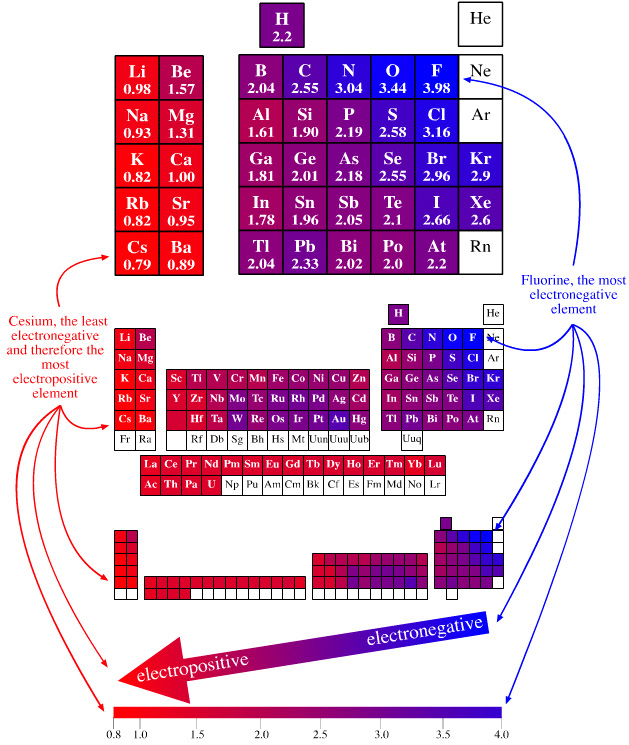
By Mark Leach
| Year: 2010 | PT id = 352, Type = formulation |
Pauling Spheron Periodic Table
The Pauling Spheron Periodic Table, can be found at APM Periodic Tables.
Linus Pauling was a brilliant physicist who tended to think outside the mainstream. One of his many contributions to science was his spheron model for the nucleus. The word "spheron" does not mean the nucleus is spherical (although it may be), it refers to Pauling's idea that clusters might form in the nucleus. For example, a nucleus may contain a stable helium nucleus within a larger uranium nucleus. Thus, when uranium decays, it releases a helium atom. Other elements, such as oxygen, may also cluster within larger elements. This makes sense since certain atoms like helium and oxygen are more strongly bound than other elements:
| Year: 2013 | PT id = 566, Type = formulation data review |
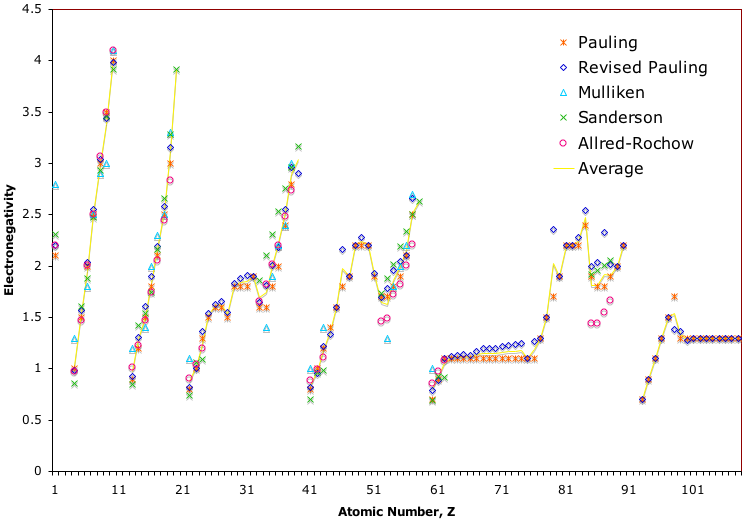
Electronegativity Chart (Leach)
From Mark R Leach's paper, Concerning electronegativity as a basic elemental property and why the periodic table is usually represented in its medium form, Journal & PDF.
Due to the importance of Pauling's electronegativity scale, as published in The Nature of The Chemical Bond (1960), where electronegativity ranges from Cs 0.7 to F 4.0, all the other electronegativity scales are routinely normalised with respect to Pauling's range.
When the Pauling, Revised Pauling, Mulliken, Sanderson and Allred-Rochow electronegativity scales are plotted together against atomic number, Z, the similarity of the data can be observed. The solid line shows the averaged data:
| Year: 2019 | PT id = 1001, Type = review |
Kultovoy's Periodic Table Book
Nicolay Kultovoy, website, as sent me a copy of his Periodic Table book, entitled [Google Translate]: Book 5. Part 11-08. A single quantum mechanical model of the structure of the atomic nucleus and the periodic table of chemical elements of D.I. Mendeleev.
In a mixture of Russian & English, the PDF of the book can be viewed here.
Chapter 1. Triune (electrons, nucleons, chemical elements) quantum mechanical model of Colt. Three
1.1 the Rules of filling of the orbits of electrons.
1.2 Pyramidal lattice.
1.3 models with cubic sieve.
1.4 models with face-centered lattice.
1.5 quantum Mechanical form of the periodic table of chemical elements.
1.6 Stowe-Janet-Scerri Periodic Table.
Chapter 2. A lattice model of the nucleus. Model 62
2.1 Berezovsky G. N.
2.2 I. Boldov
2.4 Konovalov.
2.5 Manturov V.
2.6 Semikov S. A.
2.7 alpha-partial model of the atomic nucleus.
2.8 Burtaev V.
Chapter 3. Various lattice (crystal) model of the nucleus of an atom. One hundred five
3.0 Luis Pauling.
3.1 Valery Tsimmerman. ADOMAH Periodic Table. Model 3-2.
3.2 Klishev B. V. Model 3-1.
3.3 Garai J. Model 3-1.
3.4 Winger E Model 4-2.
3.5 Norman D. Cook. Model 4-1.
3.6 Gamal A. Nasser. Model 4-1.
3.7 D. Asanbaeva Model 4-1.
3.8 Datsuk V. K.
3.9 Bolotov B.
3.10 Djibladze M. I.
3.11 Dyukin S. V.
3.12 A. N. Mishin.
3.13 M. M. Protodyakonov
3.14 Dry I. N.
3.15 Ulf-G. Meißner.
3.16 Foreign works.
Chapter 4. Long-period periodic table. One hundred eighty one
4.1 long-Period representation of the periodic table.
4.2 Artamonov, G. N.
4.3 Galiulin R. V.
4.4 E. K. Spirin
4.5. Khoroshavin L.
4.6 Step form proposed by Thomsen and Bohr.
4.7 Symmetrical shape of the periodic table.
Chapter 5. Construction of a periodic table based on the structure of orbitals. Two hundred twenty one
5.1 construction of the periodic table on the basis of orbitals.
5.2 Short V. M.
5.3 Kulakov, the Novosibirsk table of multiplets.
Chapter 6. Atomic structure. Two hundred forty eight
6.1 Table of isotopes.
6.2 the structure of the orbitals.
| Year: 2020 | PT id = 1174, Type = formulation |
Split s-, p- & d-Block Periodic Table
René Vernon presents a periodic table formulation with split s-, p- & d-blocks.
- The traditional form of periodic table is a hybrid of an electronic and a chemistry based table.
- An electronic or physics-based table would show (a) He over Be; and (b) group 3 as Sc-Y-Lu-Lr; and (c) group 13 as B-Al-Ga-In-Tl
- A chemistry-based table would show (d) He over Ne; and (e) B-Al over Sc-Y-La-Ac.
- What we have instead is a hybrid table with 1(c) and 2(d). It is not as symmetric or tidy as the pure Lu form; neither is it as irregular as the form with three split blocks.
The details: Group 3 as B-Al-Ga-In-Tl
Al over Sc has some history, which seems to have been forgotten.
Here are some other tables with B-Al-Sc-Y-La:
- Rang (1893)
- Gooch & Walker (1905)
- Cuthbertson & Metcalfe (1907)
- Baur (1911)
- Rydberg (1913)
- Black & Conant (1920)
- Lewis (1923)
- Hubbard (1924)
- Deming's table (1925), which popularised the medium-long form
- Antropoff (1926)
- LeRoy's table (1927)
- Irwin (1939)
- Seaborg (1945), with B left in group 13
- Yost & Russell (1946)
- Coryell (1952)
- Pauling's table (1960)
- Habishi's Metallurgist's Periodic Table (1992), Habishi leaves B in group 13
What was it that these luminaries knew about B-Al-Sc-Y-La-Ac that is deemed to be no longer relevant, and why is that the case?
Deming (1947, Fundamental Chemistry, 2nd ed. p. 617) located Al with the pre-transition metals in groups 1?2. Cox (2004, Inorganic Chemistry, 2nd ed. p. 185) refers to the pre-transition metals as those in groups 1 and 2, and Al. Here's that 2019 periodic table (by me), recording oxidation number trends, further suggesting B and Al are better placed over Sc.
In this vein, Rayner-Canham (2020, The periodic table: Past, present, and future, pp. 178–181) writes:
"It was Rang in 1893 who seems to have been the first, on the basis of chemical similarity, to place boron and aluminum in Group 3.
"Such an assignment seems to have been forgotten until more recent times. Greenwood and Earnshaw have discussed the way in which aluminum can be considered as belonging to Group 3 as much as to Group 13 particularly in its physical properties. Habashi has suggested that there are so many similarities between aluminum and scandium that aluminum's place in the Periodic Table should actually be shifted to Group 3.
"In terms of the electron configuration of the tripositive ions, one would indeed expect that Al3+ (electron configuration, [Ne]) would resemble Sc3+ (electron configuration, [Ar]) more than Ga3+ (electron configuration, [Ar]3d10). Also of note, the standard reduction potential for aluminum fits better with those of the Group 3 elements than the Group 13 elements (Table 9.2) – as does its melting point.
"In terms of their comparative solution behavior, aluminum resembles both scandium(III) and gallium(III). For each ion, the free hydrated cation exists only in acidic solution. On addition of hydroxide ion to the respective cation, the hydroxides are produced as gelatinous precipitates. Each of the hydroxides redissolve in excess base to give an anionic hydroxo-complex, [M(OH)4]–... There does seem to be a triangular relationship between these three elements. However, aluminum does more closely resemble scandium rather than gallium in its chemistry. If hydrogen sulfide is bubbled through a solution of the respective cation, scandium ion gives a precipitate of scandium hydroxide, and aluminum ion gives a corresponding precipitate of aluminum hydroxide. By contrast, gallium ion gives a precipitate of gallium(III) sulfide. Also, scandium and aluminum both form carbides, while gallium does not."
To answer my own question as to why group 3 as B-Al-Sc-Y-La-Ac has been forgotten.
I suspect what happened is that it was historically known that group 3 was better represented as B-Al-Sc-Y-La-[Ac]. Then, with the advent and rise of modern electronic structure theory, B-Al- got moved to the p-block because, after all, they were p-block elements, never mind the damned chemistry. And La stayed in the d-block since it was the first element to show 5d electron, and 4f did not show until Ce. And Lu stayed where it was since even thought it was learnt that the f shell become full at Yb, rather than Lu, nothing changed about the chemistry of Lu. Nowadays, this has all been forgotten.
The modern periodic table is a chemistry-physics hybrid.
Lu in group 3 demands He over Be. La in group 3 demands B-Al over Sc. Neither option gets up. The more important consideration is to teach the history and have students and chemists appreciate both perspectives.
| Year: 2022 | PT id = 1254, Type = formulation data |
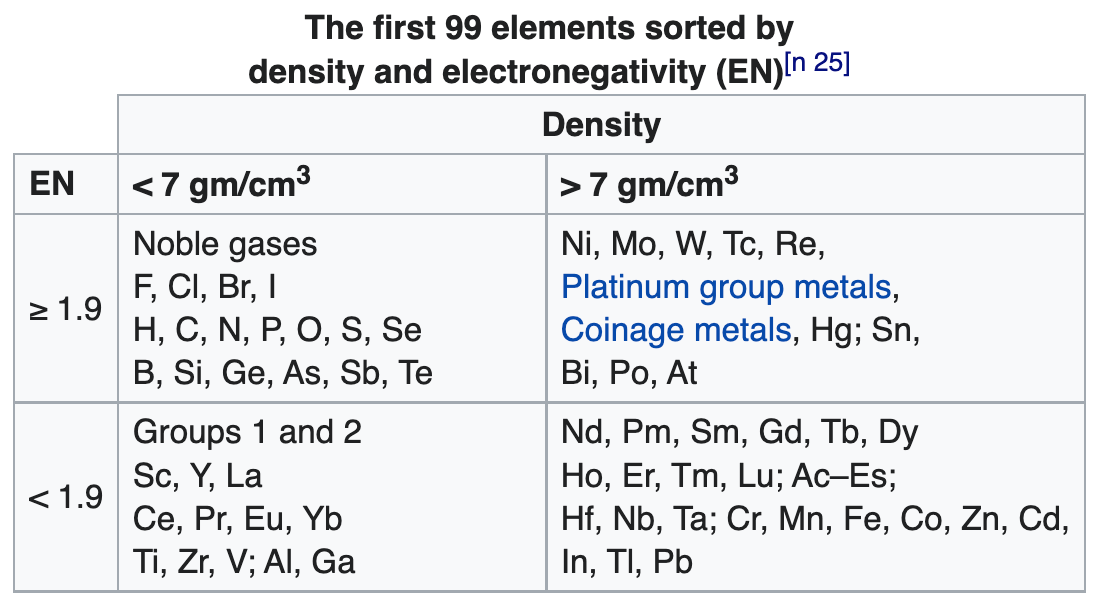
99 Elements Sorted by Density & Electronegativity
René Vernon writes:
"A little while I ago I noticed that a scatter plot of EN (revised Pauling) and density values of the elements resulted in a nice distribution, as per the table below.
"According to Hein and Arena (2013) nonmetals have low densities and relatively high EN values; the table bears this out. Nonmetallic elements occupy the top left quadrant, where densities are low and EN values are relatively high. The other three quadrants are occupied by metals. Of course, some authors further divide the elements into metals, metalloids, and nonmetals although Odberg argues that anything not a metal is, on categorisation grounds, a nonmetal.
Note 25 says:
(a) Weighable amounts of the extremely radioactive elements At (element 85), Fr (87), and elements with an atomic number higher than Es (99), have not been prepared.
(b) The density values used for At and Fr are theoretical estimates.
(c) Bjerrum (1936) classified "heavy metals" as those metals with densities above 7 g/cm^3.
(d) Vernon (2013) specified a minimum electronegativity of 1.9 for the metalloids.
- Bjerrum, N (1936). Bjerrum’s Inorganic Chemistry. London: Heinemann
- Hein, M; Arena, S (2013). Foundations of College Chemistry. Hoboken: John Wiley & Sons. pp. 226, G-6. ISBN 978-1-118-29823-7.
- Oderberg DS 2007, Real Essentialism, Routledge, New York, ISBN 978-1-134-34885-5
- Vernon R 2013, "Which elements are metalloids?", Journal of Chemical Education, vol. 90, no. 12, 1703?1707, doi:10.1021/ed3008457
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.