Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables from the year 1966:
| 1966 | Ionization Enerties |
| 1966 | Cotton and Wilkinson Periodic Table of The Elements |
| 1966 | Nobelium, Discovery of |
| 1966 | Rare Earth Pop Out Periodic Table |
| 1966 | Tottle's Periodic Table |
| Year: 1966 | PT id = 248, Type = formulation misc |
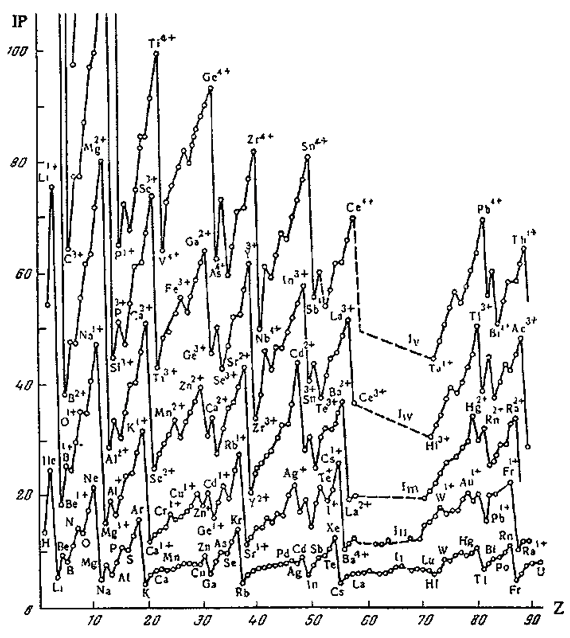
Periodic Table of Ions
From Concept of Chemical Periodicity: from Mendeleev Table to Molecular Hyper-Periodicity Patterns E. V. Babaev and Ray Hefferlin, here.
"One intriguing problem that arises from with the periodic table of atoms is the possibility of constructing periodic systems of ions, V. K. Grigorovich, Periodic Law of Mendeleev and Electronic Structure of Metals, Nauka Publ.: Moscow, 1966 (in Russian). An atom can be completely or partially ionized to a cation by removing electrons or transformed into an anion by the addition of new electrons. The energy required for a few consecutive ionisations of atoms is plotted against the atomic number. One can see that the curves are periodic, and hence it is possible to construct periodic tables for mono-, di-, and multi- charged cations. If we look at the dispositions of the maxima and minima of the curves and compare them with those for atoms, it becomes evident that the magic numbers of electrons for ions are the same as for neutral atoms. Therefore, the number of electrons (but not the charge of the nucleus) is responsible for the periodicity of ions."
| Year: 1966 | PT id = 443, Type = formulation |
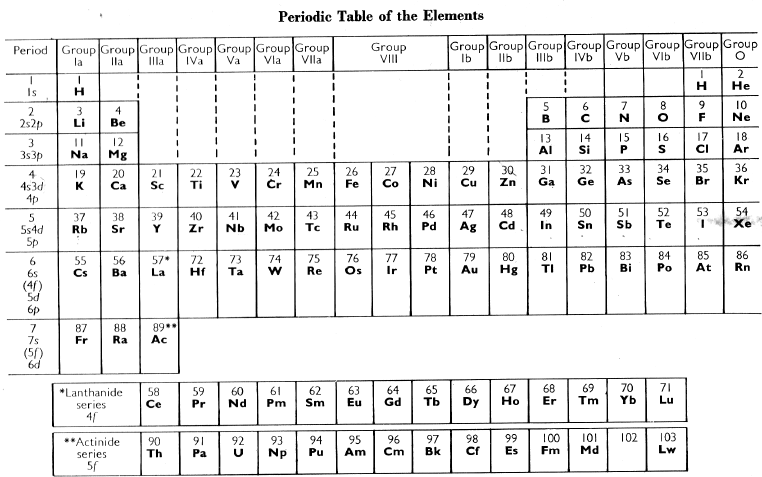
Cotton and Wilkinson Periodic Table of The Elements
From the Advanced Inorganic Chemistry 2nd Ed. textbook by Cotton and Wilkinson:
| Year: 1966 | PT id = 882, Type = element |
Discovery of Nobelium
No ![]()
Nobelium, atomic number 102, has a mass of 259 au.
Synthetic radioactive element.
Nobelium was first observed in 1966 by E. D. Donets, V. A. Shchegolev and V. A. Ermakov.
| Year: 1966 | PT id = 1265, Type = formulation review 3D |
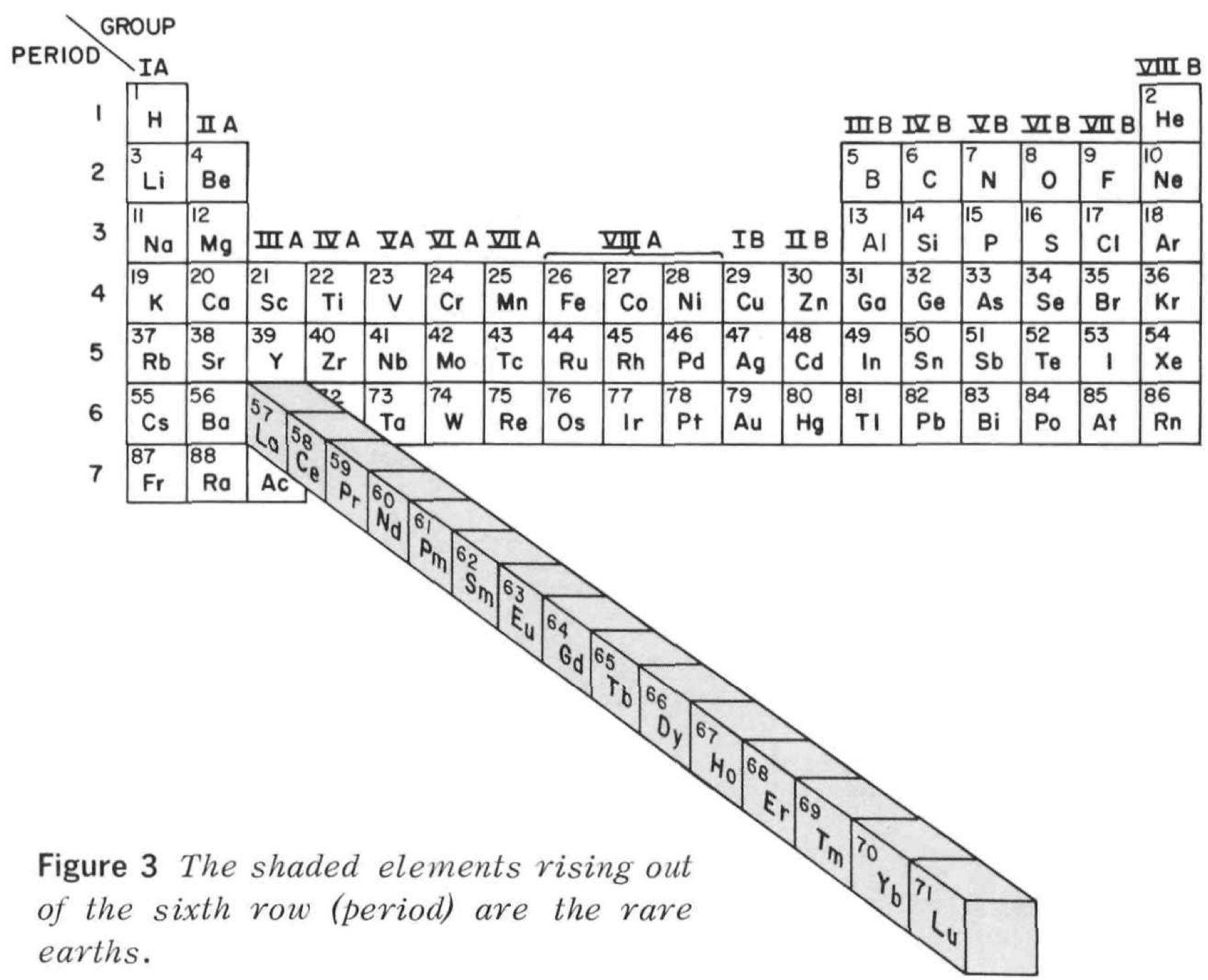
Rare Earth Pop Out Periodic Table
From Rare Earths, The Fraternal Elements by Karl A. Gschneidner Jr., United States Atomic Energy Commission Division of Technical Information Library of Congress Catalog Card Number: 65-60546 1964; 1966 (Rev.)
There is an interesting point made in the text concerning the term "Rare Earths":
"The name rare earths is actually a misnomer for these elements are neither rare nor earths. They are metals, and they are quite abundant. Cerium, which is the most abundant, ranks 28th in the abundances of the naturally occurring elements and is more plentiful than beryllium, cobalt, germanium, lead, tin, or uranium. The least abundant naturally occurring rare earth, thulium, is more plentiful than cadmium, gold, iodine, mercury, platinum, or silver. Indeed, 25% of the elements are scarcer than thulium."
Thanks to René for the tip!
| Year: 1966 | PT id = 1314, Type = formulation |
Tottle's Periodic Table
Tottle CR 1974, The Science of Engineering Materials, reprint of 1966 ed., Heinemann Educational Books, London, p. 20
René Vernon writes:
I was drawn to the attached periodic table by the strange-looking arrangement of dividing lines, one "full" and one dashed, in the p-block.
Semimetals
Ge, As, Se, Sn, Sb, Te, Bi and Po are shown as semi-metals. Tottle does not explain the basis for this division.
Showing Sn as a semi-metal or metalloid is dubious. Sure, white-Sn becomes gray-Sn at a temperature of below 13.2 °C but even here it has the electronic band structure of a semi-metal.
The same can be said for Po which has electronic band structure of a true metal, unlike the situation in As, Sb and Bi, all of which have electronic band structures of semi-metals.
Metals & Nonmetals
Starting with H, note the left to right path of the full dividing line between metals and nonmetals is continuous, except for the unique break above Be, presumably to show that there is no element above Be. This is actually not well thought-out since the metallic or nonmetallic status of the IIA elements is not then clarified.
Tottle is further interesting since, as well as referring to metals and nonmetals in the periodic table sense he later includes a chapter on Metals and alloys, and a chapter on Non-metallic materials. Some examples given by him of non-metallic materials are alumina, magnesia, graphite, beryllia, titanium carbide, glass, rubber, nylon and wood. So, he here is mixing nonmetallic elements and nonmetallic materials (which is fine).
Tottle gets into trouble in his chapter on Metals and alloys, since he includes some discussion on interstitial solid solutions, such as cementite Fe3C, which is an insulator, and intermetallic compounds, which appears fine on the surface, until one realises that some intermetallic compounds are semiconductors, such as FeGa3, RuGa3, and IrGa3. I have never heard of semiconducting or insulating metals or alloys.

 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.