Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
Periodic Tables from the year 2021:
| Year: 2021 | PT id = 1179, Type = data review misc |
Chemogenesis In 700 Seconds
The Chemogenesis analysis – by Mark Leach – tells the story of how chemical structure and reactivity emerge from the periodic table of the elements. This video is a rapid dash through the story... which unfolds over many pages of the Chemogenesis Web Book.
| Year: 2021 | PT id = 1180, Type = misc |
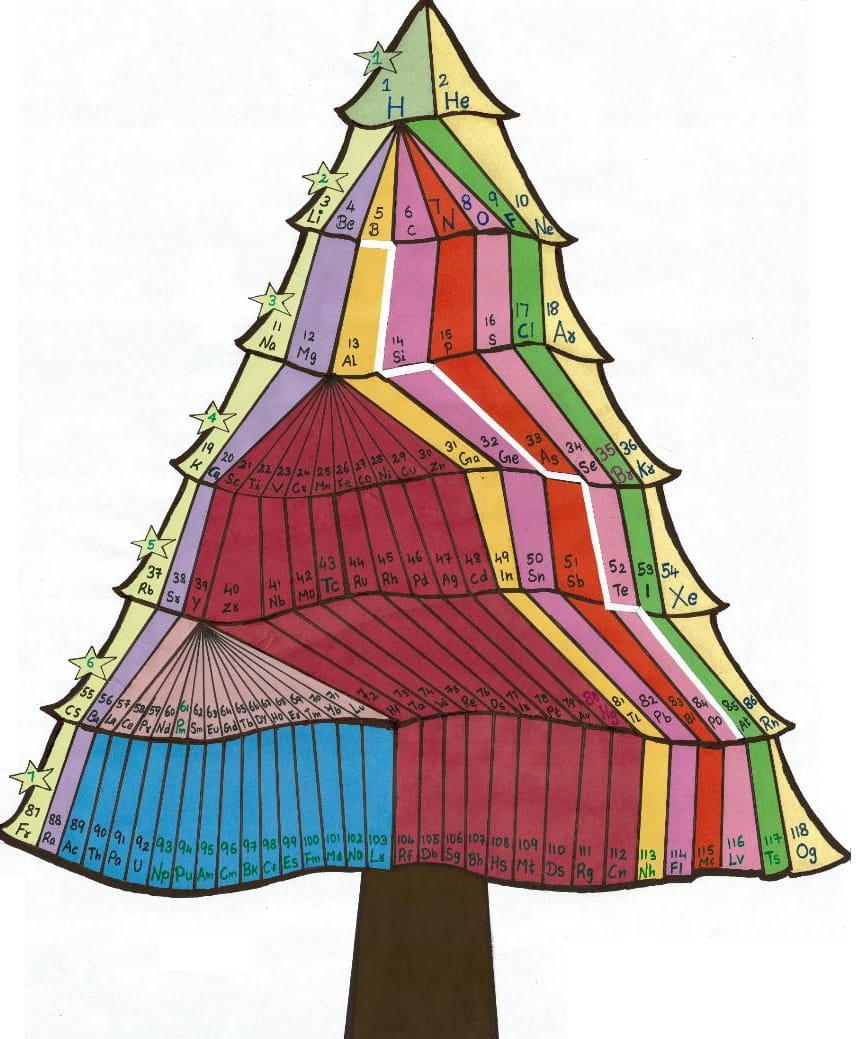
Christmas Tree Periodic Table
We have developed a new model in the form of a Christmas tree to represent periodic table. We have tried to combine both the triangle model of periodic table and modern periodic table in form of a christmas tree.
Mr G. Gnanamani Simiyon
| Year: 2021 | PT id = 1181, Type = formulation review |
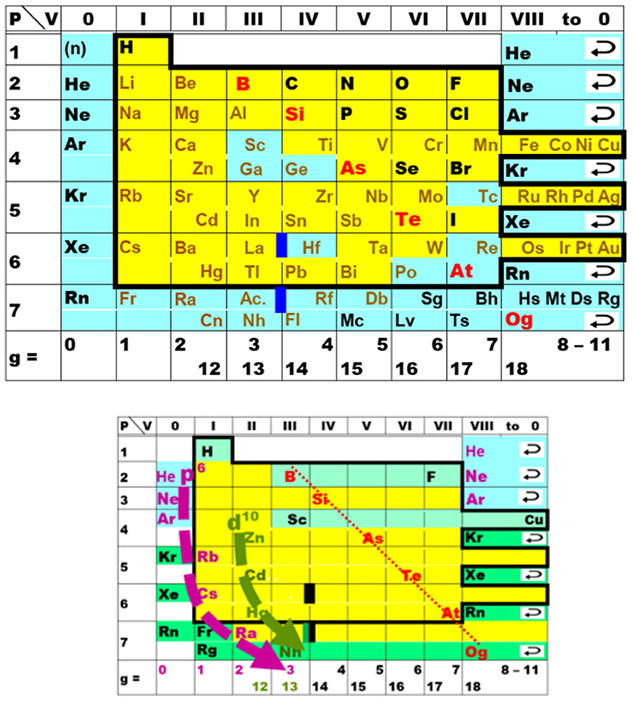
Understanding Periodic and Non-periodic Chemistry in Periodic Tables
Cao C, Vernon RE, Schwarz WHE and Li J (2021). Front. Chem. 8:813. https://doi.org/10.3389/fchem.2020.00813
Abstract:
The chemical elements are the "conserved principles" or "kernels" of chemistry that are retained when substances are altered. Comprehensive overviews of the chemistry of the elements and their compounds are needed in chemical science. To this end, a graphical display of the chemical properties of the elements, in the form of a Periodic Table, is the helpful tool. Such tables have been designed with the aim of either classifying real chemical substances or emphasizing formal and aesthetic concepts. Simplified, artistic, or economic tables are relevant to educational and cultural fields, while practicing chemists profit more from "chemical tables of chemical elements."
Such tables should incorporate four aspects:
(i) typical valence electron configurations of bonded atoms in chemical compounds (instead of the common but chemically atypical ground states of free atoms in physical vacuum);
(ii) at least three basic chemical properties (valence number, size, and energy of the valence shells), their joint variation across the elements showing principal and secondary periodicity;
(iii) elements in which the (sp)8, (d)10, and (f)14 valence shells become closed and inert under ambient chemical conditions, thereby determining the "fix-points" of chemical periodicity;
(iv) peculiar elements at the top and at the bottom of the Periodic Table.
While it is essential that Periodic Tables display important trends in element chemistry we need to keep our eyes open for unexpected chemical behavior in ambient, near ambient, or unusual conditions. The combination of experimental data and theoretical insight supports a more nuanced understanding of complex periodic trends and non-periodic phenomena.
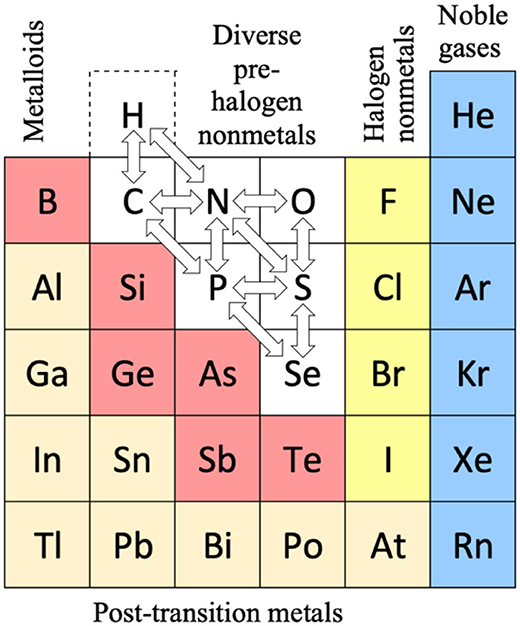
Thanks to René Vernon for the tip.
| Year: 2021 | PT id = 1183, Type = formulation data |
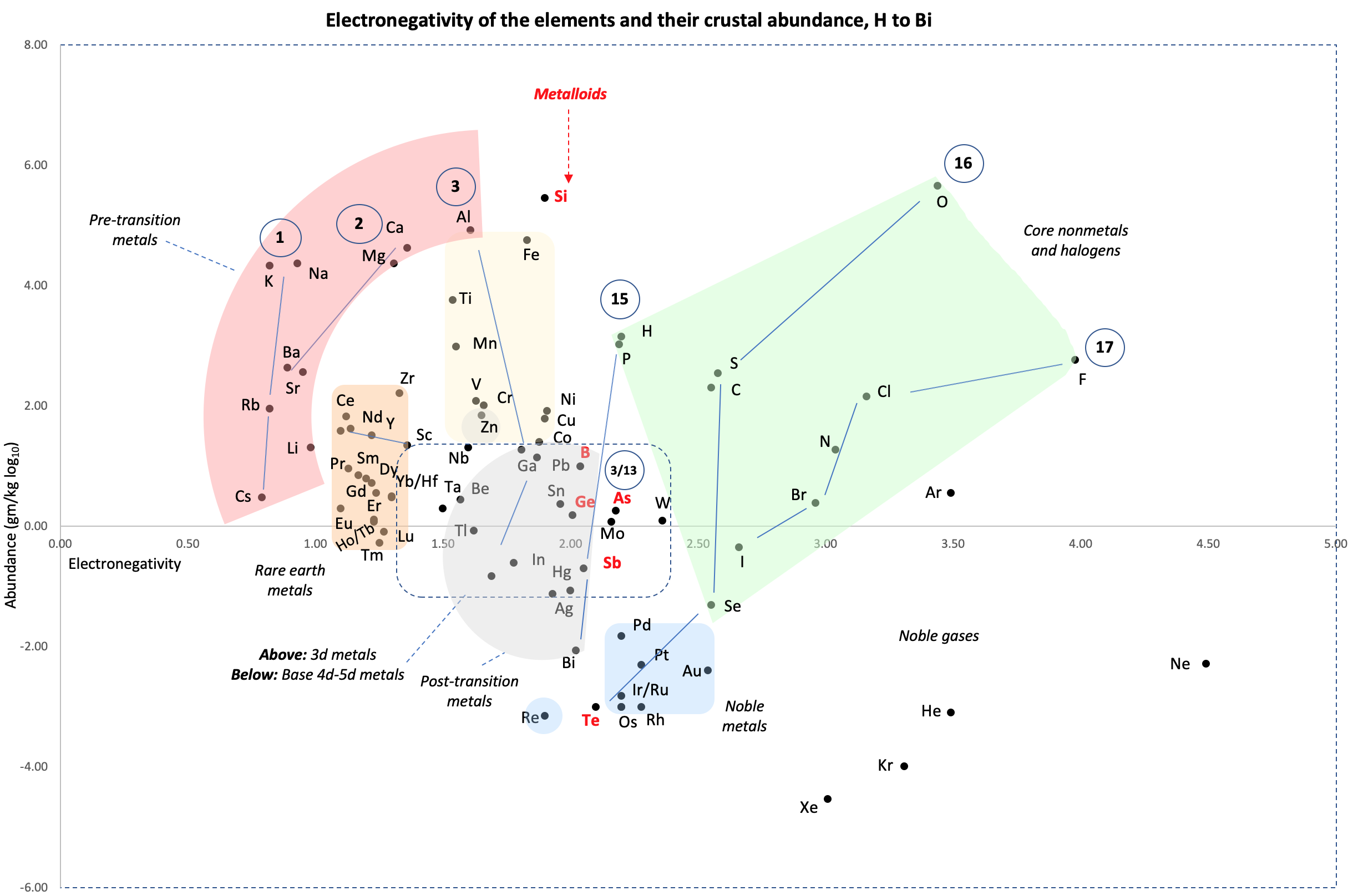
Crustal Abundance vs. Electronegativity
A chart by René Vernon of Elemental Abundance (g/kg log10) vs. Electronegativity, H to Bi.
René writes:
Below is a remarkable XY chart where x = electronegativity and y = crustal abundance (log10). It stops at the end of the s-process, at Bi. The abundance figures are from the CRC Hanbook of Physics and Chemistry (2016-2017).
I say remarkable as I had little idea what the chart would end up looking like when I started plotting the values.
As well as its coloured regions, I've marked out track lines for six of the main groups and one for group 3.
Observations
The rose-coloured arc on the left encompasses the pre-transition metals i.e. the alkali and alkaline earth metals and aluminium, followed by, in the orange rectangle, the rare earth metals. Opposite these regions, along the southern boundary of the green paddock, are the halogens.
In the pale yellow field sheltered by the pre-transition metals and the REM, are the 3d transition metals and, in the white corral, are 4d and 5d base transition metals. Opposite these regions, in the green paddock, are the core nonmetals H, C, N, O, P and S, with Se as an outlier.
Following in the grey blob are the post-transtion or poor metals, immediately adjacent to the bulk of the metalloids or poor nonmetals.
Finally, in the light blue patch, the noble metals are complemented by the noble gases frolicking in the open.
Abundance tends to decrease with increasing Z. Notable exceptions are Li, B, N and Si.
Curiosities
- H and P are almost on top of one another
- The proximity of Be to the post-transition metals, and its relative scarcity in the crust
- The metalloids, with their intermediate values of electronegativity, go down the middle. At the same time they span nearly the full range of abundance.
- B-Ga-Sc-Y-La are in a row
- N falls along the halogen line
- The abundance of O and Si, which we see in the form of silica
- F is more abundant in the crust than 85 percent of metals
- Al is the most abundant metal. Al and Fe are in the same vicinity: "Curiously, the chemistry of aluminium also resembles that of the iron(III) ion... These similarities may be ascribed to the same 3+ charge and near-identical ion radii (and hence charge density)." (Rayner-Canham 2020, p. 191)
- The abundance of Ar compared to the rest of the noble gases. Apparently this is influenced by the radioactive decay of potassium-40 in Earth's core, which is considered one of the main sources of heat powering the geodynamo that generates Earth's magnetic field. It has been suggested that a large amount of Ar may be present in the core, as the compound ArNi with an L11 Laves structure (similar to an intermetallic phase, and related to a cubic close packed lattice). ArNi is stabilised by notable electron transfer from Ni to Ar, changing their electron configurations toward 3d7 and 4s1. (Adeleke et al. 2019)
- Ti, a light yet strong metal, is about 2,500 times as abundant as Sn, a weak heavy metal
- Zn is an outlaw post-transition metal
- The most active 4d-5d transition metals (Zr, Hf) occupy a boundary overlap with the rare earth metals
- Ag, which has a largely main-group chemistry, is located in the PTM region. It is about 20 times as abundant as the noble meals
- Re is an outlaw noble metal
Comment
I was intrigued by the article referring to Ni and Ar, and the suggestion of Ar becoming somewhat anionic, albeit in extreme conditions (140 GPa, 1500 K)
References
- Adeleke AA, Kunz M, Greenberg E, Prakapenka VB, Yao Y, Stavrou R 2019, A high-pressure compound of argon and nickel: Noble gas in the Earth's core?, ACS Earth and Space Chemistry, vol. 3 no. 11, pp. 2517-2544, https://pubs.acs.org/doi/10.1021/acsearthspacechem.9b00212
- Rayner-Canham G, 2020, The periodic table: Past, present, future, World Scientific, Singapore
Correlations
I wasn't looking for these but they at least exist as follows:
- Metals with lower EN, i.e. < 1.7, or active nonmetals with higher EN, tend to be concentrated in silicate or oxide phases that are more easily found in the crust due to their lower density, and hence have higher abundances.
- Metals with moderate EN 1.7 to 2.1, say the later transition metals and post-transition metals, tend to form sulfide liquid phases; are less easily found in the crust due to their relatively higher densities; and are less abundant by about two orders of magnitude compared to the metals found in silicate or oxide phases.
- Metals with EN > 2.2, i.e. the noble metals, have an affinity for a metallic liquid phase, and are depleted in the crust since they generally sank to the core and hence have very low abundances. They are about two orders of magnitude less abundant than the sulfide metals.
My references are:
- Cox PA 1997, The elements: Their origin, abundance and distribution;
- Gill R 2014, Chemical fundamentals of geology and environmental geoscience;
- White WA 2020, Geochemistry
Thus the abundance of the metals in the crust tends to fall with increasing EN.
- For the nonmetals, the relative average abundance proportions are about 5: 700: 250: 1 for, respectively, the metalloids; the core nonmetals H, C, N, P, S, and Se; the halogen nonmetals; and the noble gases. Si and O were left out as outliers, in terms of their massive abundances.
- Thus, metalloids aside, the abundance of the nonmetals tends to fall with increasing EN. I don't know what's going on with the metalloids.
- The chart may prompt some further appreciative enquiry:
- In the case of exceptions to the initial three generalisations why do these occur?
- Why is Li so rare, compared to the other alkali metals?
- Why is Si good at forming a planetary crust?
An answer from L. Bruce Railsback, creator of the Earth Scientist's Periodic Table https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=142:
"I think I can answer one of the questions. 'Why is Si good at forming a planetary crust?' – because it's so bad at staying in the core. Silicon isn't sufficiently metallic to stay in the core. Even in the mantle and crust, it doesn't go into non-metal solids well: in cooling magmas, it's only a lesser member of the early-forming minerals (e.g., Mg2SiO4, forsterite, where it's outnumbered two to one). The mineral only of Si as a cation, SiO2 (quartz), is the LAST mineral to form as a magma cools, in essence the residuum of mineral-forming processes. At least some this thinking is at Bowen's Reaction Series and Igneous Rocks at http://railsback.org/FundamentalsIndex.html#Bowen"
- Why do the metalloids span such a wide range of abundances?
- If H is supposed to make up ca. 74% of the universe why does it have the same abundance in the Earth's crust as P?
- In what form is H found in Earth's crust—water, hydroxides?
- If H is supposed to make up ~ 74% of the universe why does it have the same abundance in the Earth's crust as P?
- Are there any chemical similarities between H and P, given both have some metalloidal character? The have virtually identically electron affinities. H is sometimes positioned above B due to chemical similarities. It then forms a diagonal relationship with C, which in turn has a diagonal relationship with P, which has a diagonal relationship with Se e.g. P reacts with Se to form a large number of compounds characterised by structural analogies derived from the white phosphorus P4 tetrahedron.
- The rare earth metals are relatively rare, having an average abundance of 1% that of the 3d metals. That being so, why is their rareness sometimes questioned? Why does the crustal abundance of the REM plummet by two orders of magnitude towards the end of the lanthanides?
Which Electronegativity Scale?
The wide variety of methods for deriving electronegativities tend to give results similar to one another.
| Year: 2021 | PT id = 1184, Type = formulation |
van Spronsen's Periodic Table: Update
René Vernon writes:
I'd never before realised how clever van Spronsen's 1969 Periodic Table is. It seems to be the ultimate logical electronic version, informed by the actual filling sequence in the gas phase atoms, rather than the idealised sequence.
So, H-He are over Li-Be.
Group 3 is Sc-Y-La-Ac since that is where the d-shell starts filling. In the rest of the d-block, there are (4+1) x d5 and (4+2) x d10.
The f-block starts with Ce, as that is where the f-shell starts filling. Notice the high degree of regularity with the 4 x f7 and the 4 x f14, and how Th is treated i.e. as 5f0.
After DIM's 8-column form, I believe the periodic family tree now looks like this:
Three split-blocks
1a. He over Ne; B-Al over Sc-Y-La-Ac = old school form
1b. H-He over F-Ne; ditto = e.g. Soddy 1914?, Kipp 1942?Two split blocks
2a. He over Ne; group 3 as Sc-Y-La-Ac = popular formOne split-block
3a. He over Ne; group 3 as Sc-Y-Lu-Lr = Lu form 3b. He over Be; group 3 as Sc-Y-La-La = forgotten van Spronsen formNo split blocks
4. He over Be = Janet equivalent
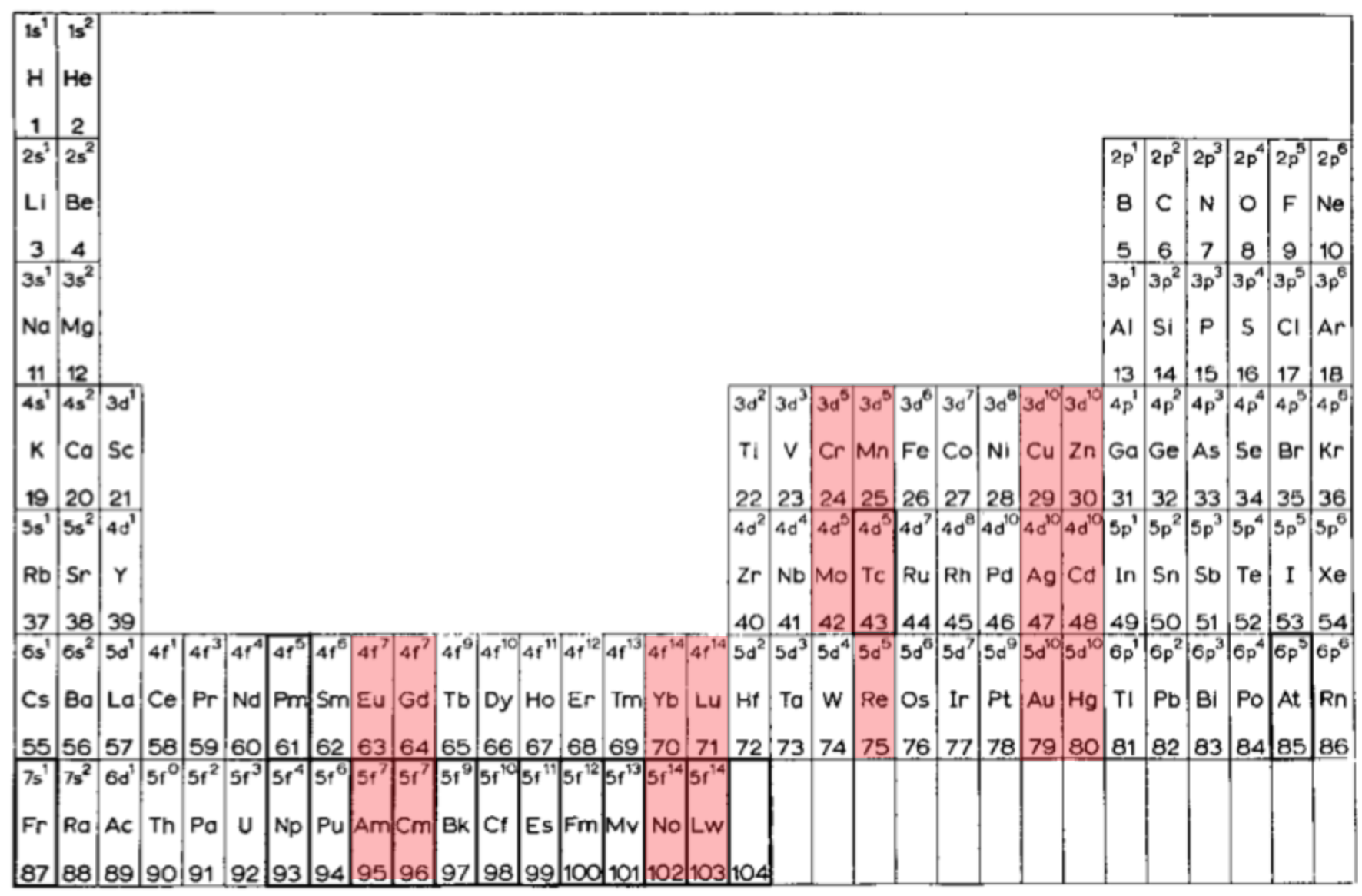
| Year: 2021 | PT id = 1185, Type = review |
150 Years of the Periodic Table
Based on the ACS 2019 symposium "150 Years of the Periodic Table". Provides an overview of how the periodic table has evolved over the past 150 years Written by leading experts in the field: Editors: Giunta, Carmen, Mainz, Vera V., Girolami, Gregory S. (Eds.)
This book provides an overview of the origins and evolution of the periodic system from its prehistory to the latest synthetic elements and possible future additions. The periodic system of the elements first emerged as a comprehensive classificatory and predictive tool for chemistry during the 1860s. Its subsequent embodiment in various versions has made it one of the most recognizable icons of science.
Based primarily on a symposium titled "150 Years of the Periodic Table" and held at the August 2019 national meeting of the American Chemical Society, this book describes the origins of the periodic law, developments that led to its acceptance, chemical families that the system struggled to accommodate, extension of the periodic system to include synthetic elements, and various cultural aspects of the system that were celebrated during the International Year of the Periodic Table.
Contributors include: Ann Robinson, Gary Patterson, Gisela Boek, Mary Virginia Orna, Simon Cotton, Jay Labinger, Kit Chapman, Virginia Trimble, Eric Scerri, William Jensen, Pekka Pyykko, Daniel Rabinovich, Carmen Giunta, Gregory Girolami, Vera Mainz
| Year: 2021 | PT id = 1186, Type = formulation |
Helix vs. Screw
Julio Antonio Gutierrez Samanez writes:
Until today, when they write about the work of Chancourtois and his telluric helix wound in a cylinder, still no one alludes to this other telluric helix wound in a cone or screw, the idea is the same: a rope that winds a geometric solid.
The first was devised in 1862, the other in 2008 (146 years later). But, there is a big epistemological difference. In the first, the elementary series presented was: 8, 8, 8, 8, 8 ..., etc., in the second it is: 2, 2, 8, 8, 18, 18, 32, 32. Furthermore, the division of conical radii is regulated by the function 2 (n ^ 2) = 2, 8, 18, 32...
Each binode has two spirals or two periods with the same number of elements, which correspond to the function 4 (n ^ 2). I don't think it is a discovery, it is just the conclusion of the contributions of Rydberg, Janet, and, of course, Chancourtois.
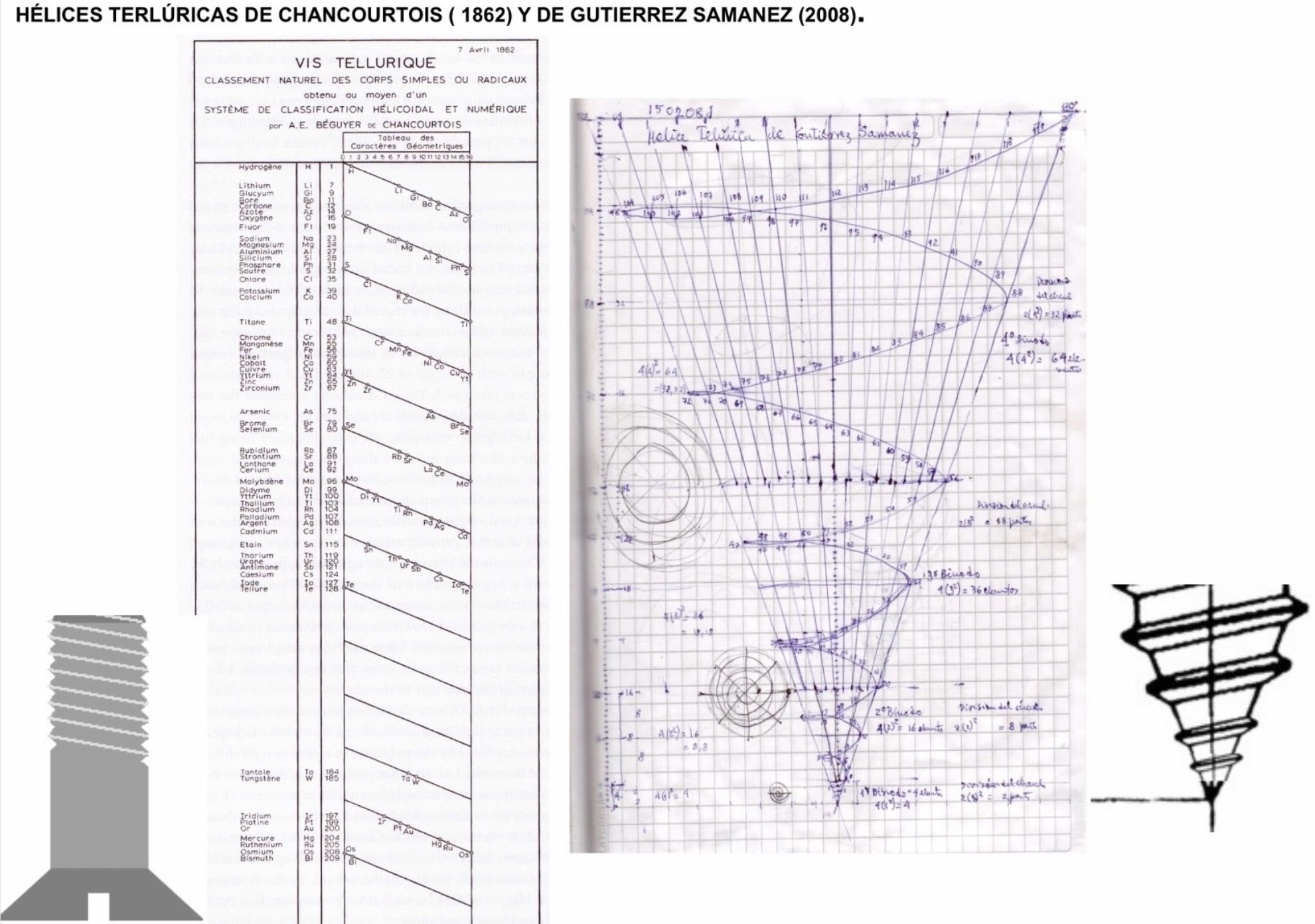
| Year: 2021 | PT id = 1187, Type = formulation |
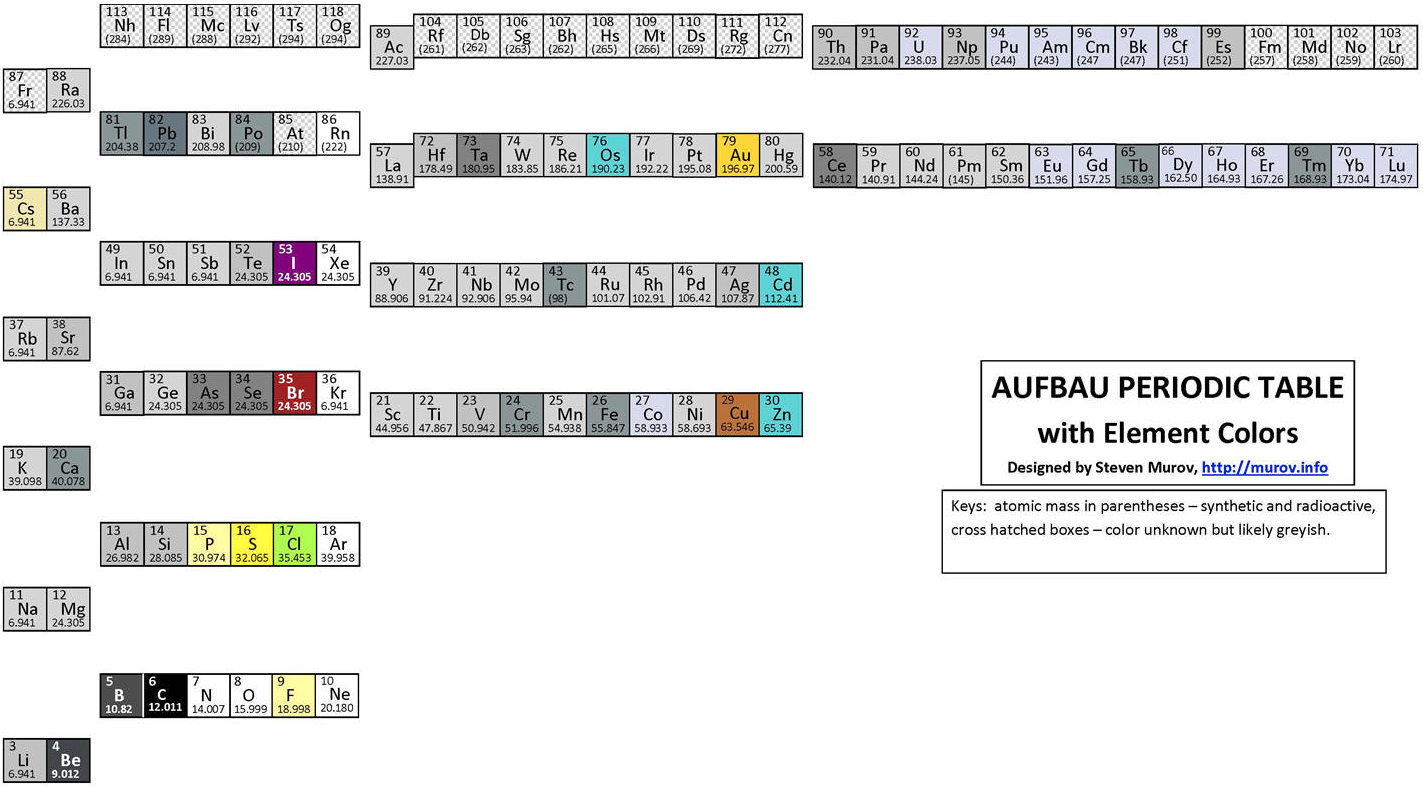
Aufbau Periodic Table
An Aufbau Periodic table designed by Steven Muov at http://murov.info/aufbaupt.htm
Steven writes:
"Science has aptly been described as a search for order in the Universe. It follows that chemistry is a search for order in matter. While the search will always be a work in progress, great strides towards the finding of order in matter resulted in 1869 when Dimitri Mendeleev stood on the shoulders of many others and published his periodic table. The table has since been modified and improved but still has a remarkable resemblance to the original Mendeleev table. Excellent compilations of many alternate periodic tables have been published that use novel and intriguing approaches (e.g., circles, spirals and 3d, but the contemporary versions of the Mendeleev table are the charts found on the walls of thousands of lecture rooms around the world. The periodic table deserves recognition as one of the milestones of science along with contributions from other sciences including but not limited to: physics by Newton and Einstein, biology by Darwin, Rosalind Franklin, Watson and Crick, astronomy by Copernicus and Galileo and geology by Wegener..."
| Year: 2021 | PT id = 1190, Type = data |
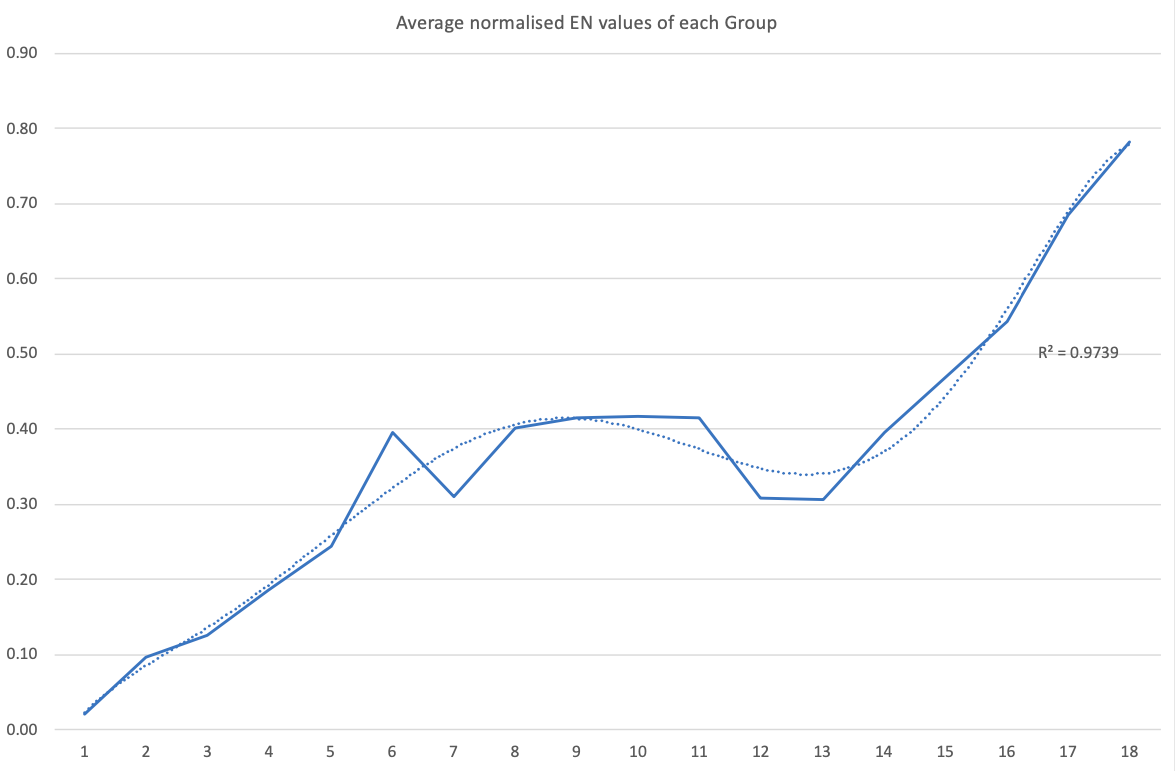
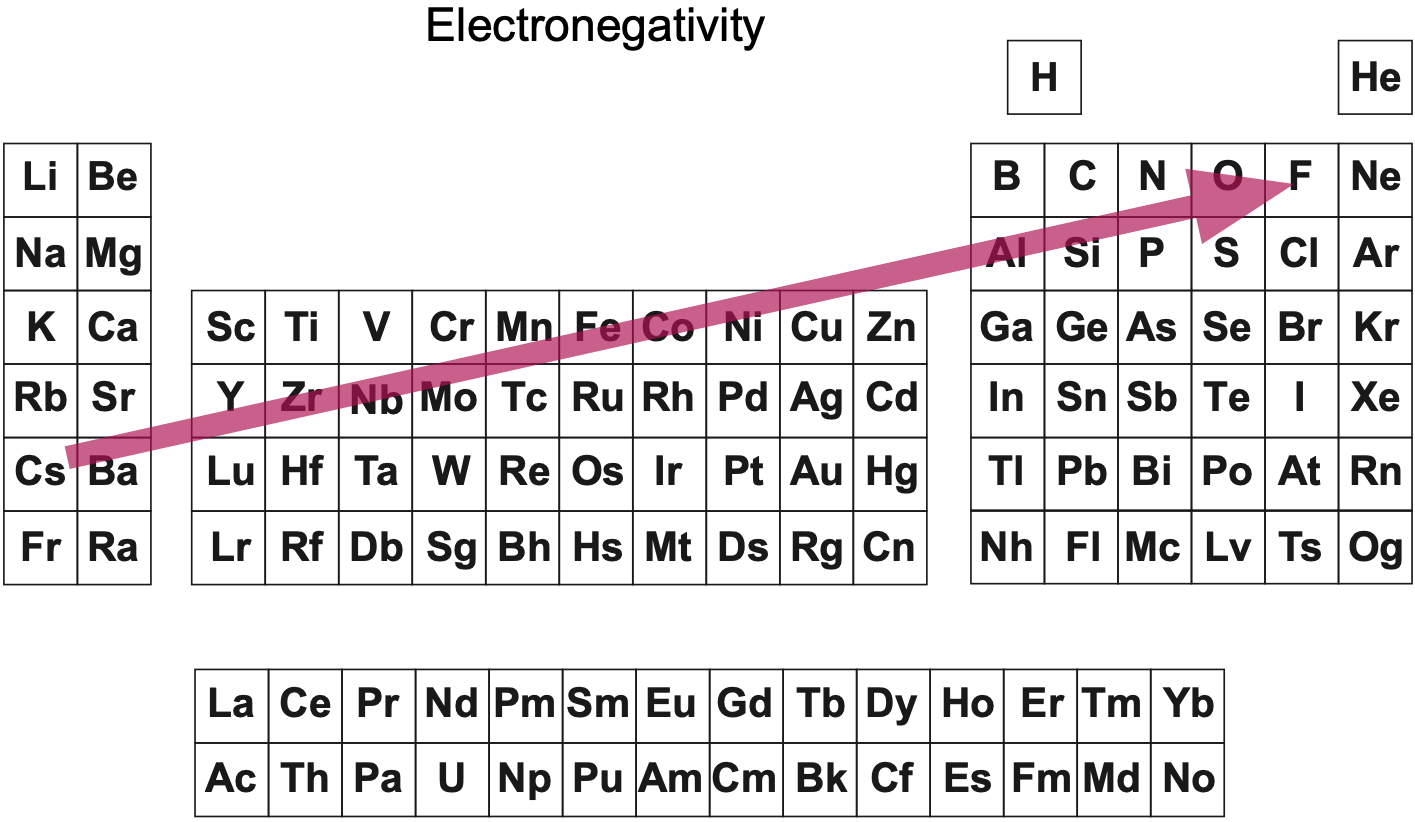
Electronegativity: A Three-Part Wave
René Vernon points out that although there is a general trend in increasing electnegativity from Cs to F, there is actually an s-curve in the data.
Electronegativity across groups 1 to 18 appears to a show a three-part wave-like pattern.
There is a rise from group 1 to group 6, followed by a fall at group 7. I guess for group 7 that the EN for Mn is based on +2 and in this state Mn has five 3d electrons. The EN for Tc and Re are presumably based on +7, in which they notionally have underlying [Kr] and [Xe] cores.
There is rise from 7 to 8 (why?); a mesa from 8 to 11 (why?) that includes the PGM; and a fall at group 12. The fall may be influenced by group 12 having a full d shell; ditto group 13.
There is a rise from 13 to 18. Whereas in group 13 there is ionic chemistry in the form of the cations of Al to Tl this is not the case for C, Si, and Ge in group 14. Sn is reluctant to form a cation expect at pH < 1, and there is no Pb4+ cation.
The R2 value of 0.9739 is a best fit value for a second order polynomial. R2 for a straight line is 0.786
| Year: 2021 | PT id = 1191, Type = formulation review |
Provisional Report on Discussions on Group 3 of The Periodic Table
Provisional Report on Discussions on Group 3 of The Periodic Table by Eric Scerri, De Gruyter | 2021
DOI: https://doi.org/10.1515/ci-2021-0115
The following article is intended as a brief progress report from the group that has been tasked with mak-ing recommendations to IUPAC about the constitu-tion of group 3 of the periodic table (https://iupac.org/project/2015-039-2-200). It is also intended as a call for feedback or suggestions from members of IUPAC and other readers.



| Year: 2021 | PT id = 1193, Type = review |
Eric Scerri's Articles, as Listed on Muck Rack
| Year: 2021 | PT id = 1196, Type = data misc |
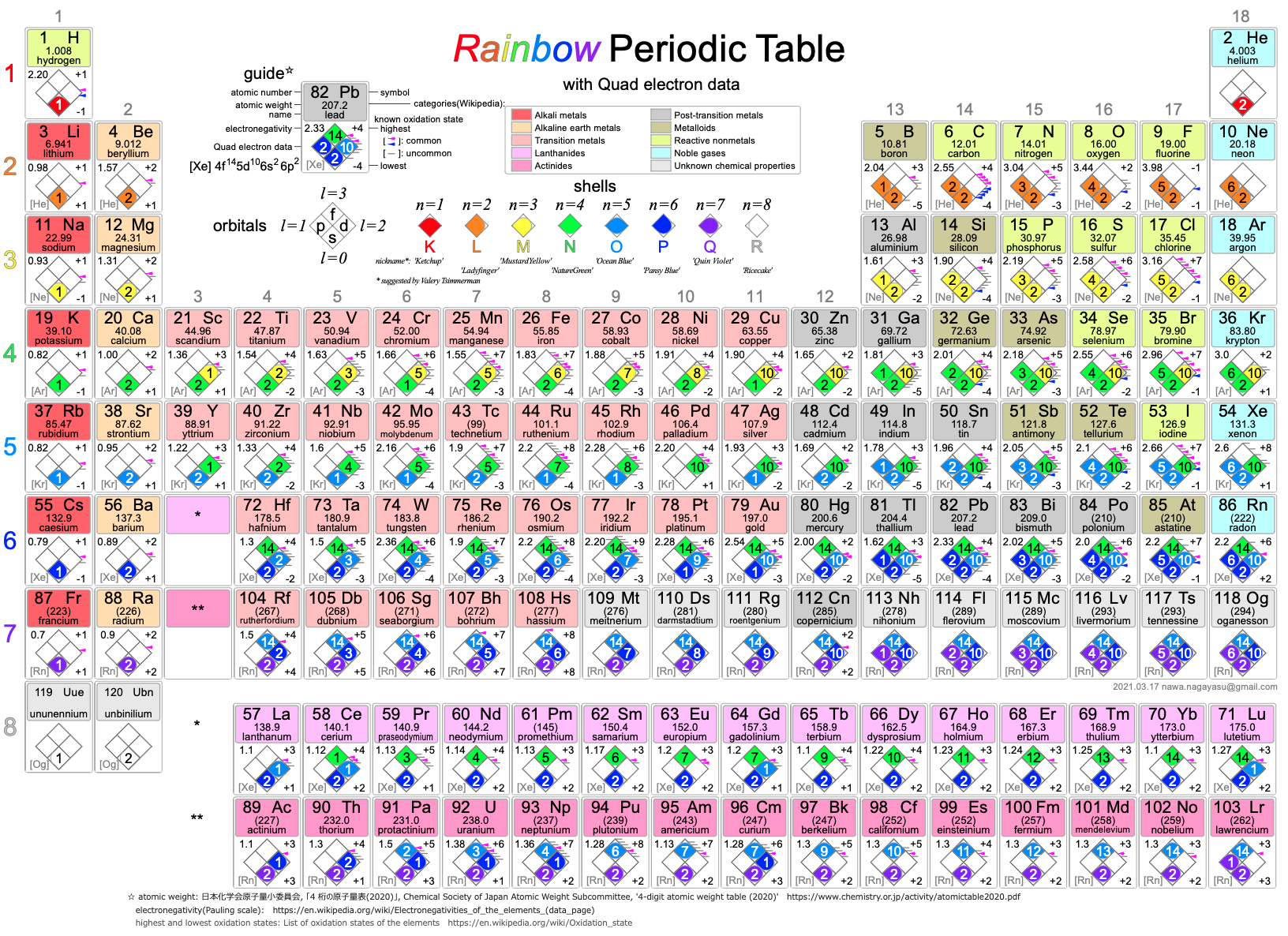
Nawa's Rainbow Periodic Table
Nagayasu Nawa - "A Japanese school teacher and periodic table designer" - has developed a Rainbow Periodic Table that is stuffed full of data.
Click here to download the .pdf file.
| Year: 2021 | PT id = 1197, Type = formulation data spiral |
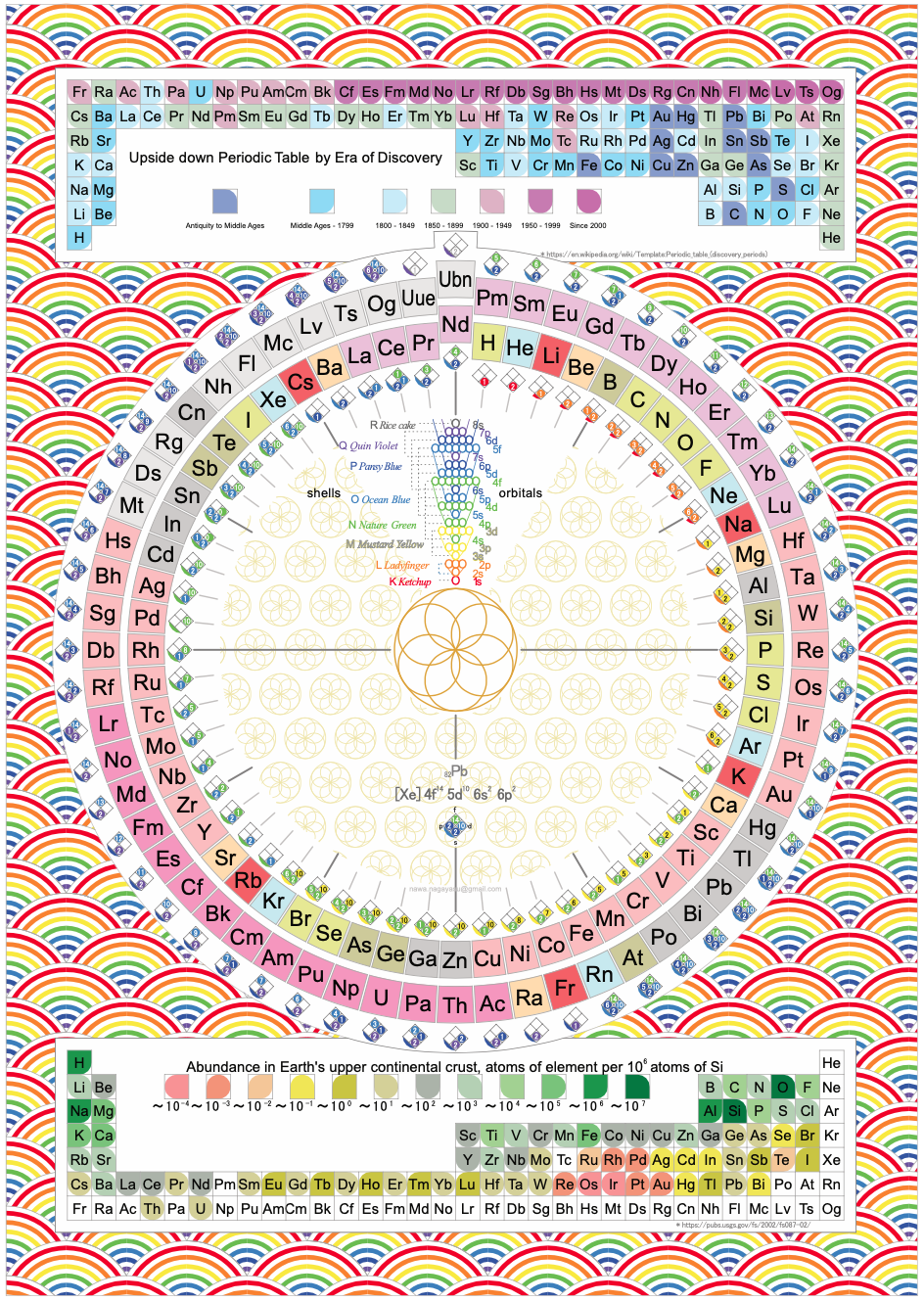
Nawa's Multi Periodic Table
Nagayasu Nawa - "A Japanese school teacher and periodic table designer" - has developed a "Multi" Periodic Table with three formulations: long-form, upsidedown long-form & circular with era of discovery, electronic structure and abundance data.
Click here to download the .pdf file.
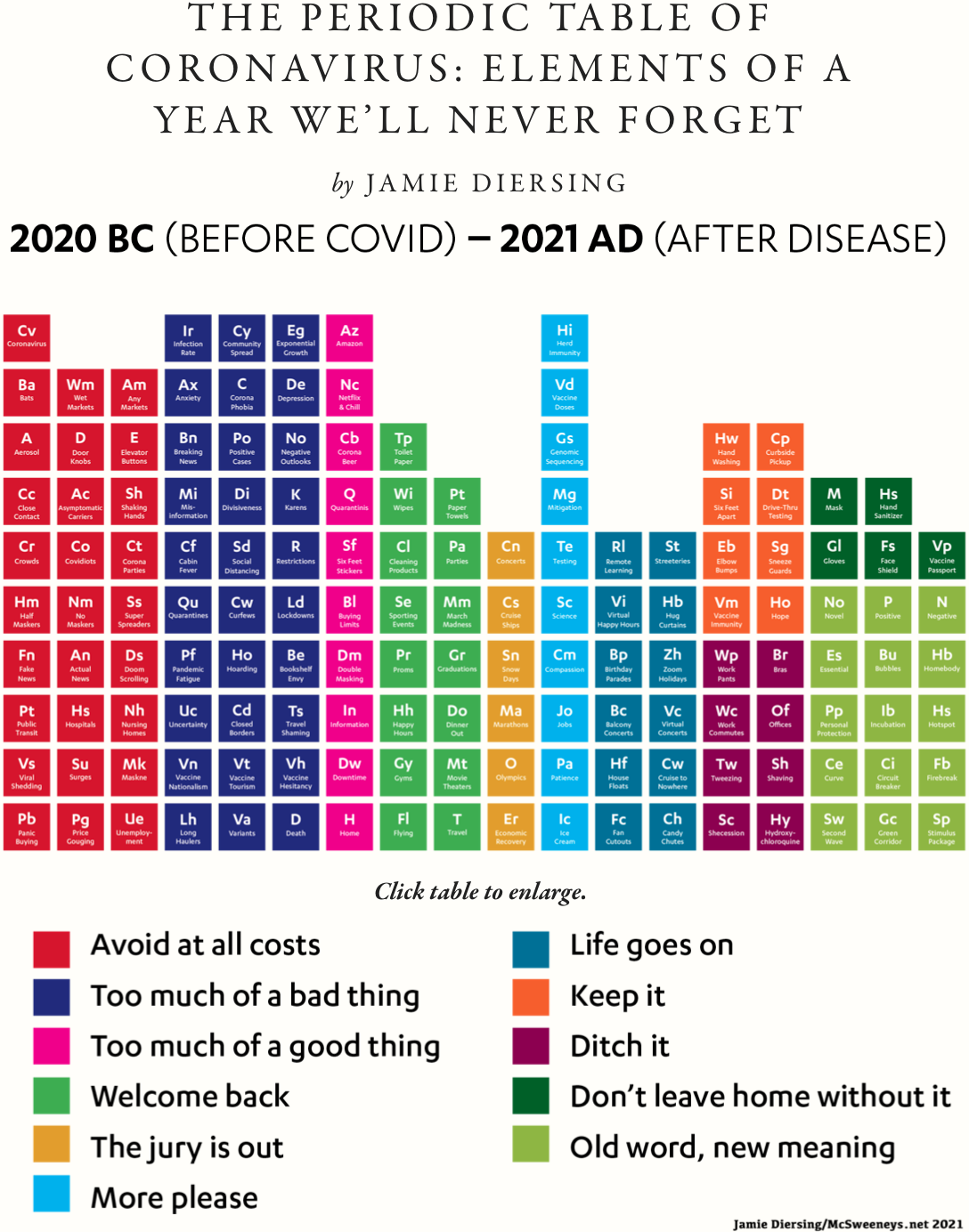
| Year: 2021 | PT id = 1199, Type = non-chem |
Coronavirus, Periodic Table of: Elements of a Year We'll Never Forget
By Jamie Diersing and posted on McSweeneys, The Periodic Table of Coronavirus: Elements of a Year We'll Never Forget
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2021 | PT id = 1200, Type = non-chem misc data |
Map of Fundemental Particles
By Domain of Science – Posters & YouTube Channel – a periodic table of the fundamental particles that make up the periodic table.
Domain of Science is produced by physicist Dominic Walliman who is on a quest to make science as easy to understand as possible.

| Year: 2021 | PT id = 1203, Type = formulation |
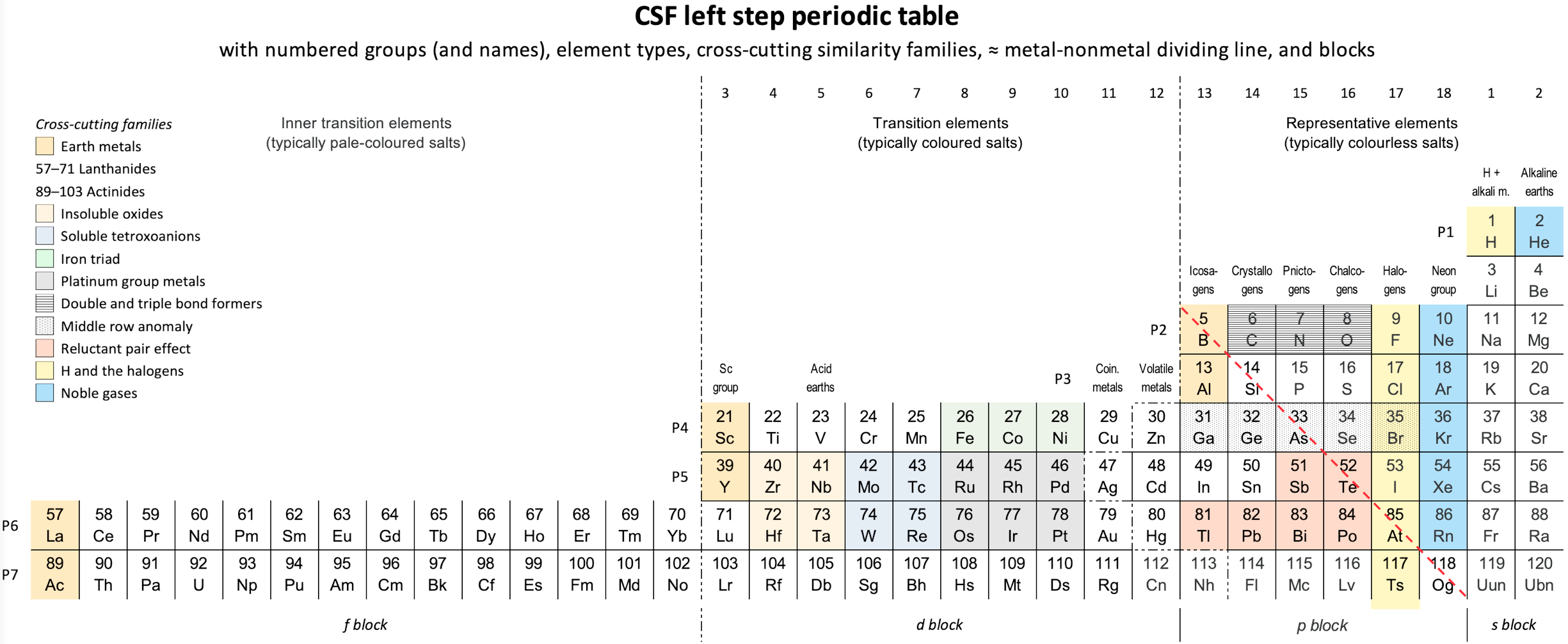
Vernon's CSF Left-Step Periodic Table.
René Vernon's CSF Left-Step Periodic Table.
"I was prompted to switch to He-Be and [to develop a Janet type] left-step periodic table. I suggest it remediates concerns about H and He, and Lu in group 3.
Pros
- There is symmetry in this version.
- The physiochemical relationship of He to Ne is retained.
Cons
- There is a loss of physiochemical regularity in placing He over Be. Even if helium can be enticed to become chemically active, it will still be very much better located in group 18.
- While the d, p, and s blocks start with the appearance of the relevant electron, there is a loss of consistency with La at the start of the f-block. This is confusing to students since there is no such inconsistency in the La form.
- In terms of predominant differentiating electrons in each block, this form is less consistent than an La table.
- There is one less form of "element block-type" symmetry, than in the La form.
| Year: 2021 | PT id = 1204, Type = formulation 3D |
Cubical-Stair Periodic Table
Sarthak Gupta's Cubical-Stair Periodic Table (Into a Whole New Dimension):
"Looking at the Modern periodic Table, somethings always bug you. The huge gap between the s and p-block when they should be side by side. The whole f-block floating around in air when it should be there in period 6 and 7. So why not experiment with shapes and structures and come up with something more space efficient?
"The cubical Periodic table paves the way taking the periodic table into a whole new dimension. Yes! from the 118 squares, we are going to transition into 67 cubes stacked onto each other like stairs."
The Cubical-Stair Periodic Table Explained:
- The table is made up of 67 cubes stacked onto each other, having three sides exposed(top, left and right)
- The top faces contain s and p-block elements
- The left side faces contain d block and right side faces contain f block
- There are two different Major Groups: A and B
- Major Group A is divided into 14 minor groups (from -1 to 12) and Major group B is divided into 8 minor groups (from 3 to 12)
- Major Group A applies to s, p & f-block. Major Group B is exclusively for d-block.
- To go down a group we follow the arrow and descend the stair in the given direction
Advantage over the Modern PT
- Although being 3 dimensional, it can be easily represented in 2 dimensions in the form of trisected hexagons
- All the elements of the same period lie in the same line (unlike MPT where f-block elements had to be depicted separately due to lack of space).
- Viewing the table from 3 different directions makes only one or two blocks visible:
1. From top: s & p-block
2. From left: d-block
3. From right: f-block - This helps in diffrentiating between the blocks easily
- The disturbing gap between s and p-block of traditional periodic table is not simply there. All advantages of Modern Periodic Table remain conserved.

| Year: 2021 | PT id = 1206, Type = non-chem |
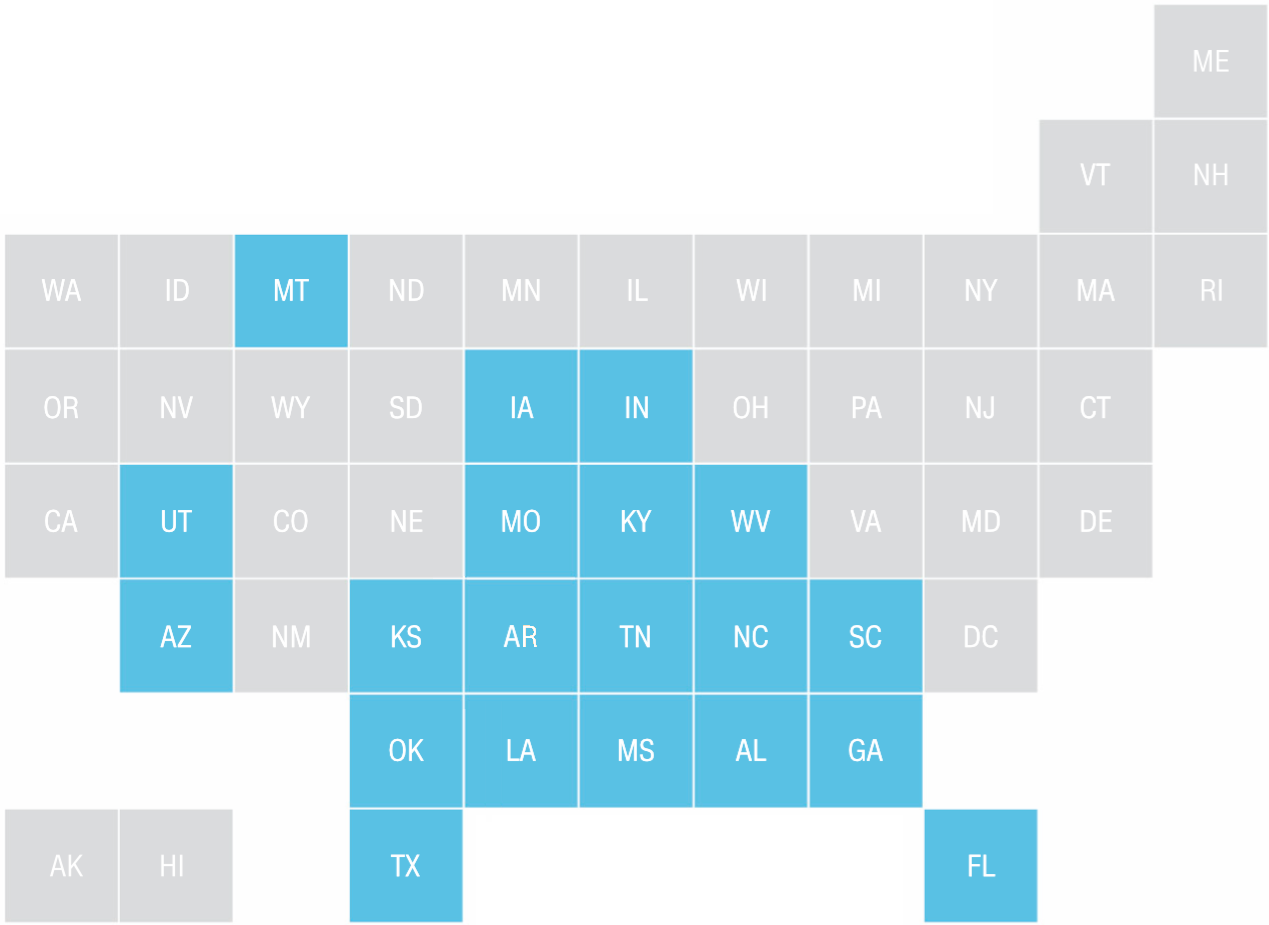
USA as Periodic Table Infographic
An periodic table inspired infographic of the USA (from CNN):
| Year: 2021 | PT id = 1207, Type = misc |
World's Largest Periodic Table Created on ECU's Science Building
From architectureanddesign.com.au:
"The new science building at Edith Cowen University (ECU), Joondalup Campus in Perth stands out for its striking façade, which features the world's largest periodic table. The thoughtful design by Silver Thomas Hanley Architects responded to the brief to deliver a bold and sophisticated architectural statement in the urban setting. The façade featuring the periodic table celebrates the building's purpose as a centre of scientific research and learning. Based on the university vice chancellor Professor Steve Chapman's idea, the periodic table is an enormous 662 square metres, spanning the entire front façade of the building."

Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2021 | PT id = 1210, Type = formulation data |
Vernon's Eight-Fold Way Periodic Table
René Vernon suggests that the chemical elements can be grouped into eight classes: four metallic (Active, Transition, Post-Transition and Noble) and four non-metallic (Halogen, Biogen, Metalloid and Noble gas):
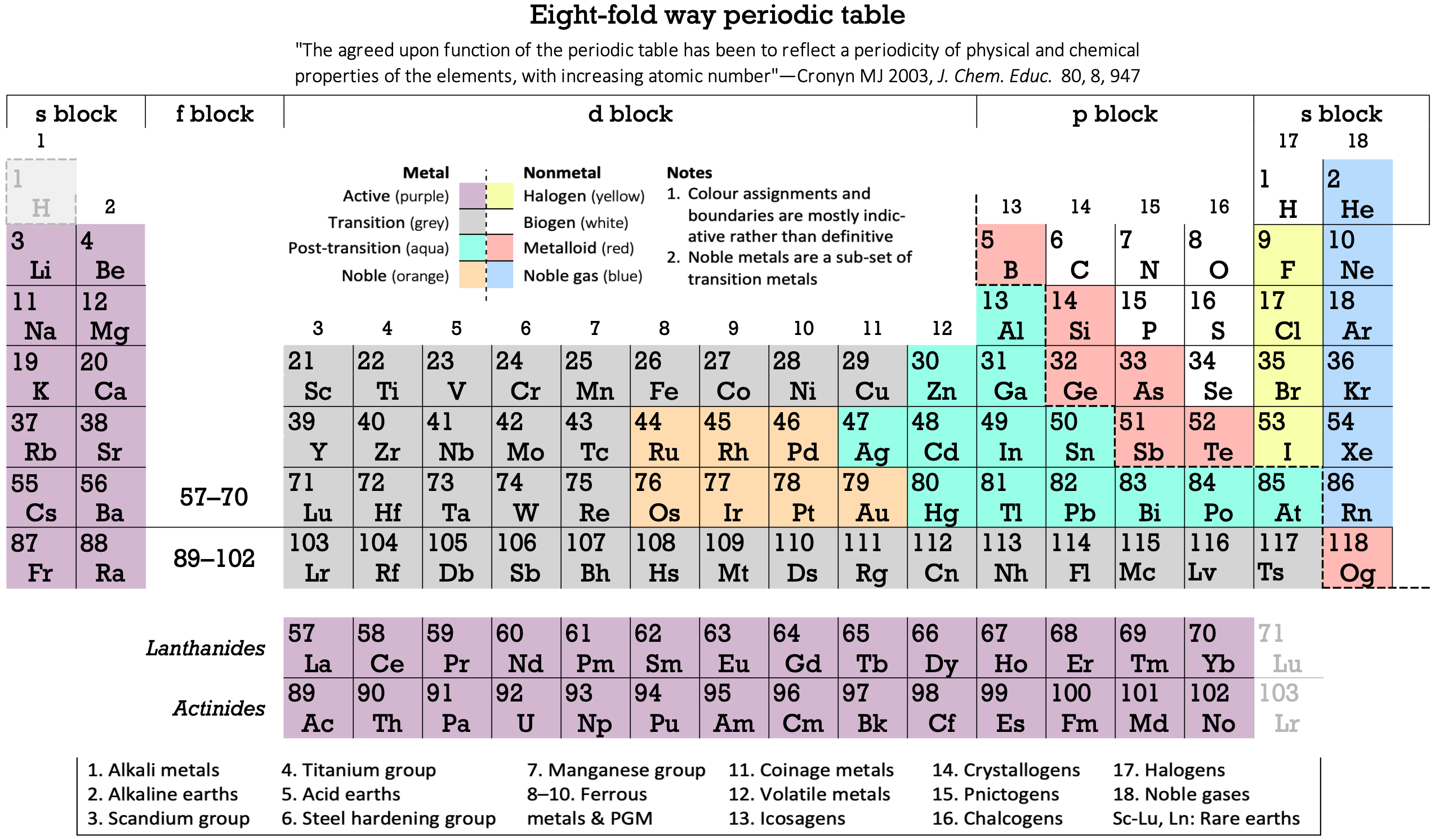
| Year: 2021 | PT id = 1212, Type = formulation |
Term & Spin State Periodic Table
A Tern & Spin State periodic table by Gnanamani Simiyon who writes:
"We tried to arrange the elements based on the ground state term and spin state. I attached picture of the periodic table drawn. For example, I notice that alkali metals and coinage metals grouped up indicating some relationship between the groups. Similarly with respect to alkaline Earth metals and Zinc group. We are unable to further understand other groupings based on the ground state term and spin state."
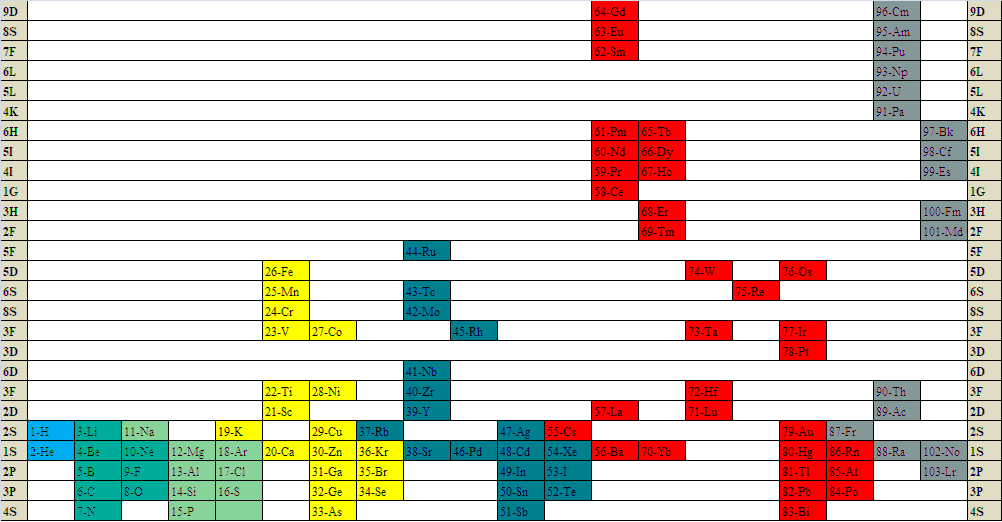
| Year: 2021 | PT id = 1213, Type = formulation review |
Mendeleyev Revisited
An Open Access paper: Marks, E.G., Marks, J.A. Mendeleyev revisited. Found Chem 23, 215-223 (2021).
https://doi.org/10.1007/s10698-021-09398-4
"Despite the periodic table having been discovered by chemists half a century before the discovery of electronic structure, modern designs are invariably based on physicists' definition of periods. This table is a chemists' table, reverting to the phenomenal periods that led to the table's discovery. In doing so, the position of hydrogen is clarified."
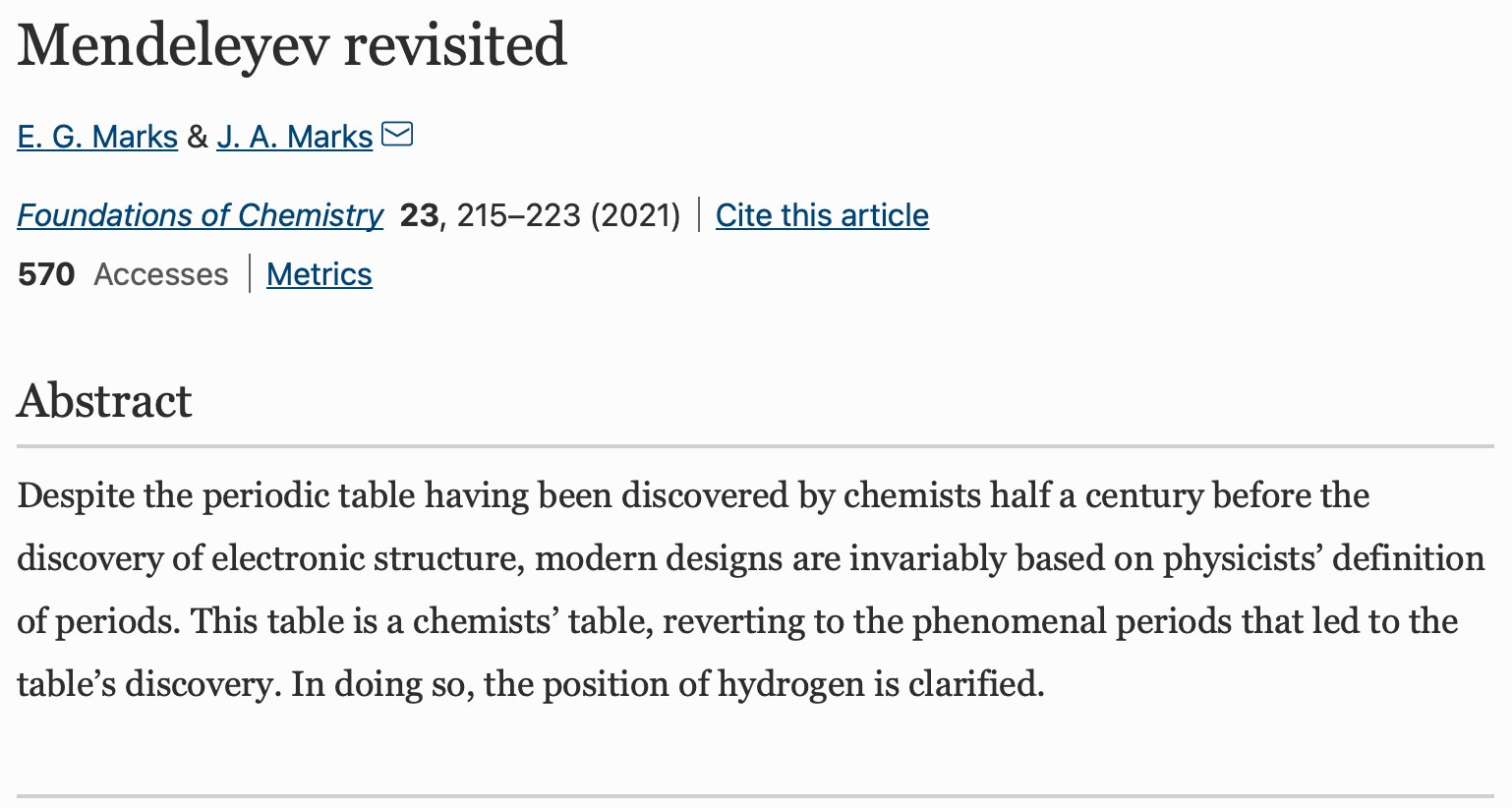
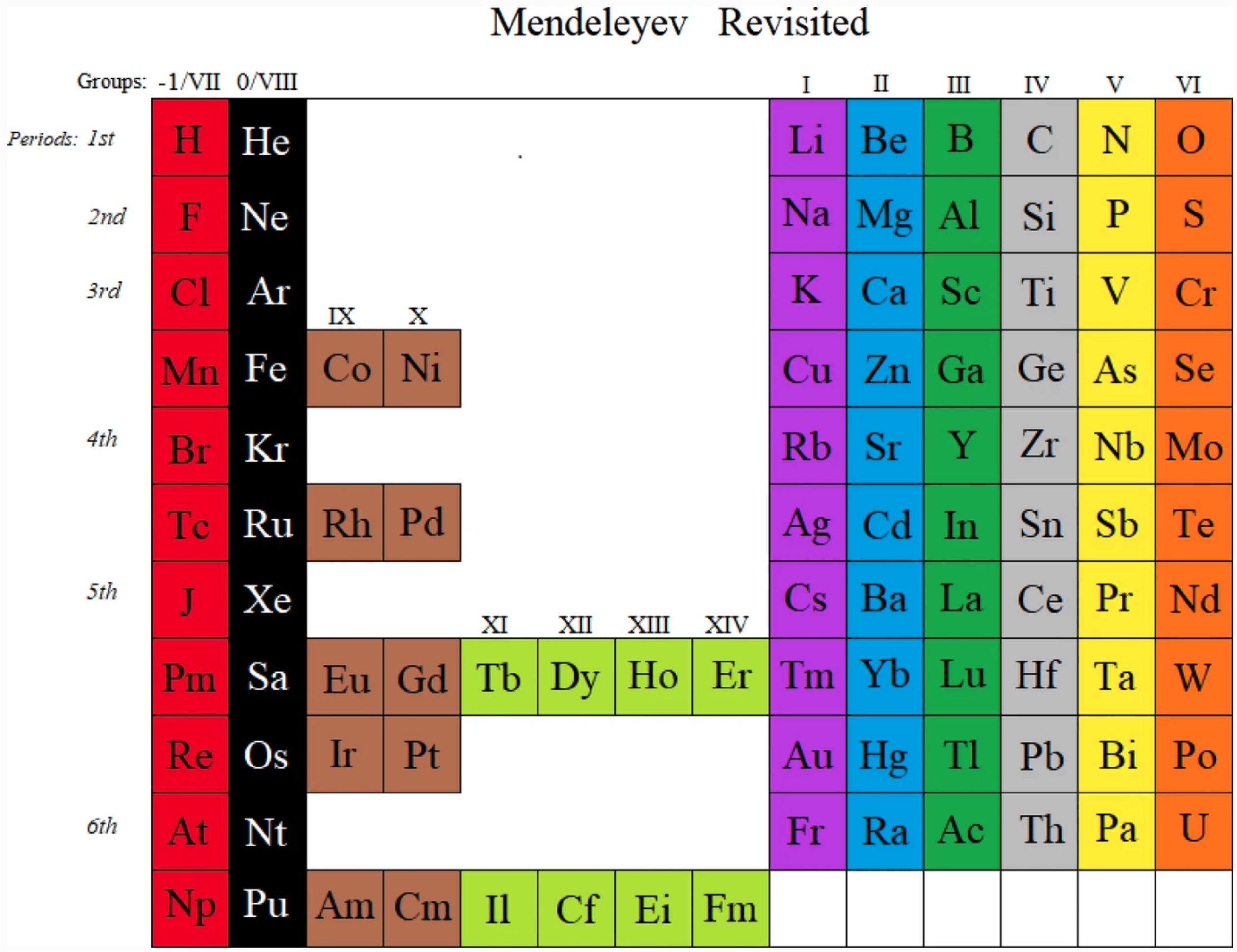
| Year: 2021 | PT id = 1215, Type = misc |
Largest Periodic Table in Eurasia Created in Dubna
From The Times of India:
"The largest [PT] in Eurasia, the Periodic Table of Mendeleev opened in Dubna near Moscow. The event is timed to coincide with the 65th anniversary of the Joint Institute for Nuclear Research located here and the city itself. It is noteworthy that it is at JINR, in the Laboratory of Nuclear Reactions. G N Flyorov under the guidance of Academician of the Russian Academy of Sciences Yuri Oganesyan, all known to date superheavy elements were obtained – from 113th to 118th (the latter is even named after the scientist – 'Oganeson Og'). Oganesyan is the second scientist in the world, after whom a new element of the Periodic Table was named during his lifetime (the first was the American scientist Glenn Theodore Seaborg)."

Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2021 | PT id = 1217, Type = data element misc |
History [of the] Elements and Periodic Table
From the Royal Society of Chemistry (RSC) an interactive Elements and Perioid Table History web page:

Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2021 | PT id = 1218, Type = formulation |
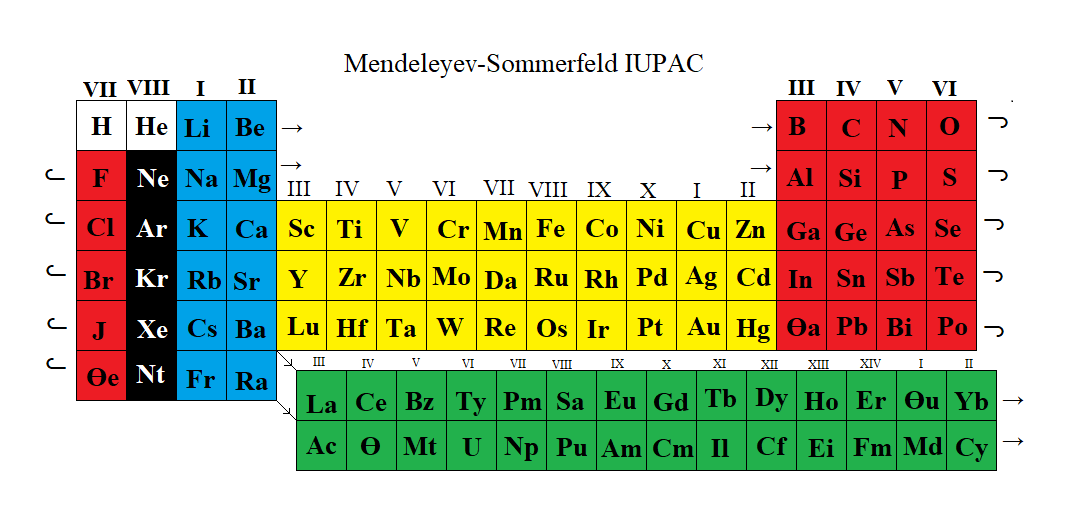
Mendeleyev-Sommerfeld IUPAC Periodic Table
From John Marks' updated Mendeleyev-Sommerfeld IUPAC Periodic Table.
John writes:
This is an adaptation of Fig. 4 [from https://link.springer.com/article/10.1007/s10698-021-09398-4] to match IUPAC's 18-column table. The yellow (transition metals) are Sommerfeld's 'A'-subgroups and the green (rare earths) are Sommerfeld's 'B'-subgroups.
| Year: 2021 | PT id = 1219, Type = formulation spiral |
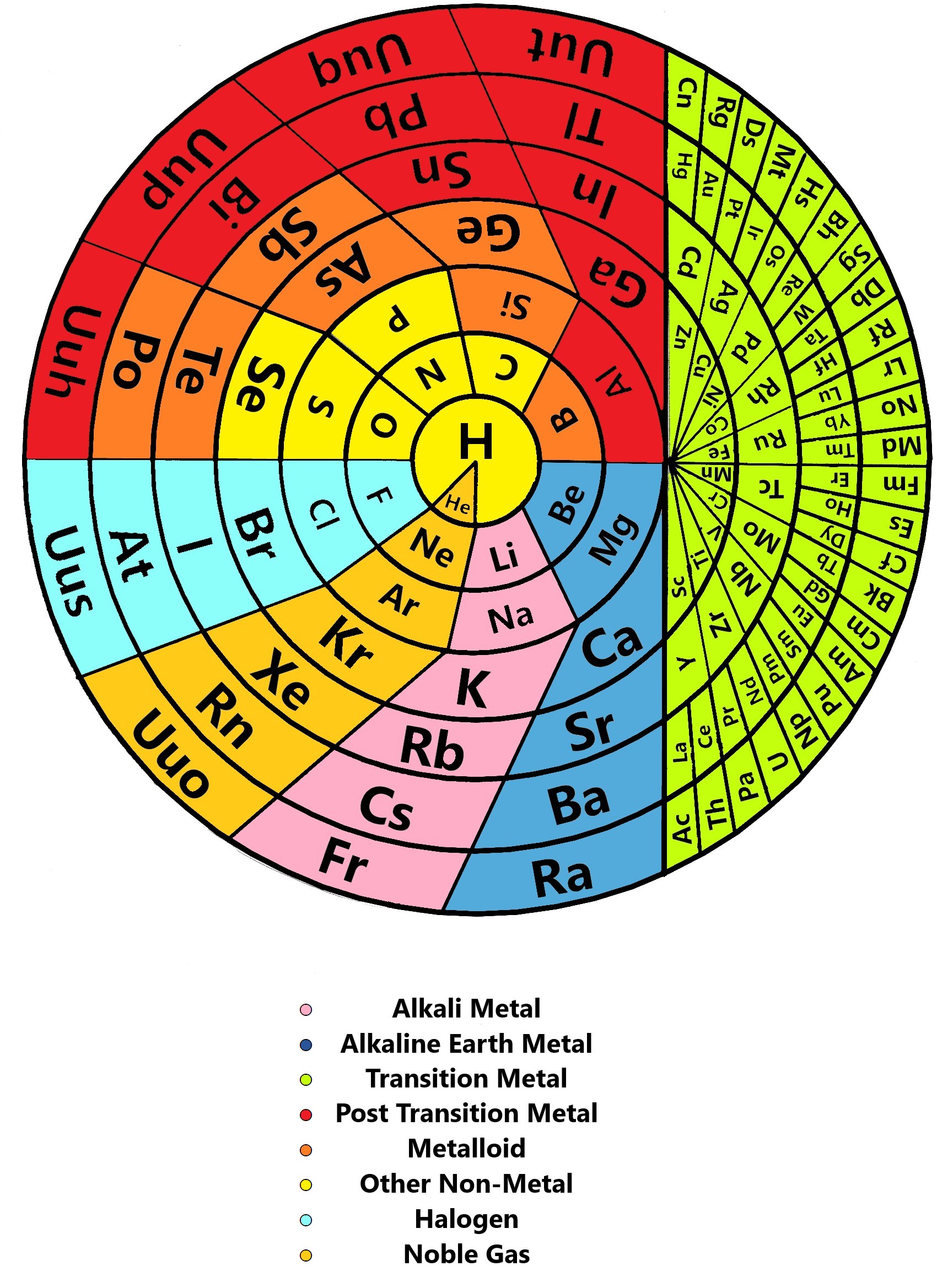
Discoid Periodic Table of The Elements
Statement: "The orbital periodicity of the elements are the periodic function of their atomic number." By Muzzammil Qureshi.
Muzzammil writes:
"Years before Mendeleev's publications, there was plenty of experimentation with alternative layouts for the elements. Even after the table got its permanent right-angle flip, folks suggested some weird and wonderful twists.
"One of them are Circular in shapes. Discoid means circular in shape, and there is a great reason for choosing such a shape. The term "Periodicity" itself means "To occur in intervals", and if you walk around in a circle, you will find that you will return to the point from where you started at. Similarly, if the elements are also arranged in such way, then we shall experience more periodicity in the elements than before..."
| Year: 2021 | PT id = 1222, Type = formulation 3D |
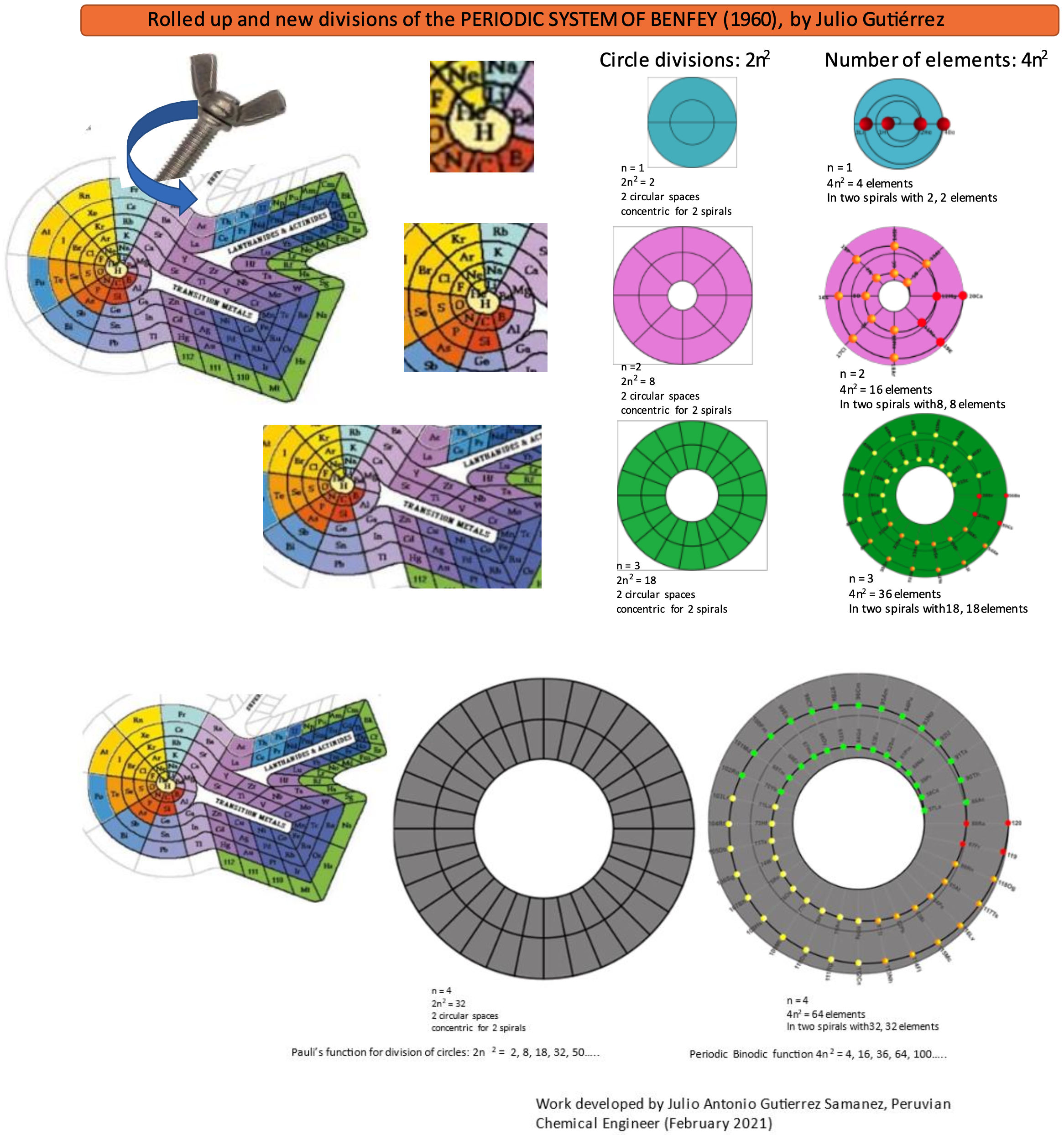
Rolled-up Version of Benfey's Periodic System
Rolled-up Version of Benfey's Periodic System by Julio Antonio Gutiérrez Samanez. More on the YouTube video here.
| Year: 2021 | PT id = 1224, Type = formulation |
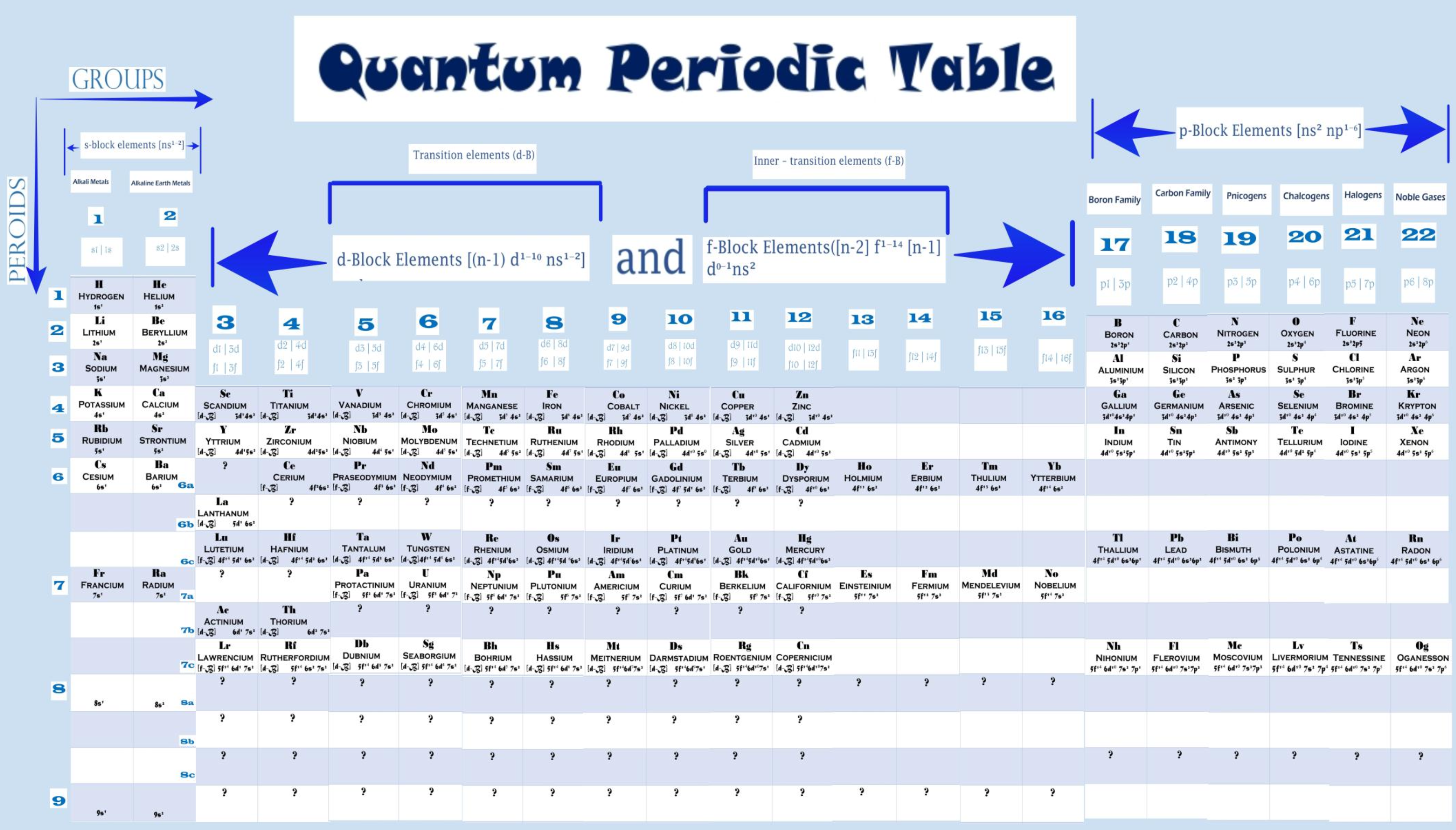
Quantum Periodic Table
Shriya Tiwari & D. K. Awasthi, Quantum Periodic Table, wjpmr, 2021, 7(4), 124-130
The authors write:
"In [the] quantum periodic table, The elements are arranged according to the order of electron-shell filling, by classifying the energy levels of the atoms in the order they are filled, to create a layout based on electronic configuration. The classification of the elements is done purely on the basis clarified above, without giving any weight age to the atomic numbers. With the advent of electronic configurations and quantum mechanics, many attempts have been tried in this periodic table to unlock all the problems related with the placement of elements, which have been remained as the topic of debate by generations of chemists."
| Year: 2021 | PT id = 1225, Type = formulation |
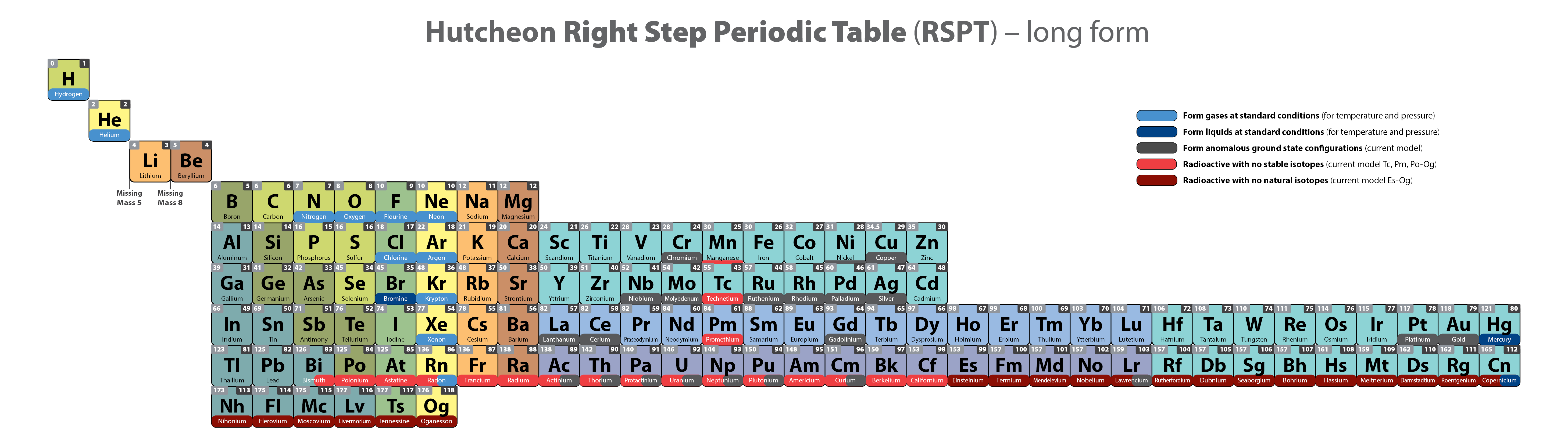
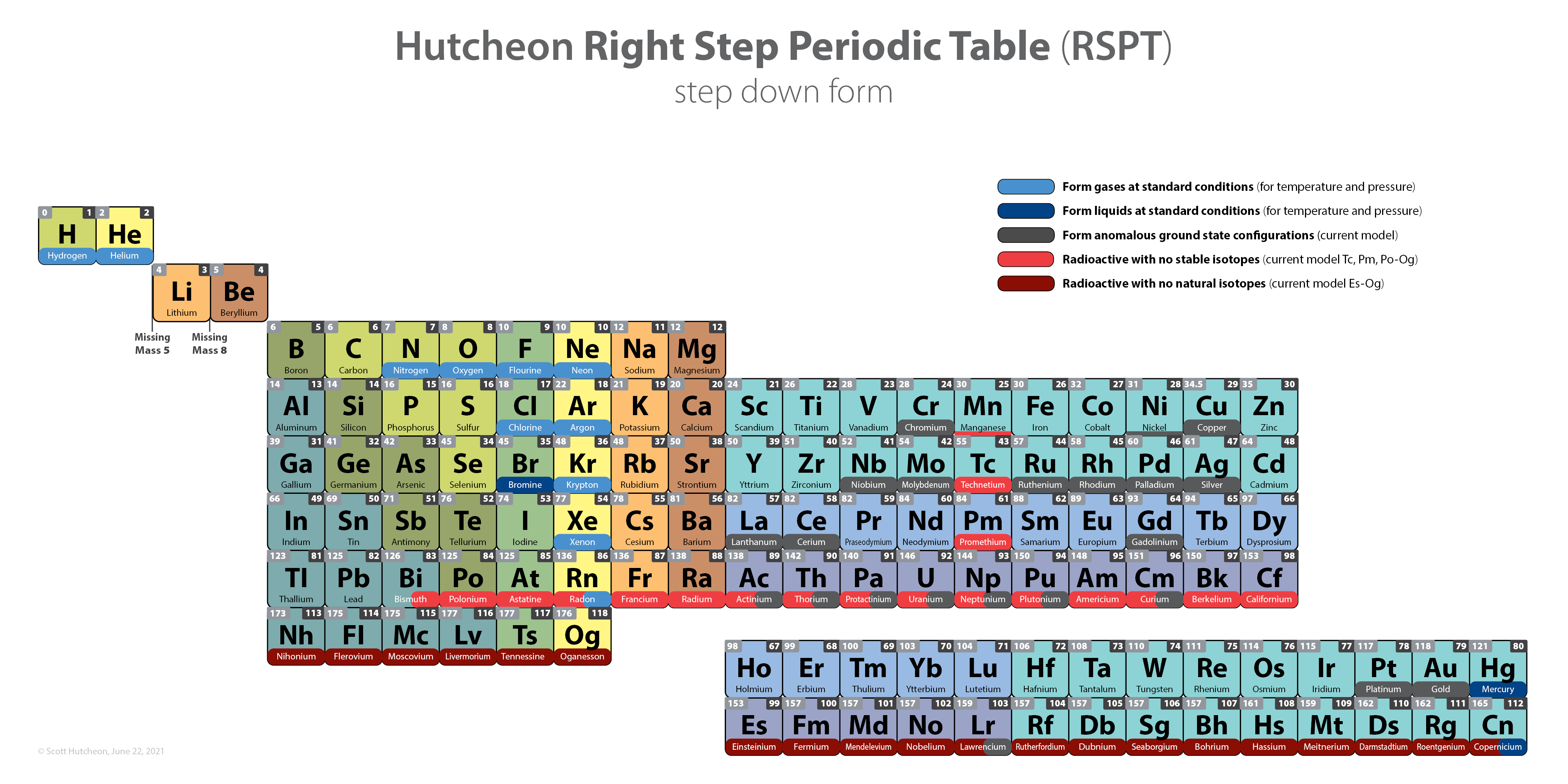
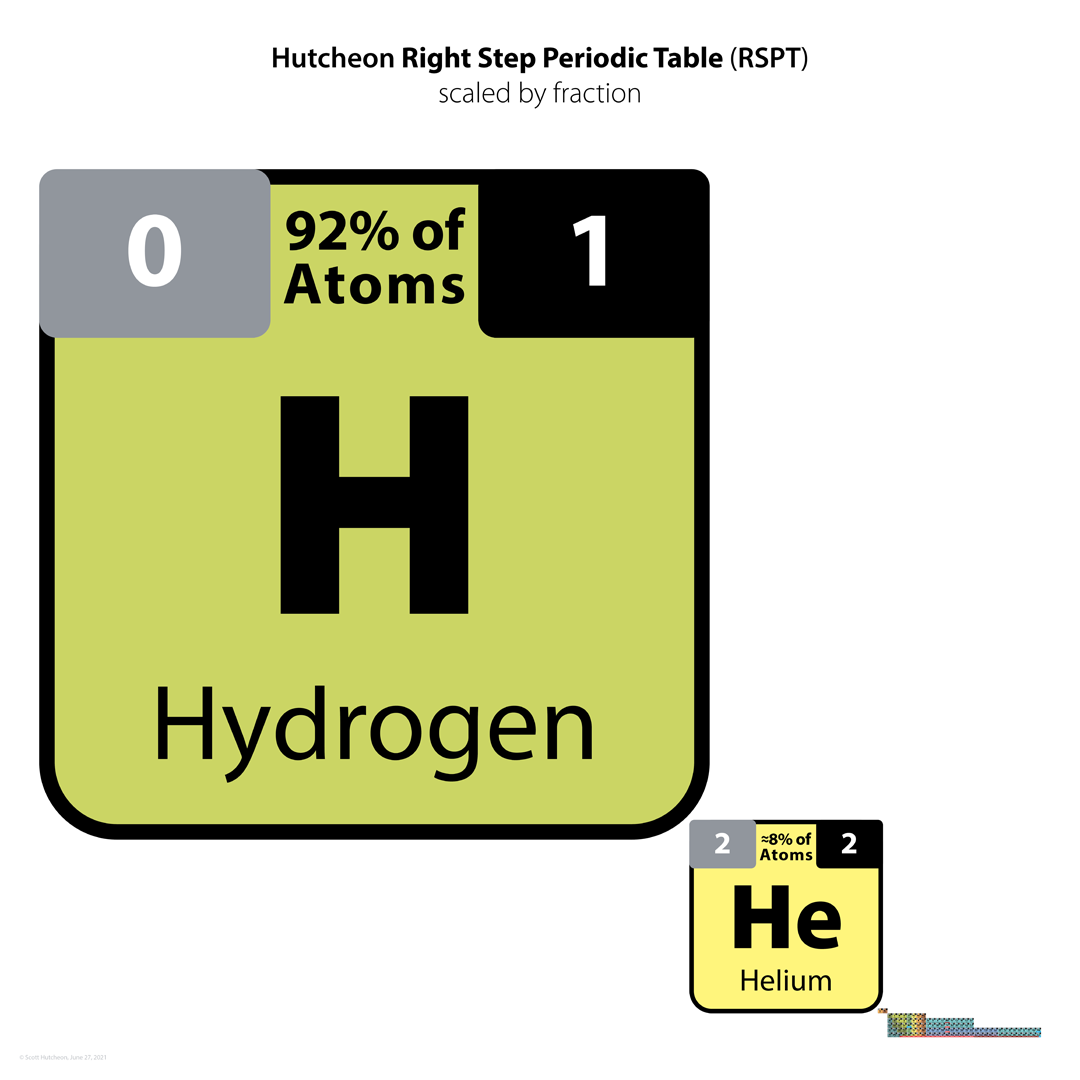
Hutcheon Right Step Periodic Table
From Scott Hutcheon's Linkedin Website:
Abstract:
"Built from first principles, the RSPT is the only Periodic Table (so far) that reflects the periodicity of radioactive elements (natural and artificial) including Tc-Np and Cf/Es, the periodicity of liquids and gases (at standard and other conditions), depicts the 5 and 8 mass roadblocks, and finally clarifies the positions of the initial propagating elements H, He, and Li-Be/B in accordance with their cosmic and stellar evolutionary origins.
"Name inspired by the Janet Left Step Periodic Table (LSPT).
"Bonus Easter Egg: SPOCH BONSe, pronounced Spock Bones, is a new mnemonic device to remember the updated elements considered most essential for human life. Also considered POSCH SeNOB and SNOBS ePOCH."
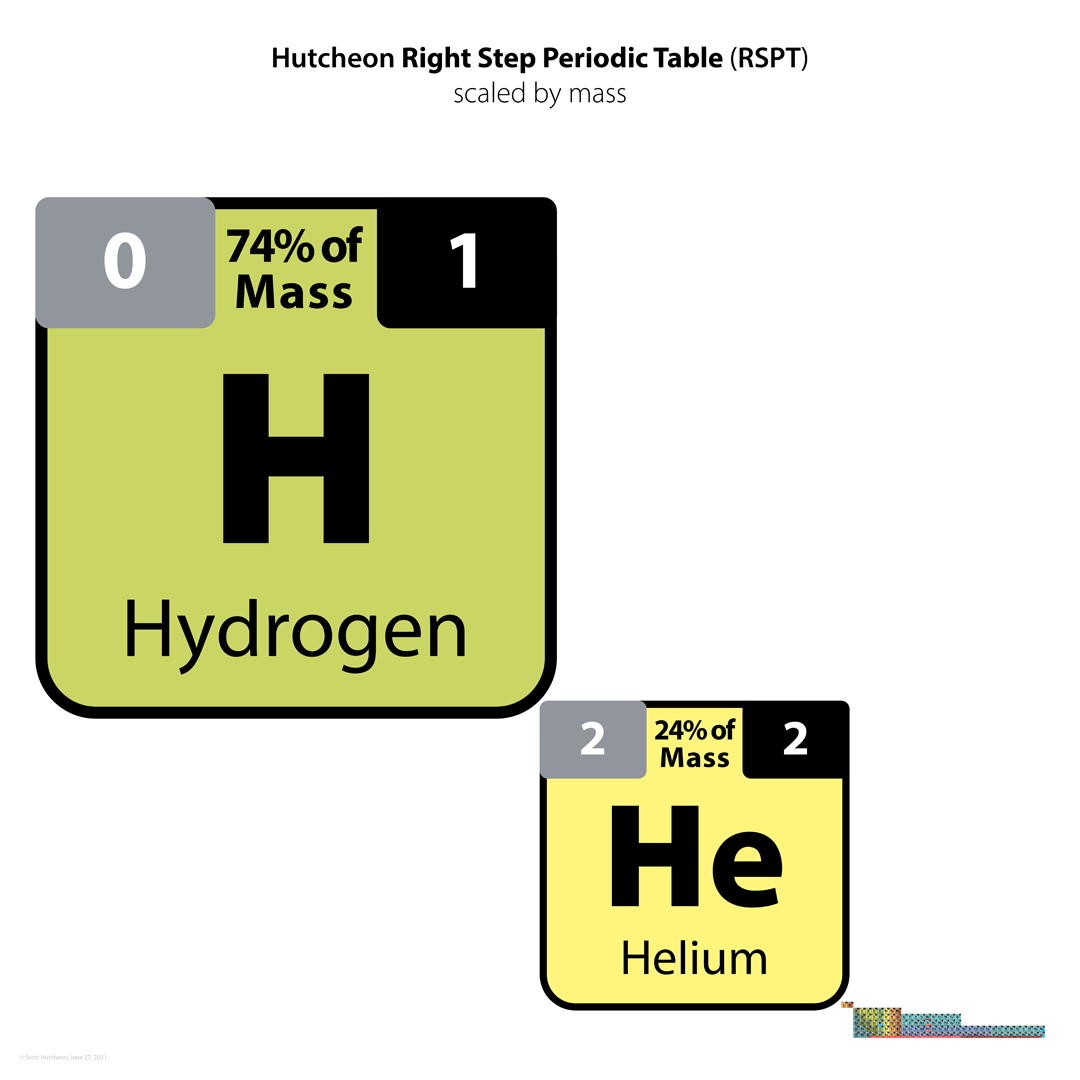
| Year: 2021 | PT id = 1232, Type = formulation review |
The Periodic Table: Is it Perfect, is it Fractured or is it Broken?
A video from Mark Leach, who writes:
The periodic table is an icon of science. Indeed, all chemical matter is made from periodic table stuff. The periodic table of the elements is often presented as being:
- With 118 elements the periodic table is now complete
- The periodic table is perfectly described (fully explained) by the application of four quantum numbers with the and some simple rules
- Chemical structure & reactivity can be deduced from the periodicity of the Groups & Periods
However, the chemistry of the chemical elements is actually a little more complicated than this. So, where & why does the predictability 'break'?
| Year: 2021 | PT id = 1244, Type = data |
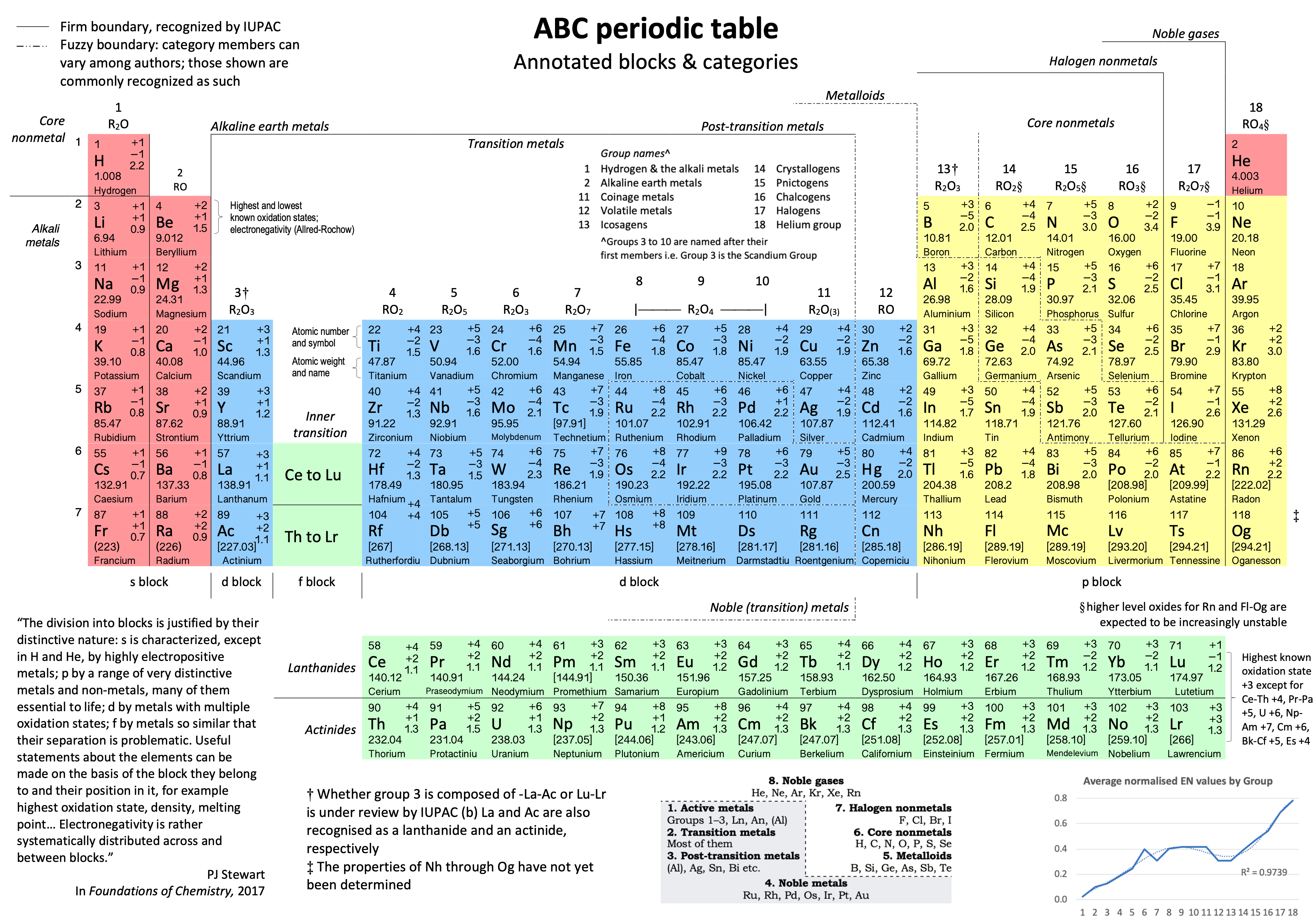
Vernon's ABC Periodic Table
The Annotated Blocks & Categories (ABC) Periodic Table by René Vernon.
| Year: 2021 | PT id = 1248, Type = review |
Meyer or Mendeleev: Who created the periodic table?
An article in Academic Influence by Eric Scerri: Meyer or Mendeleev: Who created the periodic table?
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.