Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables referencing the text string "Mazurs", listed by date:
| Year: 1782 | PT id = 297, Type = formulation |
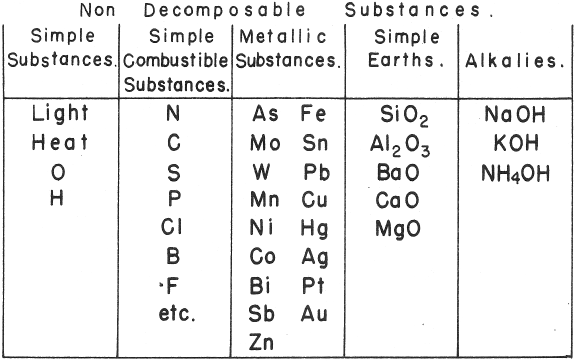
de Morveau's Table of Chemically Simple Substances
de Morveau's table of chemically simple substances (updated with modern representations by Mazurs):
| Year: 1905 | PT id = 585, Type = formulation 3D spiral |
Gooch & Walker Periodic Table
Mazurs' reproduction (p. 82) of a periodic table formulation by Frank Austin Gooch and Claude Frederic Walker, from Outlines of Inorganic Chemistry, Macmillan, London and New York, p. 8/9, 1905 (ref Mazurs p.188):
Thanks to Laurie Palmer for the tip, and to Philip Stewart for the corrections and details.
| Year: 1913 | PT id = 973, Type = formulation |
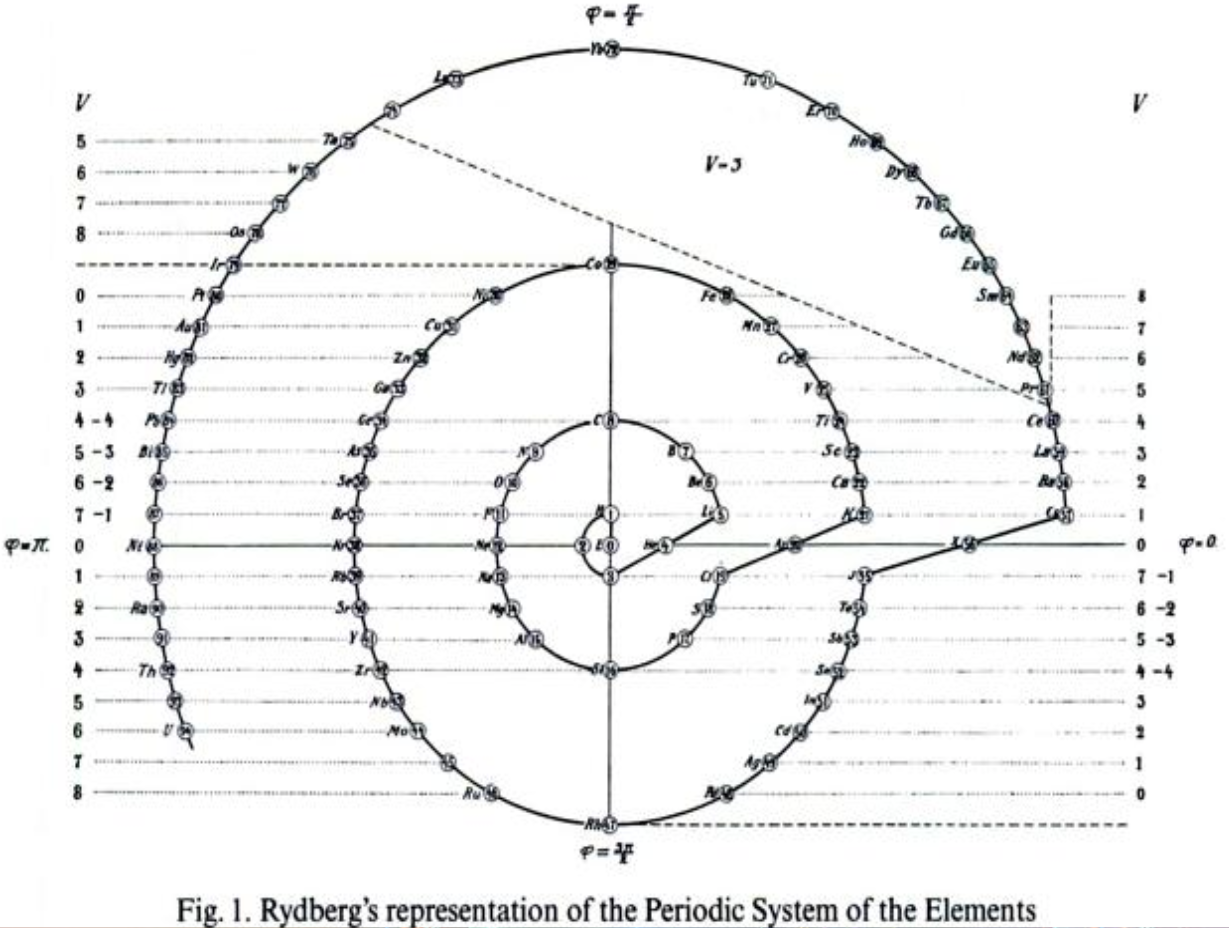
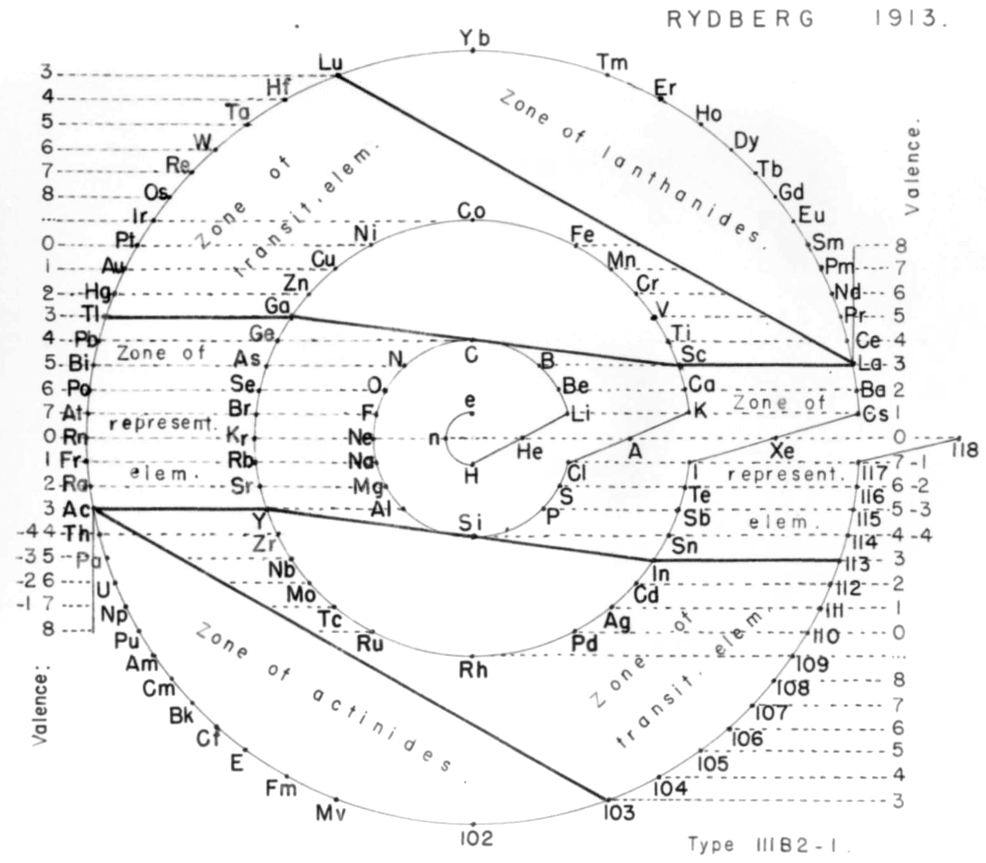
Rydberg's Periodic Table in style of Spiral with Four Revolutions
Periodic table in style of spiral with four revolutions circa 1913 (Original design) and 1957 (Date attributed to slide).
This table was originated by Swedish physicist Johannes Rydberg (1854-1919) in 1913 and classified by chemist Edward G. Mazurs as Type IIIB2-1 in his seminal work Types of Graphic Representation of the Periodic System of Chemical Elements (1957). The lower version of the table appears as Figure 63 on page 132 of Mazurs' 1957 publication.
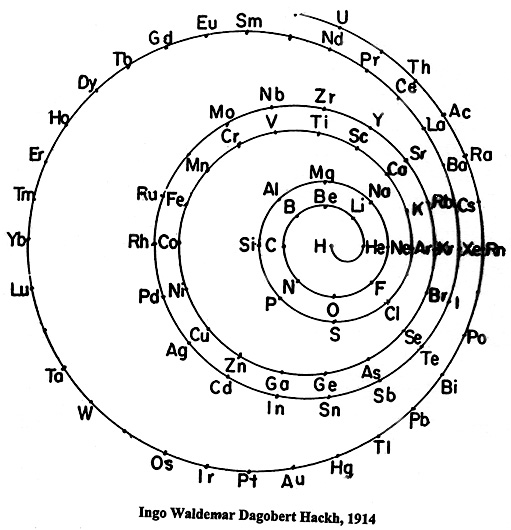
| Year: 1914 | PT id = 23, Type = formulation spiral |
Hackh's Spiral Periodic Table
Ingo Hackh's spiral periodic table of 1914, from Das Synthetisches System der Atome, Hamburg, Hephaestos.
Philip Stewart says:
"I believe that Hackh's 1914 spiral is of special interest it is the first spiral to take account of Mosley's atomic numbers, and the first to show successively larger pairs of coils. It is also interesting because H stands alone in the centre. I have only seen Mazurs' redrawn (as usual!) version, but Mazurs gives SciAm Supplement 1919 as one reference."
This is the Mazurs version:
| Year: 1928 | PT id = 305, Type = formulation spiral |
Janet's "Lemniscate" Formulation
From in The Helicoidal Classification of the Elements, Chemical News vol. 138, 21 June 1929, Fig. XI, p. 392:
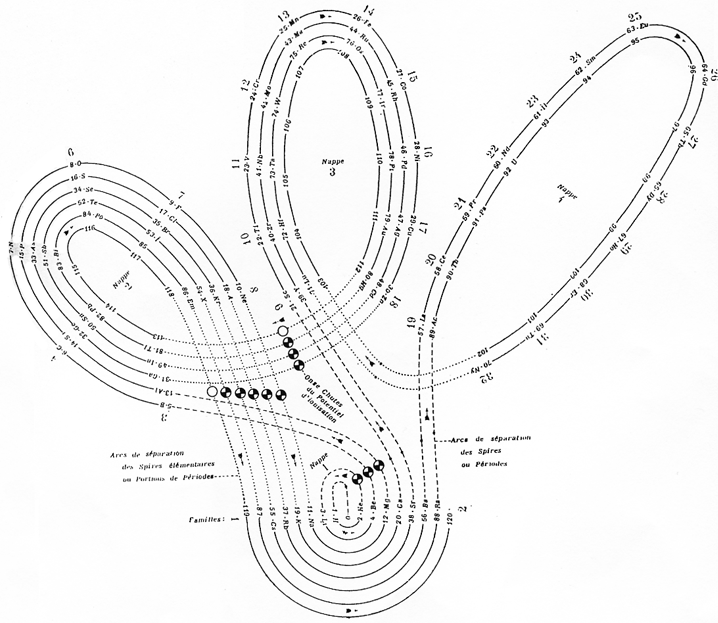
Philip Stewart points out that this formulation is an 'end on' view of the Janet Cylinder or Three-Dimensional Spiral-Tube System formulation, and the term "lemniscate" comes from Mazurs.
| Year: 1930 | PT id = 696, Type = formulation |
Gardner & Mazzucchelli's Periodic System Elaborated as Electronic Configuration
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
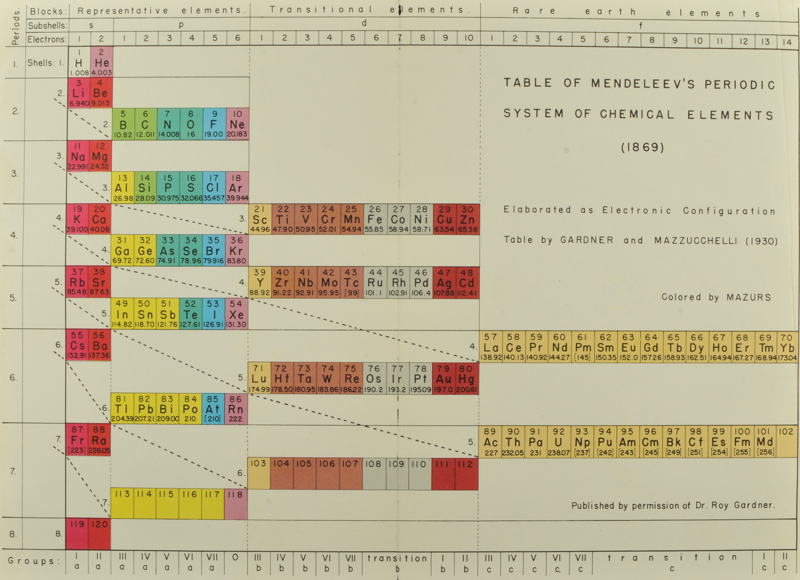
Thanks to Philip Stewart for the tip!
| Year: 1939 | PT id = 1056, Type = formulation |
Foster's Periodic Arrangement
L.S. Foster, "Why not modernise the textbooks also? I. The periodic table", Journal of Chemical Education, vol. 16, no. 9, pp. 409–412, https://pubs.acs.org/doi/10.1021/ed016p409
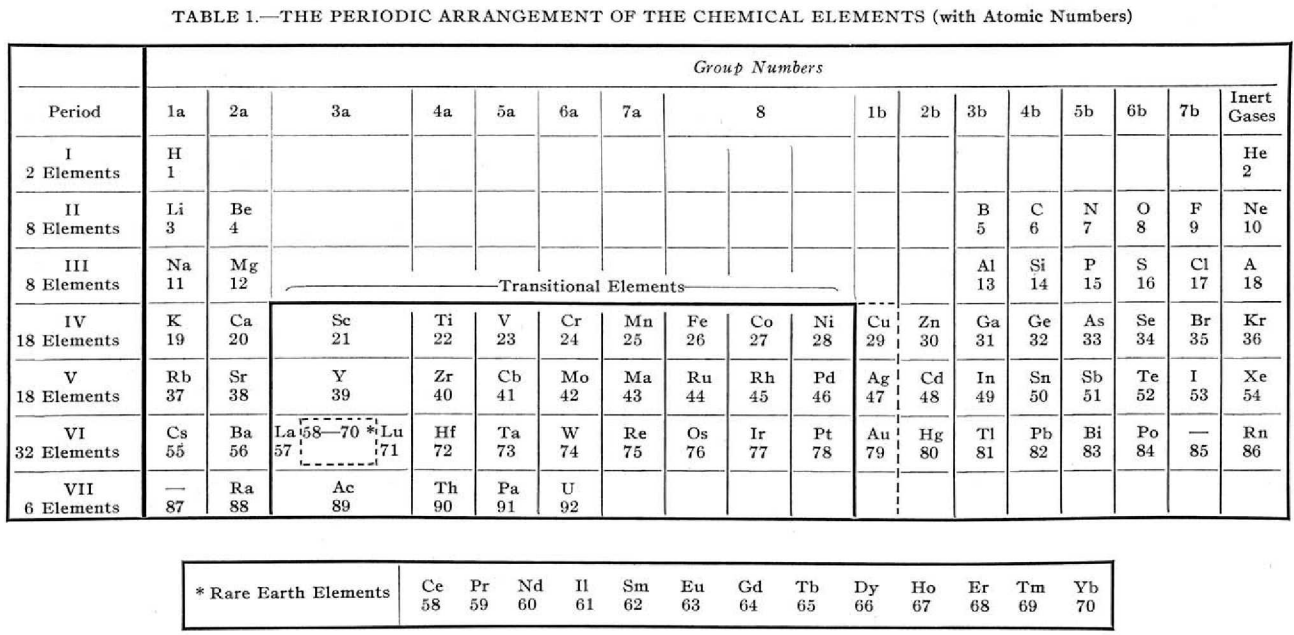
Foster writes:
"The [above] modern periodic table is simply an orderly array of the elements with all unnecessary ornamentation omitted, has been found highly satisfactory for instructional purposes.
"The transitional elements, with two unfilled electron shells, are separated from the non-metallic elements.
"The rare-earth elements, defined as those with three incomplete electron shells, are shown to be those of atomic numbers 58 to 70, while La and Lu, which have only two incomplete electron shells are classified as transitional elements.
"Copper, silver, and gold act as transitional elements except when the state of oxidation is one."
René Vernon writes:
Foster couldn't show the coinage metals – with their full d10 complements – as transitional elements, but by adding a broken line around them he was showing they had the capacity to act as if they were.
I tried to work out how he distinguished La & Lu from Ce to Yb. Foster seems to be saying that La 5d1 6s2, has incomplete 5th and 6th (ie. 6p) shells.
Same for Lu 4f14 5d1 6s2 having incomplete 5th and 6th shells. Whereas, for example, Ce 4f1 5d1 6s2 has incomplete 4th, 5th and 6th shells. Presumably this was in the years before the fact that the 4f shell became full at Yb was widely appreciated. So, strictly speaking, group 3a should have read:
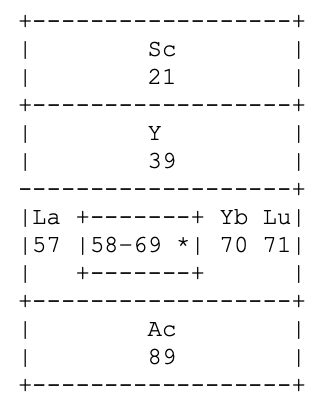
On the other hand, Yb3+ has an f13 configuration, so it does meet his three unfilled shells criterion. Had he known, he probably would've put a broken line around Yb to indicate its full f14 complement but that it normally acted as a rare earth, with an incomplete 4f shell; whereas neither La nor Lu have this capacity.
Good to see Foster put so much thought into organising his table, and his experience with using it for instructional purposes.
Van Spronsen does not mention Forster's table. Mazurs has a reference to Foster's table but lumps it in with the other medium-long tables, not appreciating its subtlety.
Mark Leach writes:
This formulation is very much like the XBL 769-10601, Periodic Table Before World War II used by Seaborg and the Manhattan project and is a precursor to the modern periodic table.
| Year: 1942 | PT id = 1081, Type = formulation spiral |
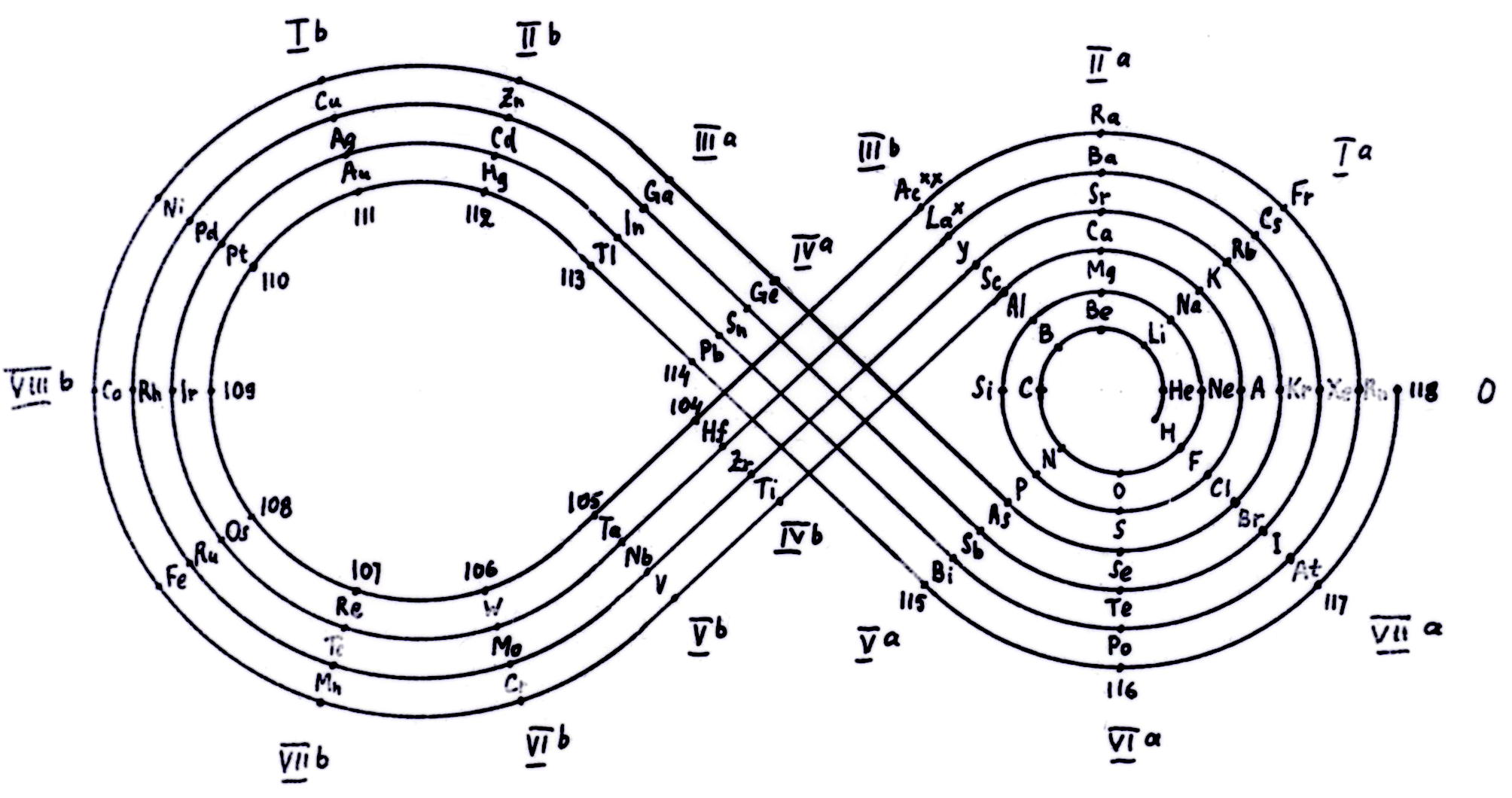
Kipp (& Mazurs') Periodic Table in Style of Spiral and Plane Lemniscate
Kipp, Friedrich, and Edward G. Mazurs. "Periodic Table in Style of Spiral and Plane Lemniscate". Glass, circa 1942–1957. Edward G. Mazurs Collection of Periodic Systems Images, Box 1. Science History Institute, Philadelphia. https://digital.sciencehistory.org/works/nz806022g
Periodic table in style of spiral and plane lemniscate 1942 (Original design) circa 1957 (Date attributed to slide).
This table was originated by Friedrich Kipp in 1942 and classified by chemist Edward G. Mazurs as Type IIB2-2 in his seminal work Types of Graphic Representation of the Periodic System of Chemical Elements (1957).A version of this table appears as Figure 49 on page 122 of Mazurs' 1957 publication.
Thanks to Dhr. J.G. van Gils for the tip!
| Year: 1954 | PT id = 1317, Type = formulation |
New Periodic Table of the Elements Based on the Structure of the Atom
Tomkeieff SI, 1954, A New Periodic Table of the Elements Based on the Structure of the Atom, Chapman & Hall, London.
Thanks to René Vernon for the tip, who writes:
It is a helix wrapped on the surface of a cone. The shadow on the left is from the edge of my hand holding down the table; the shadow on the right is from the edge of a different book, again used to hold down the table into some semblance of flatness.
Mazurs said: "This is not a very successful table".
First, there is the cumbersome nature of a table on a cone, Secondly, see how the eight main group numbers at the top are sort of mushed into the 18 A and B series group numbers. This does not work well.
The colour scheme shows the dominant acid-base properties of the elements:
Dark blue — strong bases
Light blue — weak bases
Light red — weak acids
Dark red — strong acids
White — Inert gasesSince nonmetals never form basic oxides it is interesting to note that the (23) nonmetals fall on the right side of the table:
H He
B C N O F Ne
Si P S Cl Ar
Ge As Se Br Kr
Sb Te I Xe
Rn[Water is amphoteric; hydrogen peroxide is weakly acid.]
While the underlined elements are sometimes called metalloids, it is has been known for over 100 years that metalloids predominately behave chemically like nonmetals.
Astatine would’ve been a nonmetal but for relativistic effects. Immediately following its production in 1940, early investigators considered it a metal.

| Year: 1955 | PT id = 300, Type = formulation |
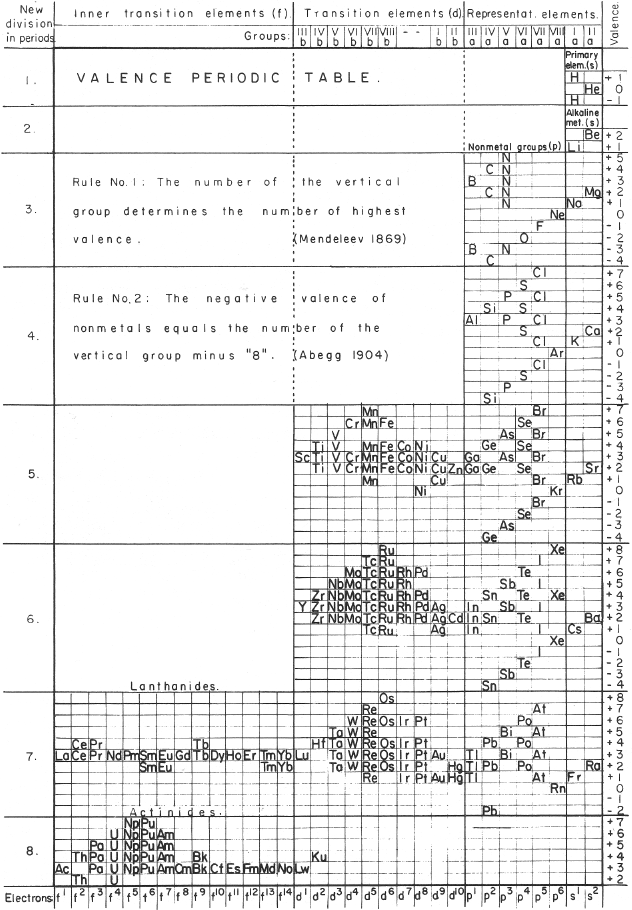
Mazurs' Valence Periodic Table
In his 1974 book Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press (2nd edition) Edward G. Mazurs presents a valence periodic table. He classifies this as a Subtype IIIC3-6a formulation:
| Year: 1955 | PT id = 301, Type = formulation |
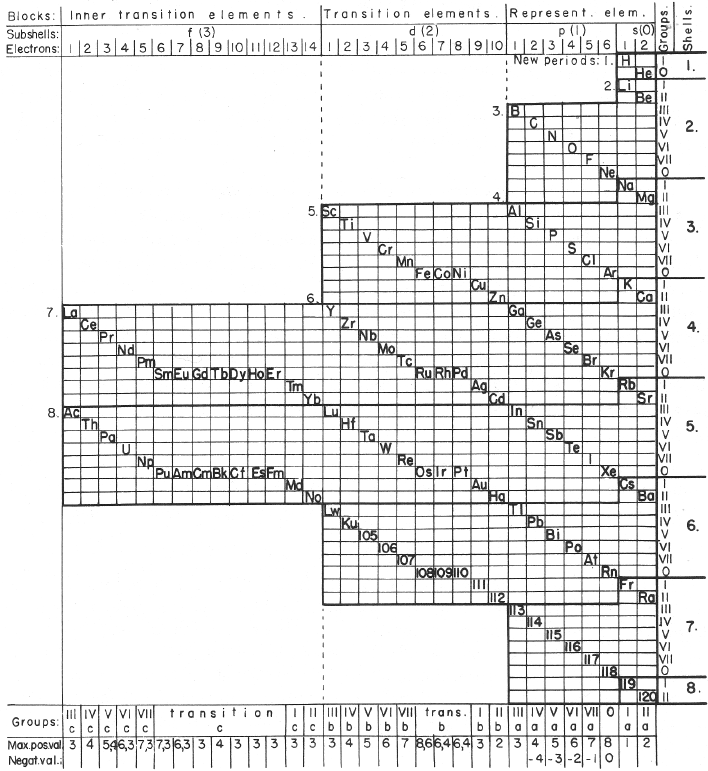
Mazurs' Valence Periodic Table
In his 1974 book Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press (2nd edition) Edward G. Mazurs presents a periodic table he classifies as a Subtype IIIC3-6b formulation:
| Year: 1955 | PT id = 691, Type = formulation |
Mazurs' 1955 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
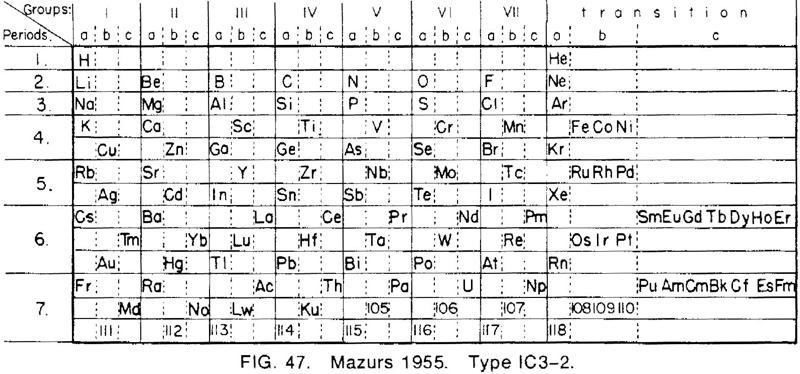
Thanks to Philip Stewart for the tip!
| Year: 1957 | PT id = 110, Type = review |
Mazurs' Graphical Representations of The Periodic System During 100 Years
Edward Mazurs, Graphical Representations of The Periodic System During 100 Years, University of Alabama Press, 1957.
There is an internet archive: Edward G. Mazurs Collection of Periodic Systems Images.
This book gives a very full analysis and classification of periodic table formulations. Most of the formulations are redrawn.
However, anybody who is seriously interested in periodic table formulations will want to see/read/own this book. Read more about Mazrus on the Elements Unearthed blog.
1955 |
Mazurs' Valence Periodic Table (1974, p.94) |
1955 |
Mazurs' Periodic Table (1974, p. 95) |
1955 |
Mazurs' 1955 Formulation (1974, p. 44) |
1958 |
Mazurs' 1958-73 Formulation (1974, endpaper) |
1965 |
Mazurs' 1965 Formulation (1974, p/ 134) |
1967 |
Mazurs' 1967 Formulation (1974. Inside front cover) |
1967 |
Mazurs' other 1967 Formulation (1974, p. 126) |
1967 |
Mazurs' another 1967 Formulation (1974, p. 134) |
1969 |
Mazurs' Perio |
1974 |
Mazurs' Version of Janet's "Lemniscate" Formulation (1974, p.80) |
1974 |
Marzus' Wooden Version of Mendeleev's Periodic Table (Chem. Heritage Foundn.) |
1974 |
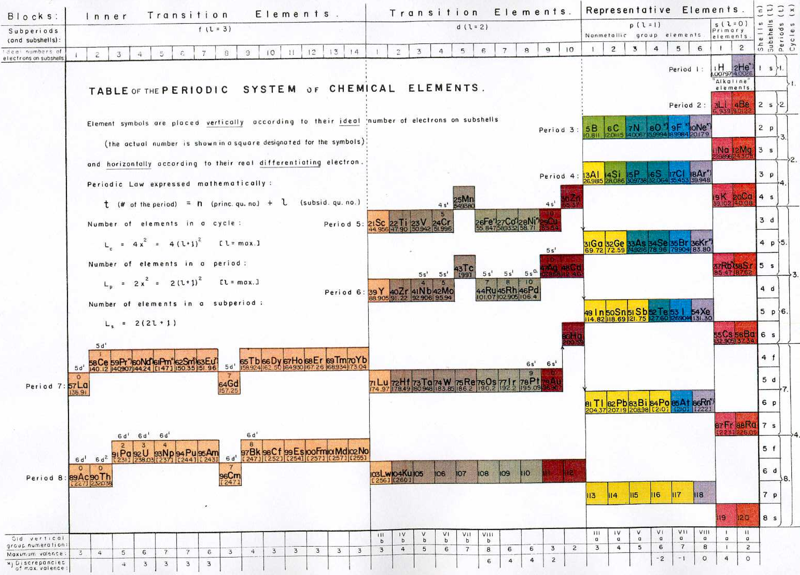
Mazurs' PT Formulation Analysis (1974, pp.15-16) |
Many thanks to Philip Stewart for preparing the links table above.
| Year: 1958 | PT id = 693, Type = formulation |
Mazurs' 1958-73 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
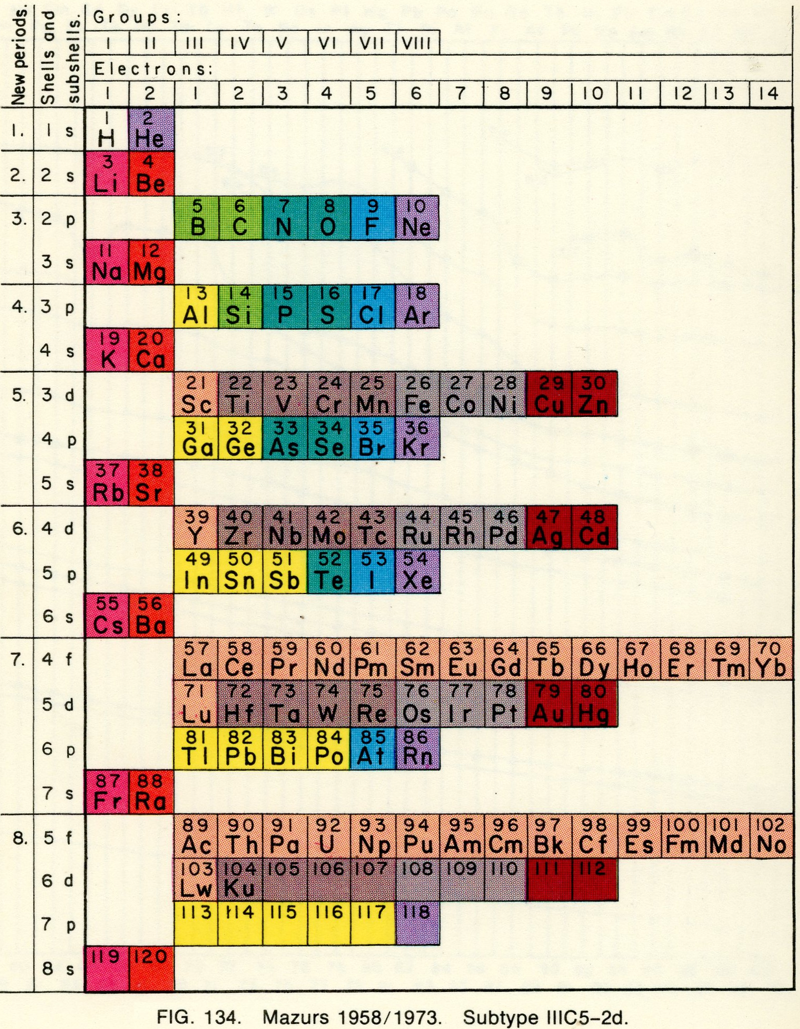
Thanks to Philip Stewart for the tip!
| Year: 1965 | PT id = 692, Type = formulation |
Mazurs' 1965 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
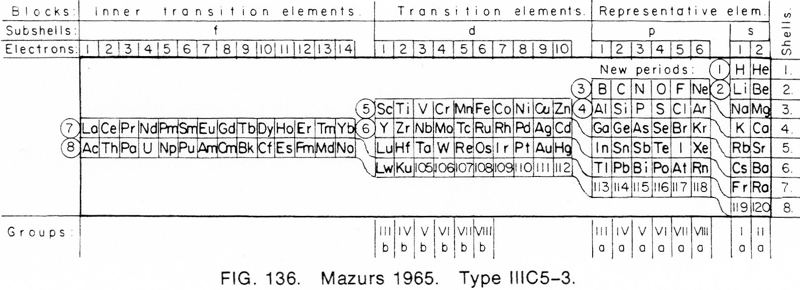
Thanks to Philip Stewart for the tip!
| Year: 1967 | PT id = 298, Type = formulation |
Mazurs' 1967 Formulation
From the front cover of Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
Thanks to Philip Stewart for the tip!
| Year: 1967 | PT id = 694, Type = formulation 3d spiral |
Mazurs' other 1967 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
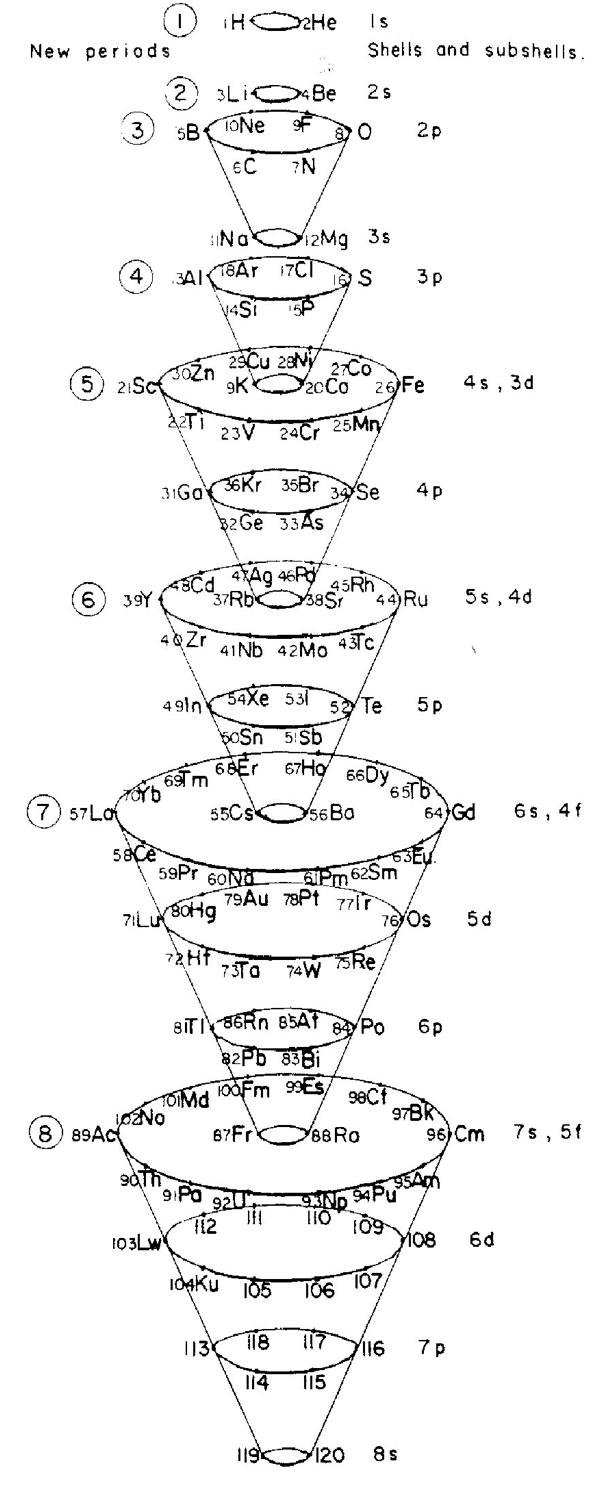
Thanks to Philip Stewart for the tip!
| Year: 1967 | PT id = 695, Type = formulation |
Mazurs' another 1967 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press.
This formulation is the basis of Philip Stewart's Janet Rajeuni:
Thanks to Philip Stewart for the tip!
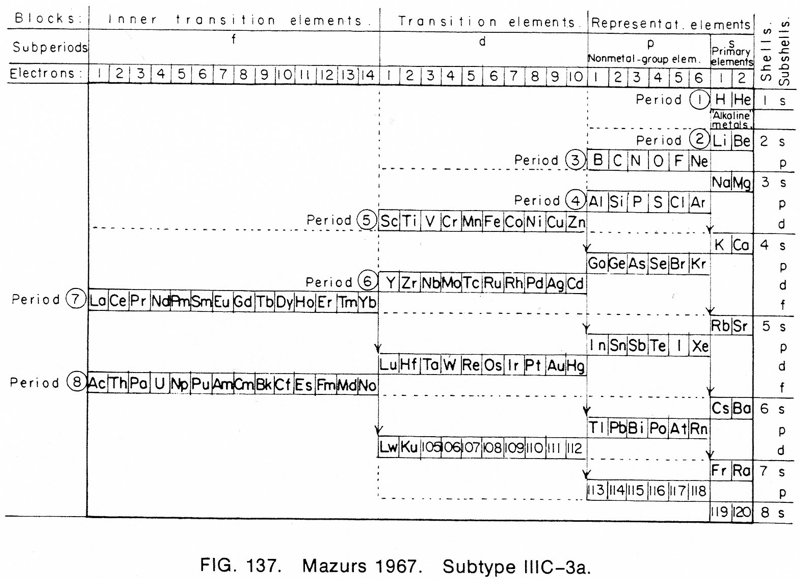
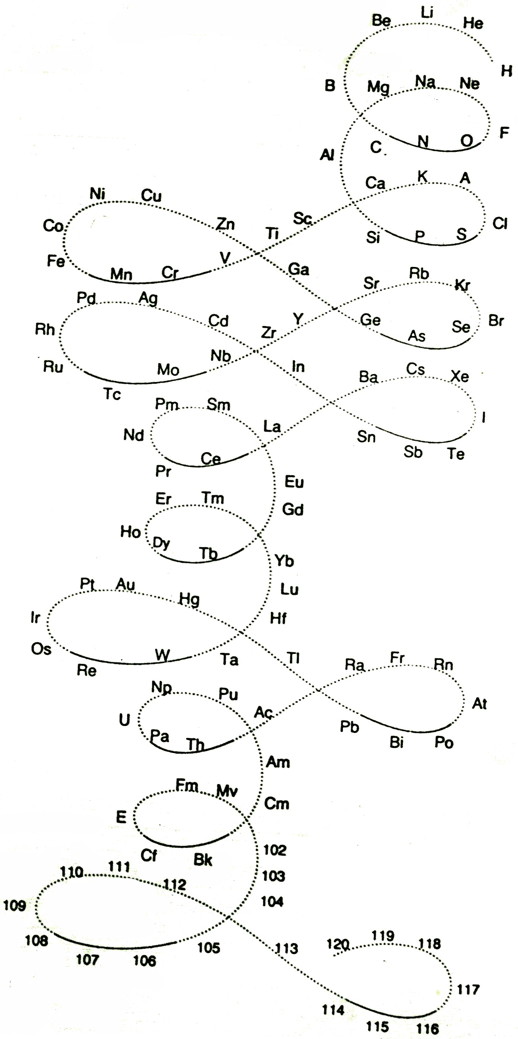
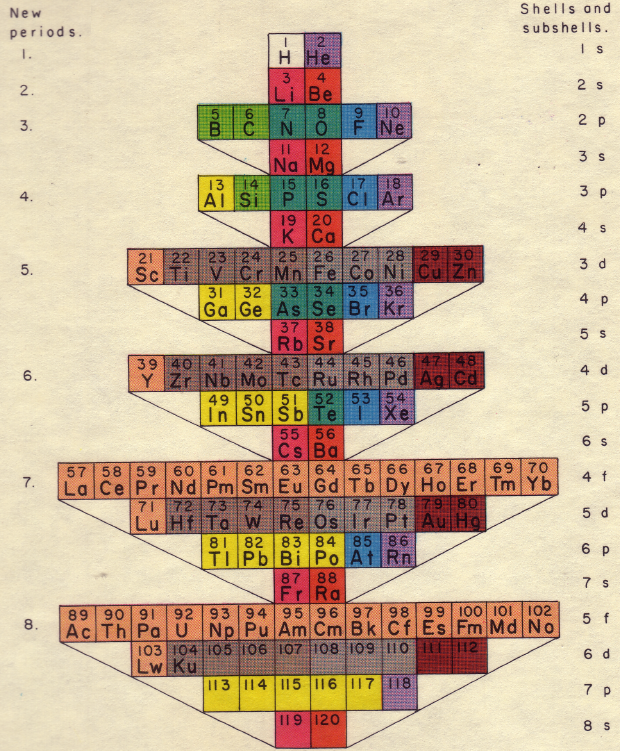
| Year: 1969 | PT id = 624, Type = formulation |
Mazurs Periodic System of Chemical Elements
A foldout from the Mazurs book, Graphical Representations of The Periodic System During 100 Years.
Mazurs said he drew it in 1967 and published it in 1969: ref. E Mazurs, A new numeration of periods in the periodic system and the Kessler Principle for the construction of the periodic table, Canad. Chem. Edu. 4(3), 21-23, 1969.
It is a Janet's modified system to show the irregularities – Lu, Cr, Pd etc. Click here for a larger version:
Thanks to Philip Stewart for the tip!
| Year: 1974 | PT id = 260, Type = formulation spiral |
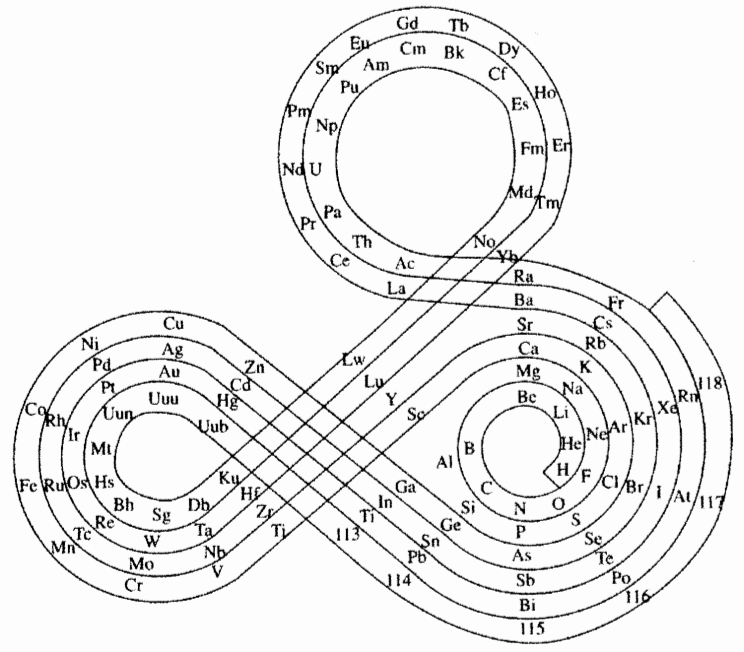
Mazurs Version of Janet's "Lemniscate" Formulation
Janet's lemniscate formulation periodic table as modified by E.G. Mazur in his Graphic Representations of the Periodic System during One Hundred Years (1974), cited in Punyashloke Mishra's The Role of Abstraction in Scientific Illustration: Implications for Pedagogy (1999) republished in Carolyn Handa's Visual Rhetoric in a Digital World: A Critical Sourcebook", from the Island94 blog, here:
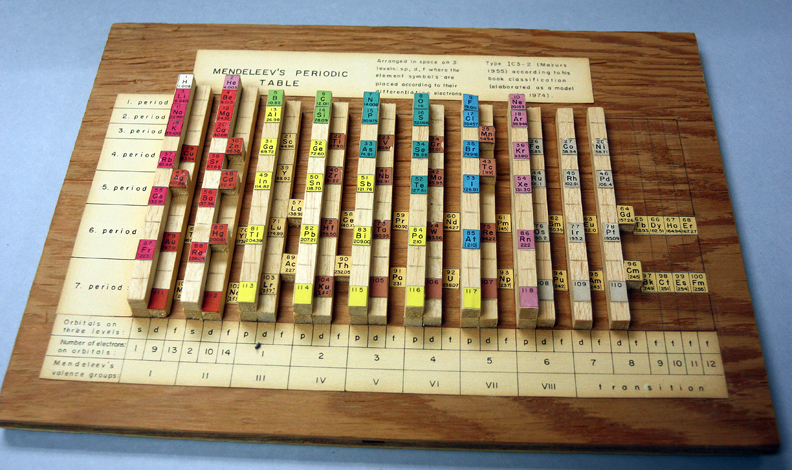
| Year: 1974 | PT id = 267, Type = formulation 3D |
Mazurs Wooden Version of Mendeleev's Periodic Table
There is a posting in the The Elements Unearthed blog by David V Black concerning a view of the Marzus archive:
"My biggest discovery this week has been a collection in our archives of the notes of Edward Mazurs, who wrote the definitive work on classifying different systems of periodic tables in 1957 with a revised edition in 1974 (Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press). He collected articles and wrote extensive, detailed notes on every version of the periodic table he could find as it developed from its start in the early 1860s with the work of de Chancourtois through 1974. All of those notes have been donated to Chemical Heritage Foundation and fill up ten binders, with meticulous drawings, charts, tables, and frequent additions and changes. There are also some pieces of the original artwork prepared for the book, and a wooden model of the periodic table Mazurs built himself. "
| Year: 1974 | PT id = 1058, Type = formulation spiral |
Mazurs' Redrawing of Stedman's Formulation
An spiral formulation by Mazurs, cited as being after Janet (1928). However, it is actually, it is after Stedman (1947).
In an article Bull. Hist. Chem., VOLUME 34, Number 2 (2009) O.T. Benfey writes:
"After we had developed our own [Periodic Snail] spiral design, we found that E. G. Mazurs had published a spiral with a separate protrusion for the lanthanides which, under the image, he misleadingly ascribed to Charles Janet in 1928, the same year that Janet had published a simple circular form also shown by Mazurs. The Mazurs diagram with the lanthanide protrusion was reprinted in [the journal] Chemistry. However, [Philip] Stewart informed me that the Mazurs figure bears no resemblance to the Janet diagram he indicated nor to any other of his designs. Detailed references given a few pages later by Mazurs suggested correctly that the spiral derives from Stedman and is so identified and depicted by van Spronsen. The Mazurs diagram is a mirror image of the Stedman spiral, updated to include elements discovered since 1947." [For references, see the article.]"
Mazurs (p. 77) writes:
"Subtype IIIA3–1a Helix on a modified cone. The transition and inner transition elements have special revolutions in the form of loops. This table, originated by Stedman in 1947 is not a successful one."
Thanks to René for the tip and information!
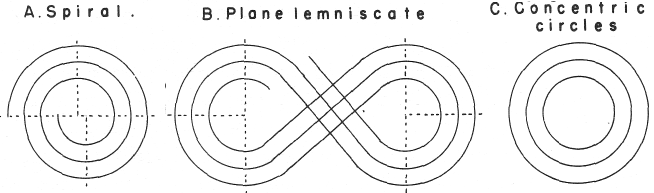
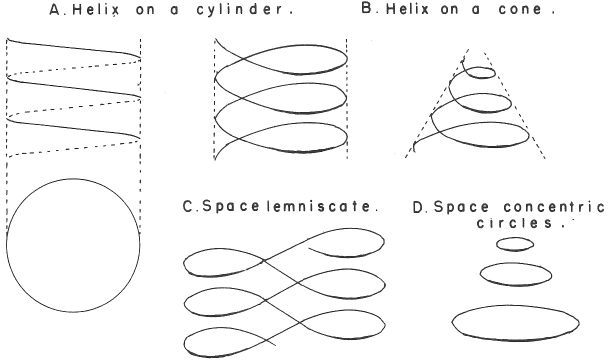
| Year: 1974 | PT id = 299, Type = formulation spiral 3D misc |
Mazurs' PT Formulation Analysis
In his 1974 book Edward G. Mazurs (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press gives a comprehensive analysis of periodic table formulations.
Mazurs identifies most PT formulations as being:
- Spiral
- Plane lemniscate
- Concentric circles
- Helix on a cylinder
- Helix on a cone
- Space lemniscate
- Space concentric circles


| Year: 2010 | PT id = 302, Type = review |
Before & After Mendeleev: Periodic Table Videos
Two videos by the Chemical Heritage Foundation:
- Part 1 Before Mendeleev (17min) covers the events leading up to Mendeleev's invention of the periodic table, including the work of several precursors such as de Chancourtois, Newlands, Odling, Hinrichs, and Meyer.
- Part 2 Mendeleeve & Beyond (20 min). The second part covers Mendeleev's working out of his periodic system and the work of his successors, as well as some interesting questions such as whether the periodic table can be entirely deduced from quantum mechanics and the mystery of the Knight's Move pattern of properties.
The videos feature interviews with Dr. Eric Scerri of UCLA, with added narration, animations, illustrations, photos, captions, etc. by David V. Black as well as publication artwork and notes by Edward G. Mazurs.
| Year: 2012 | PT id = 501, Type = review |
Books on the Chemical Elements and the Periodic Table/System
From Eric Scerri's forthcoming book A Tale of Seven Elements (Oxford University Press, 2013) and used by permission of the author, is the most complete and up-to-date list of Books on the Chemical Elements and the Periodic Table/System, including some titles in foreign languages.
Additional books in other languages can be found listed in Mazurs, 1974
- H. Alderesey-Williams, Periodic Tales, Viking Press, 2011
- N.P. Agafoshin, Ley Periódica y Sistema Periódico de los Elementos de Mendeleiev, Ed. Reverté S.A., Barcelona, 1977
- I. Asimov, The Building Blocks of the Universe, Lancer Books, New York, 1966
- P.W. Atkins, The Periodic Kingdom, Basic Books, New York, NY, 1995
- O. Baca Mendoza, Leyes Geneticas de los Elementos Quimicos. Nuevo Sistema Periodico, Universidad Nacional de Cuzco, Cuzco, Peru, 1953
- P. Ball, A Guided Tour of the Ingredients, Oxford University Press, Oxford, 2002
- P. Ball, A Very Short Introduction to the Elements, Oxford University Press, 2004
- I. Barber, Sorting The Elements: The Periodic Table at Work, Rourke Publishing, Vero Beach, Florida, US, 2008
- R. Baum (ed), Celebrating the Periodic Table, Chemical & Engineering News, A Special Collector's Issue, September 8, 2003
- H.A. Bent, New Ideas in Chemistry from Fresh Energy for the Periodic Law, Author House, Bloomington IN, 2006
- J. Bernstein, Plutonium, Joseph Henry, Washington DC, 2007
- J. C.A. Boeyens, D.C. Lavendis, Number Theory and the Periodicity of Matter, Springer, Berlin, 2008
- N. Bohr, Collected Works Vol 4. The Periodic System (1920-1923), Nielsen J Rud (Editor), North Holland Publishing Company, 1977
- T. Bondora, The Periodic Table of Elements Coloring Book, Bondora Educational Media Publications, 2010
- D.G. Cooper, The Periodic Table, 3rd edition. Butterworths, London, 1964
- P.A. Cox, The Elements, Oxford University Press, Oxford, 1989
- P. Depovere, La Classification périodique des éléments, De Boeck, Bruxelles, 2002
- H. Dingle and G.R. Martin, Chemistry and Beyond: Collected Essays of F.A. Paneth, Interscience, New York, NY, 1964
- S. Dockx, Theorie Fondamentale du Systeme Periodique des Elements, Office Internationale de Librairie, Bruxelles, 1950
- A. Ducrocq, Les éléments au pouvoir, Julliard, Paris, 1976
- A. Ede, The Chemical Elements, Greenwood Press, Westport, CT, 2006
- J. Emsley, The Elements, 3rd edition. Clarendon, Oxford University Press, 1998
- J. Emsley, Nature's Building Blocks, An A-Z Guide to the Elements, Oxford University Press, Oxford, 2001
- P. Enghag, Encyclopedia of the Elements, Wiley-VCH, Weinheim, 2004
- D.E. Fisher, Much Ado About (Practically) Nothing, The History of the Noble Gases, Oxford University Press, New York, 2010
- I. Freund, The Study of Chemical Composition: An Account of its Method and Historical Development, Dover Publications, Inc., New York, NY, 1968
- J. García-Sancho & F. Ortega-Chicote, Periodicidad Química, Trillas, México, 1984
- A. E. Garrett, The Periodic Law, D. Appleton & Co., New York, 1909
- L. Garzon Ruiperez, De Mendeleiev a Los Superelementos, Universidad de Oviedo, Oviedo, 1988
- L. Gonik, C. Criddle, The Cartoon Guide to Chemistry, Harper Resource, New York, 2005
- M. Gordin, A Well-Ordered Thing, Dimitrii Mendeleev and the Shadow of the Periodic Table, Basic Books, New York, 2004
- T. Gray, The Elements: A Visual Exploration of Every Known Atom in the Universe, Black Dog & Leventhal, 2009
- D. Green, The Elements, The Building Blocks of the Universe, Scholastic Inc. New York, 2012
- R. Hefferlin, Periodic Systems and their Relation to the Systematic Analysis of Molecular Data, Edwin Mellen Press, Lewiston, NY, 1989
- D.L. Heiserman, Exploring the Chemical Elements and their Compounds, McGraw-Hill New York, 1991
- S. Hofmann, Beyond Uranium, Taylor & Francis, London, 2002
- F. Hund, Linienspektren und Periodisches System der Elemente, Verlag von Julius Springer, Berlin, 1927
- W.B. Jensen, Mendeleev on the Periodic Law: Selected Writings, 1869-1905, Dover, Mineola, NY, 2005
- S. Kean, The Disappearing Spoon, Little, Brown & Co., New York, 2010
- D.M. Knight, Classical Scientific Papers, Chemistry Second Series, American, Elsevier, New York, NY
- P.K. Kuroda, The Origin of the Chemical Elements, and the Oklo Phenomenon, Springer-Verlag, Berlin, 1982
- H.M. Leicester and H.S. Klickstein, A Source Book in Chemistry 1400-1900, 1st Edition, McGraw-Hill Book Company Inc., London, 1952
- M.F. L'Annunziata, Radioactivity, Introduction and History, Elsevier, 2007
- S.E.V. Lemus, Clasificación periódica de Mendelejew, Guatemalan Ministry of Public Education, Guatemala, 1959
- P. Levi, The Periodic Table, 1st American Edition. Schocken Books, New York, NY, 1984
- R. Luft, Dictionnaire des Corps Simples de la Chimie, Association Cultures et Techniques, Nantes, 1997
- J. Marshall, Discovery of the Elements, Pearson Custom Publishing, 1998
- E. Mazurs, Graphic Representation of the Periodic System During One Hundred Years, Alabama University Press, Tuscaloosa, AL, 1974
- D. Mendeleeff, An Attempt Towards A Chemical Conception of the Ether, translated by G. Kamensky. Longmans, Green, and Co., London, 1904
- D. Mendeleeff, The Principles of Chemistry, translated by G. Kamensky, 5th Edition, vol. 2. Longmans, Green, and Co., London, 1891
- L. Meyer, Modern Theories of Chemistry, 5th Edition, translated by P.P. Bedson, Longmans, Green, and Co., London, 1888
- L. Meyer, Outlines of Theoretical Chemistry, 2nd Edition, translated by P.P. Bedson and W.C. William. Longmans, Green, and Co., London, 1899
- F. Mohr, (E), Gold Chemistry, Wiley-VCH, 2009
- D. Morris, The Last Sorcerers, The Path from Alchemy to the Periodic Table, Joseph Henry Press, New York, 2003
- I. Nechaev, G.W. Jenkins, The Chemical Elements, Tarquin Publications, Norfolk, UK, 1997
- R.D. Osorio Giraldo, M.V. Alzate Cano, La Tabla Periodica, Bogota, Colombia, 2010
- M.J. Pentz, (General Editor), The Periodic Table and Chemical Bonding, Open University Press, Bletchley, Buckinghamshire, UK, 1971
- I.V. Peryanov, D.N. Trifonov, Elementary Order: Mendeleev's Periodic System, translated from the Russian by Nicholas Weinstein, Mir Publishers, Moscow, 1984
- J.S.F. Pode, The Periodic Table, John Wiley, New York, NY, 1971
- R.J. Puddephatt, The Periodic Table of the Elements, Oxford University Press, Oxford, 1972
- R.J. Puddephatt and P.K. Monaghan, The Periodic Table of the Elements, 2nd edition. Oxford University Press, Oxford, 1986
- H.-J. Quadbeck-Seeger, World of the Elements, Wiley-VCH, Weinheim, 2007
- E. Rabinowitsch, E. Thilo, Periodisches System, Geschichte und Theorie, Stuttgart, 1930
- R. Rich, Periodic Correlations, Benjamin, New York, 1965
- J. Ridgen, Hydrogen, The Essential Element, Harvard University Press, Cambridge, MA, 2002
- H. Rossotti, Diverse Atoms, Oxford University Press, Oxford, 1998
- D.H. Rouvray, R.B. King, The Periodic Table Into the 21st Century, Research Studies Press, Baldock, UK, 2004
- D.H. Rouvray, R.B. King, The Mathematics of the Periodic Table, Nova Scientific Publishers, New York, 2006
- G. Rudorf, The Periodic Classification and the Problem of Chemical Evolution, Whittaker & Co., London, New York, 1900
- G. Rudorf, Das periodische System, seine Geschichte und Bedeutung für die chemische Sysytematik, Hamburg-Leipzig, 1904
- O. Sacks, Uncle Tungsten, Vintage Books, New York, 2001
- R.T. Sanderson, Periodic Table of the Chemical Elements, School Technical Publishers, Ann Arbor, MI, 1971
- S. E. Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009
- E.R. Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, New York, 2007
- E.R. Scerri, Selected Papers on the Periodic Table, Imperial College Press, London and Singapore, 2009
- E.R. Scerri, A Very Short Introduction to the Periodic Table, Oxford University Press, Oxford, 2011; Also translated into Spanish and Arabic.
- E.R. Scerri, Le Tableau Périodique, Son Histoire et sa Signification, EDP Sciences, 2011, (translated by R. Luft); Japanese Translation by Hisao Mabuchi et. al.
- C. Schmidt, Das periodische System der chemischen Elementen, Leipzig, 1917.
- G.T. Seaborg, W.D. Loveland, The Elements Beyond Uranium, Wiley, New York, 1990
- M.S. Sethi, M. Satake, Periodic Tables and Periodic Properties, Discovery Publishing House, Delhi, India, 1992
- H.H. Sisler, Electronic Structure, Properties, and the Periodic Law, Reinhold, New York, 1963
- P. Strathern, Mendeleyev's Dream, Hamish-Hamilton, London, 1999
- R.S. Timmreck, The Power of the Periodic Table, Royal Palm Publishing, 1991
- M. Tweed, Essential Elements, Walker and Company, New York, 2003
- F.P. Venable, The Development of the Periodic Law, Chemical Publishing Co., Easton, PA, 1896
- M.E. Weeks, Discovery of the Elements, Journal of Chemical Education, Easton PA, 1960
- B.D. Wilker, The Mystery of the Periodic Table, Bethlehem Books, New York, 2003
- J. Van Spronsen, The Periodic System of the Chemical Elements, A History of the First Hundred Years, Elsevier, Amsterdam, 1969
- T. Zoellner, Uranium, Penguin Books, London, 2009
- A. Zwertska, The Elements, Oxford University Press, Oxford, 1998
Works by D. I. Mendeleev
- Nauchnyi arkhiv. Periodicheskii zakon, t. I, ed. B. M. Kedrov. Moscow: Izd. AN SSSR, 1953
- Periodicheskii zakon. Dopolnitel'nye materialy. Klassiki nauki, ed. B. M. Kedrov. Moscow: Izd. AN SSSR, 1960
- Periodicheskii zakon. Klassiki nauki, ed. B. M. Kedrov. Moscow: Izd. AN SSSR, 1958
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2013 | PT id = 598, Type = formulation 3D spiral |
Bernard Periodic Spiral
The Bernard Periodic Spiral of the Elements (BPSE), depicts a novel rendition of the Periodic Table that replaces the flat rectangular format with a continuous unidirectional spiral that maintains all the properties of Group and Period formation.
Comparisons may be made with similar models spanning the last three decades of the 20th century (Alexander, 1971; Mazurs, 1974; & Kaufman, 1999).
In the chart form, this new rendition is referred to as the Elliptical Periodic Chart of the Elements. In the three-dimensional form, the model resembles a Christmas tree in shape with the 7 Periods represented as circular platforms situated at various levels with the elements placed appropriately at the outer edges of each of these platforms as a Period builds up. The elements may be represented as spherical objects or flat discs with radii proportionate to atomic radii (or reasonable approximations). Color schemes accentuate the four different Blocks of elements: the s-Block (green), the p-Block (blue, with the exception that the last Group is red signifying the end of a Period), d-Block (orange), and the f-Block (yellow). The grey section, called the Group-Period Interchange, is where the end of a particular Period connects to the beginning of the next Period, and, at the same time, transitions from Group 18 to Group 1.
Watch the video here:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2014 | PT id = 664, Type = formulation |
Janet Rajeuni
By Philip Stewart:
Janet Rejuvenated, with acknowledgement to Mazurs and to Valery Tsimmerman for the idea of using one square per orbital and of shifting the blocks so that each row represents one value of n, the principal quantum number.
The main objection people make to Janet is that He is placed at the head of the alkaline earth metals although it behaves as a noble gas. The essential answer is that electronic structure explains behaviour and not vice versa; like Ne (and unlike Ar, Kr, Xe and Rn), He has a complete shell. Similarly H, like C, is half way between a full and an empty shell, unlike the alkali metals and the halogens. I suggest a new argument: nobody finds it strange that the p block has a row of non-metals at its head (and that half its members are non-metals), so why not the s block?
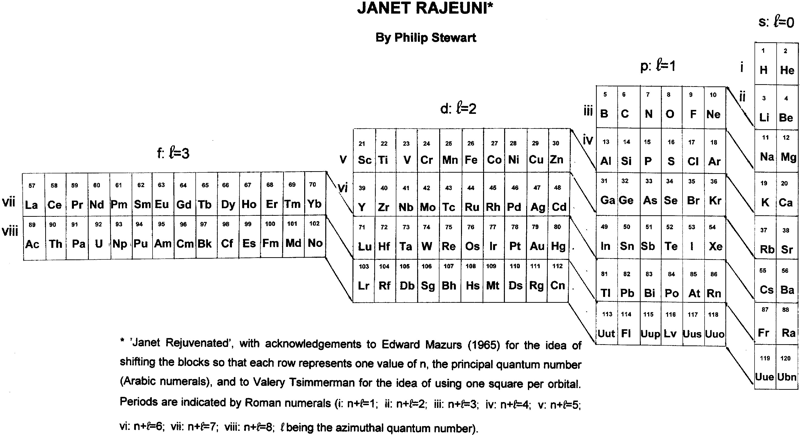
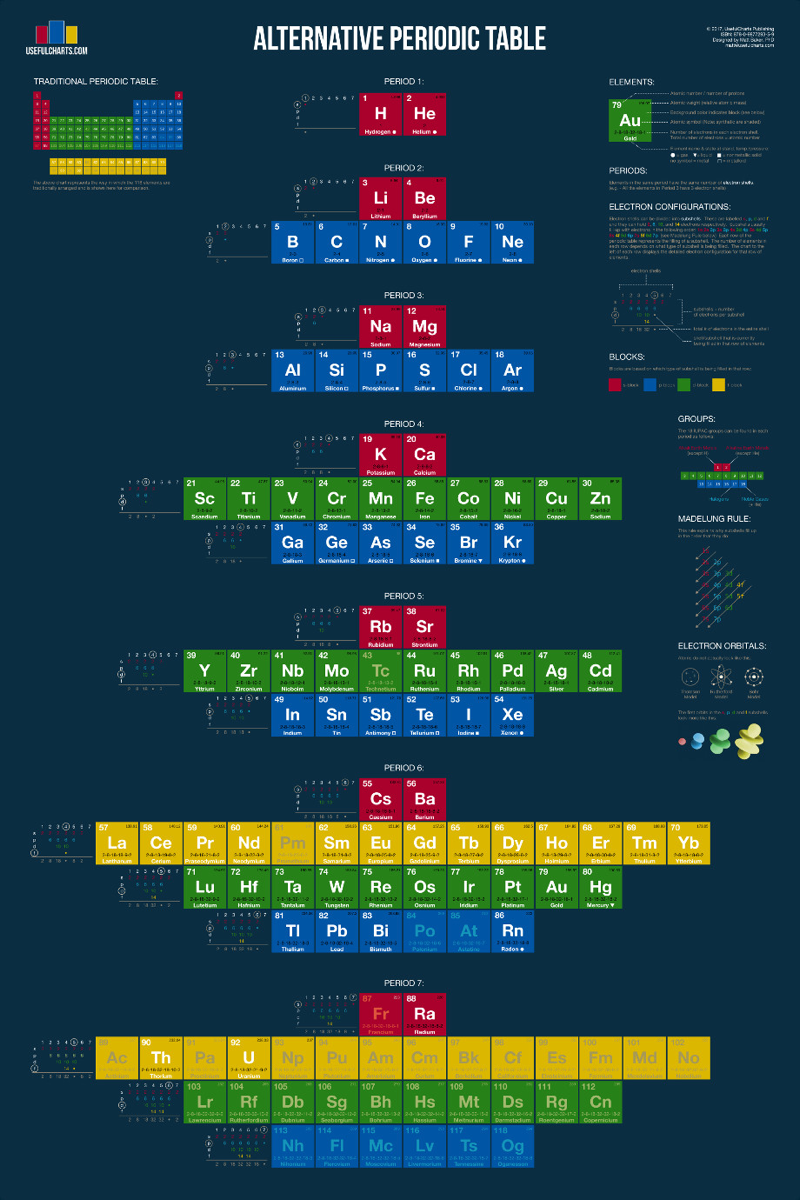
| Year: 2017 | PT id = 739, Type = formulation |
Alternative Periodic Table
From Useful Charts:
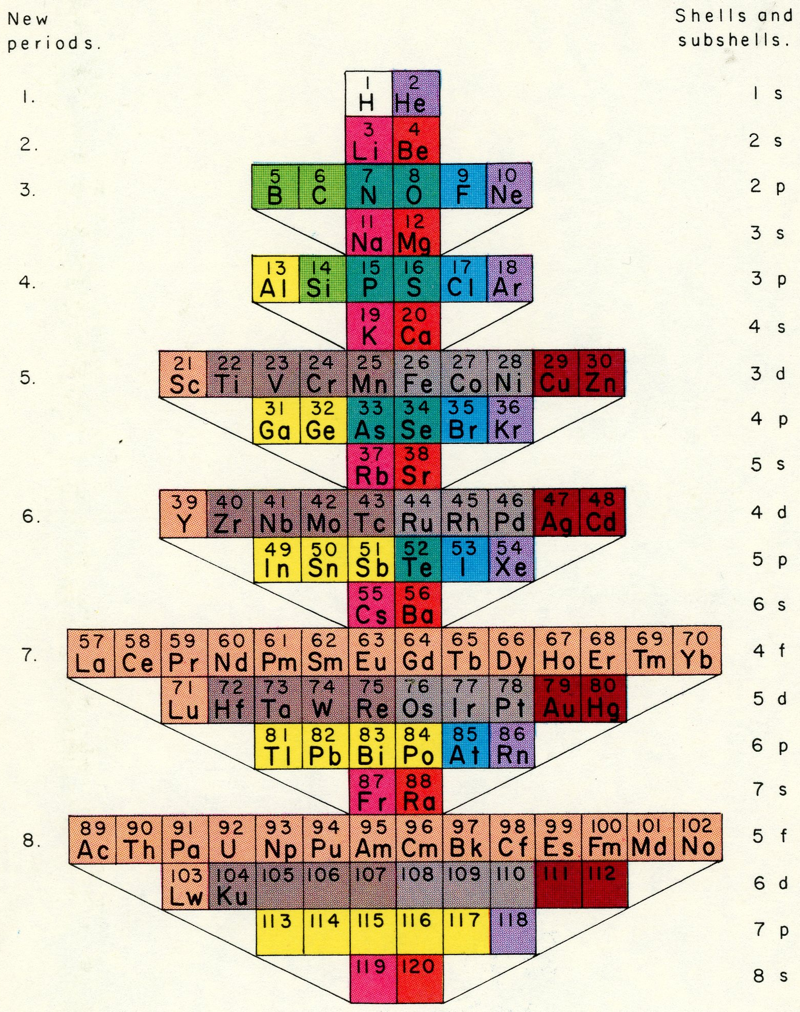
You'll notice that this periodic table looks quite a bit different from the one you're used to. The traditional periodic table is designed to emphasize the concept of valence, which is important for knowing which elements can easily combine with others to form compounds. In contrast, the periodic table below is designed to simply emphasize the way in which atoms are "built" (specifically, how electrons group together into shells and subshells).
It's based on a design proposed by Edward Mazurs in the 1960s. Like the traditional table, this alternative version can be used to find an elements name, number, atomic weight, state of matter, period, group, and block. However, it also contains detailed information on electron configurations and the different types of electron subshells.
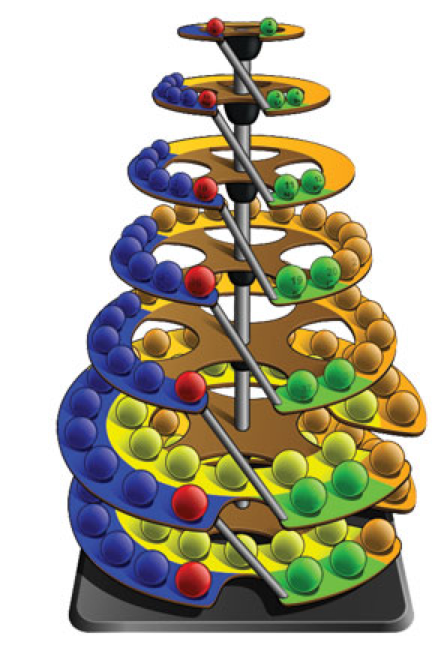
| Year: 2017 | PT id = 761, Type = formulation 3D |
Stewart's Chemosphere
P J Stewart, a good friend of the periodic table database, has mapped a PT onto a sphere.
PJS writes: "It is Janet Rajeuni 2014 wrapped round a sphere, going back to Mazurs 1965, and Tsimmerman 2006. Arabic numerals indicate shells (values of principal quantum number); Roman numerals indicate periods."
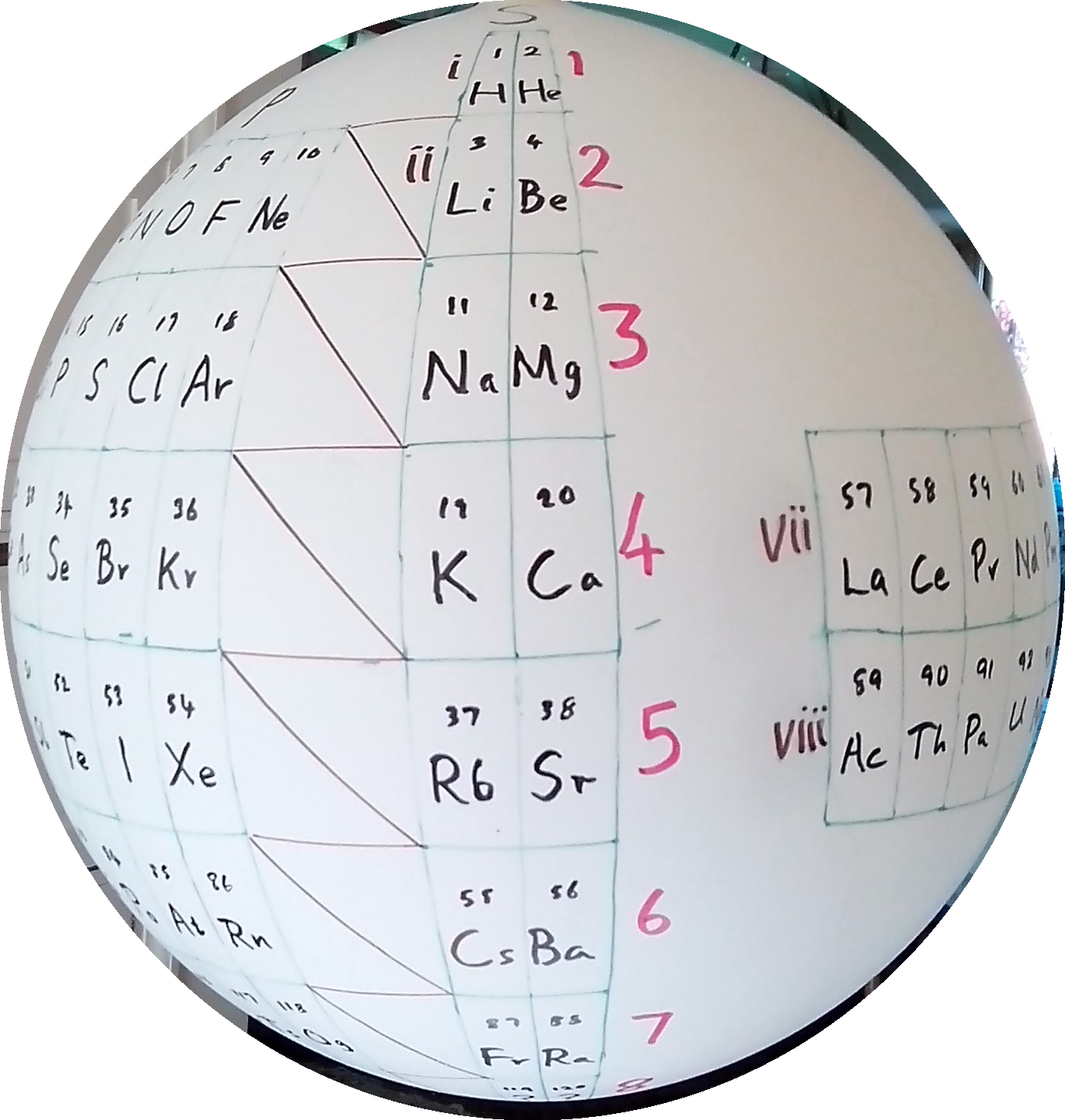
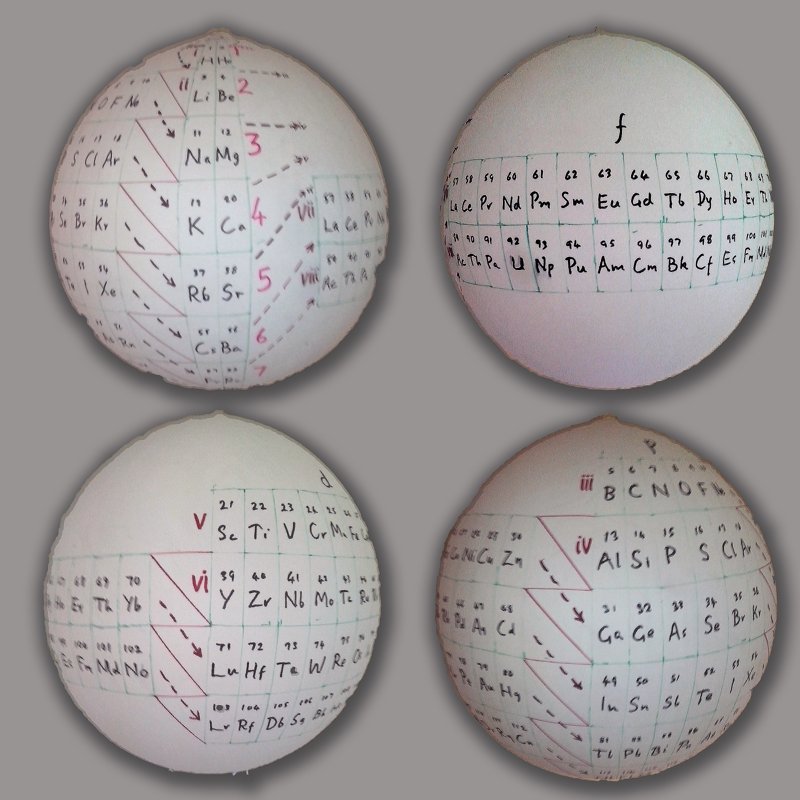
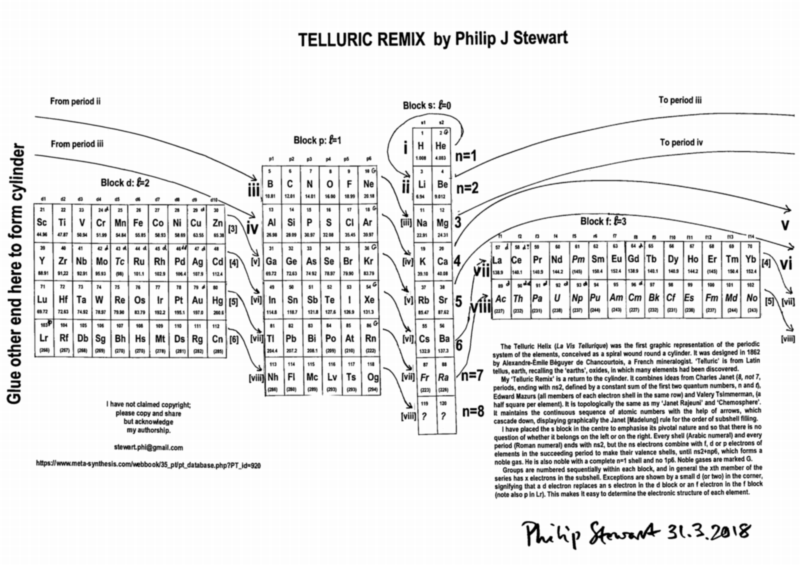
| Year: 2018 | PT id = 920, Type = formulation 3d |
Telluric Remix
Philip Stewart writes:
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version.
The printable version is available (click here for the full size version) to make your own:
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
| Year: 2019 | PT id = 984, Type = formulation 3D spiral |
Telluric Remix in Colour
Philip Stewart writes (this is the same text that accompanies the 2018 B/W version):
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version (pdf).
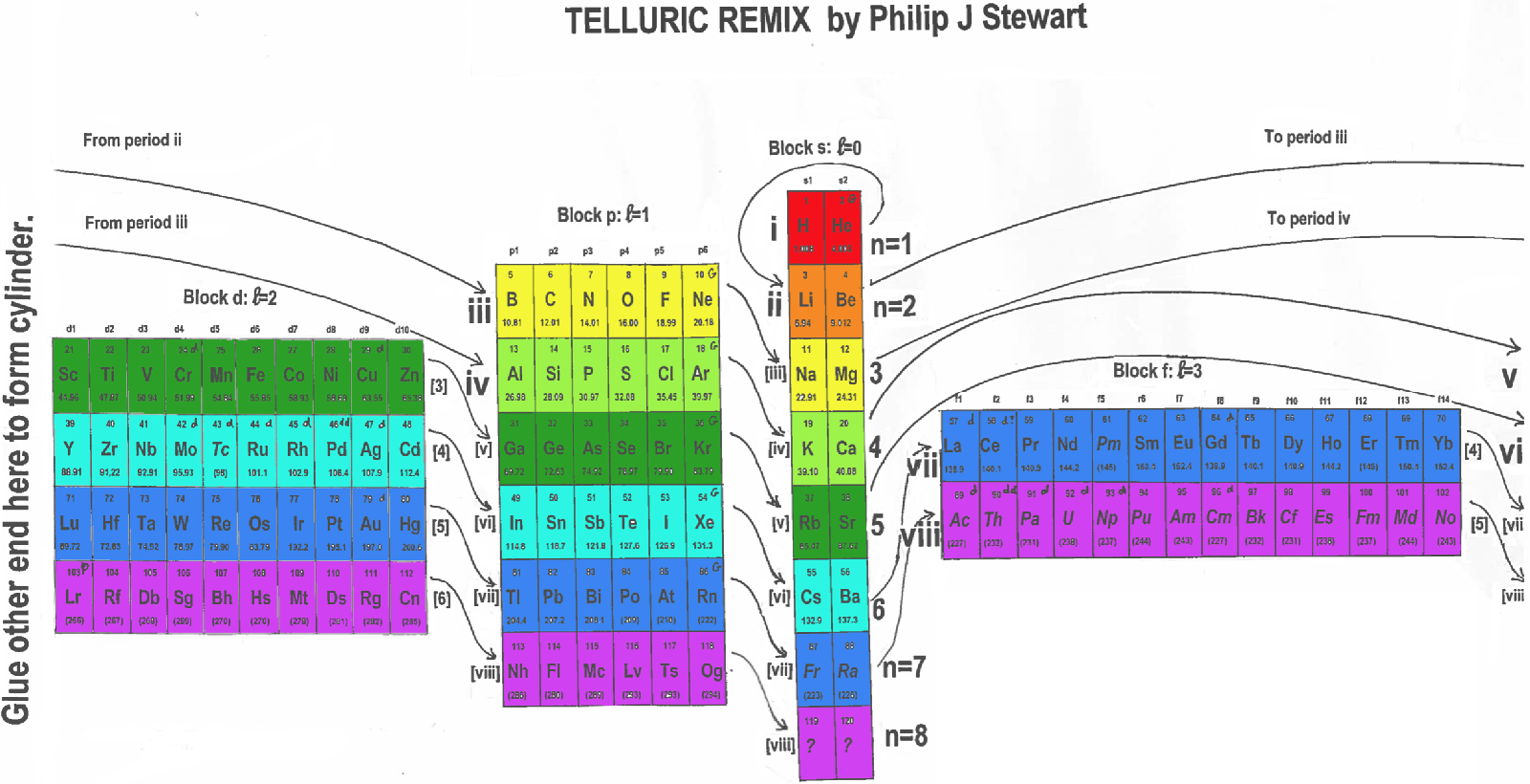
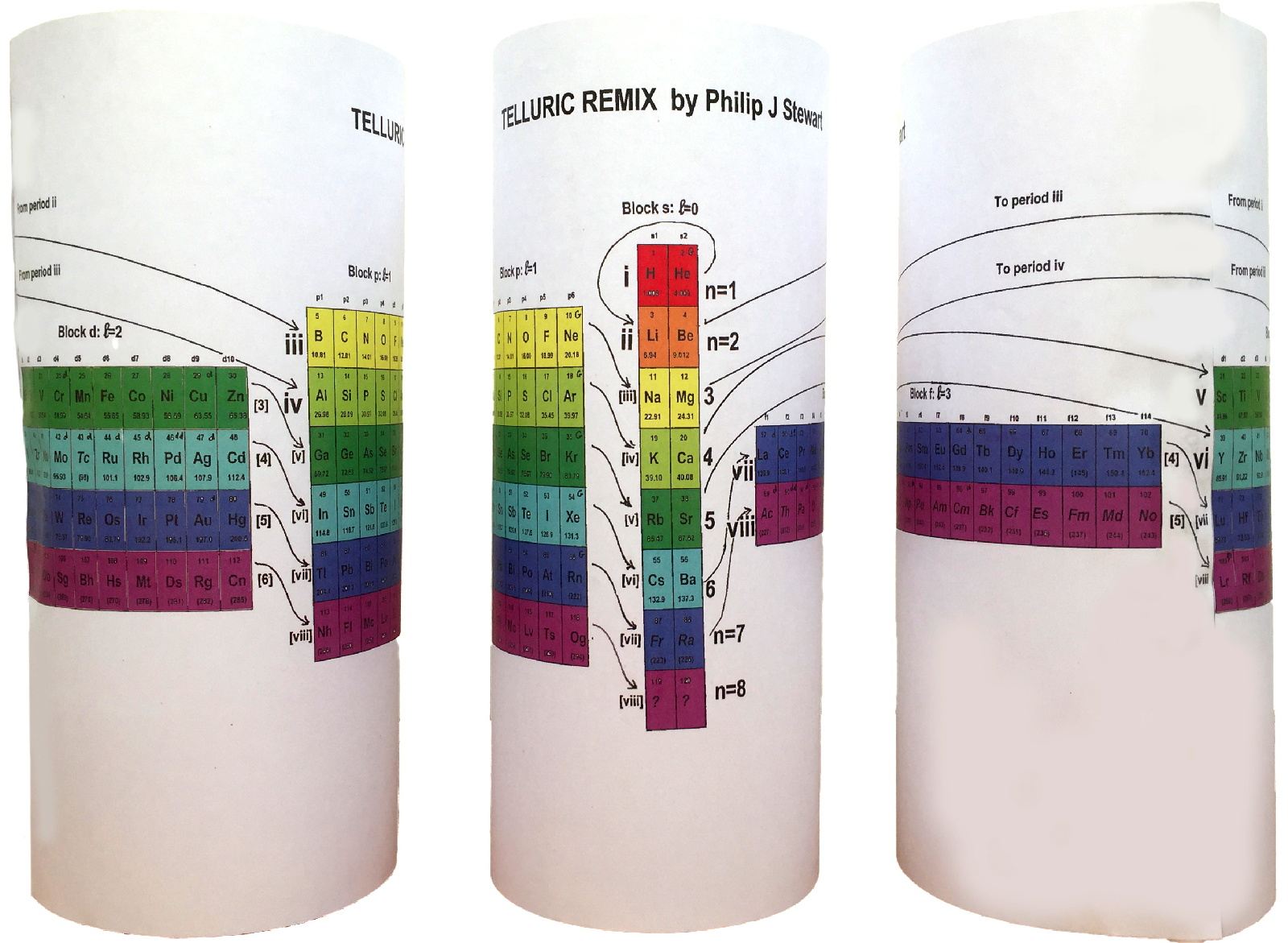
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
| Year: 2020 | PT id = 1149, Type = misc review formulation |
Scerri's Periodic Table of Books About The Periodic Table & The Chemical Elements
From Eric Scerri, a periodic table of books about the periodic table & the chemical elements... many by Eric Scerri himself.
Eric Scerri, UCLA, Department of Chemistry & Biochemistry. See the website EricScerri.com and Eric's Twitter Feed.
There is no particular connection between each of the elements and the book associated with it in the table, with the exception of: H, He, N, Ti, V, Nb, Ag, La, Au, Ac, U, Pu & Og.
The following is a list of references for each of the 118 books featured on Periodic Table of Books About The Periodic Table & The Chemical Elements. Books published in languages other than English are. They include the Catalan, Croatian, French, German, Italian, Norwegian & Spanish languages:
| 1 | H | J. Ridgen, Hydrogen, the Essential Element, Harvard University Press, Cambridge, MA, 2002. |
| 2 | He | W.M. Sears Jr., Helium, The Disappearing Element, Springer, Berlin, 2015. |
| 3 | Li | K. Lew, The Alkali Metals, Rosen Central, New York, 2009. |
| 4 | Be | S. Esteban Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009. (Spanish) |
| 5 | B | E.R. Scerri. The Periodic Table, Its Story and Its Significance, 2nd edition, Oxford University Press, New York, 2020. |
| 6 | C | U. Lagerkvist, The Periodic Table and a Missed Nobel Prize, World Scientific, Singapore, 2012. |
| 7 | N | W.B. Jensen, Mendeleev on the Periodic Law: Selected Writings, 1869–1905, Dover, Mineola, NY, 2005. |
| 8 | O | M. Kaji, H. Kragh, G. Pallo, (eds.), Early Responses to the Periodic System, Oxford University, Press, New York, 2015. |
| 9 | F | E. Mazurs, Graphic Representation of the Periodic System During One Hundred Years, Alabama University Press, Tuscaloosa, AL, 1974. |
| 10 | Ne | T. Gray, The Elements: A Visual Exploration of Every Known Atom in the Universe, Black Dog & Leventhal, 2009. |
| 11 | Na | N.C. Norman, Periodicity and the s- and p-Block Elements, Oxford University Press, Oxford, 2007. |
| 12 | Mg | M. Gordin, A Well-Ordered Thing, Dimitrii Mendeleev and the Shadow of the Periodic Table, 2nd edition, Basic Books, New York, 2019. |
| 13 | Al | S. Kean, The Disappearing Spoon, Little, Brown & Co., New York, 2010. |
| 14 | Si | P.A. Cox, The Elements, Oxford University Press, Oxford, 1989. |
| 15 | P | J. Emsley, The 13th Element: The Sordid Tale of Murder, Fire, and Phosphorus, Wiley, New York, 2002. |
| 16 | S | P. Parsons, G. Dixon, The Periodic Table: A Field Guide to the Elements, Qurcus, London, 2014. |
| 17 | Cl | P. Levi, The Periodic Table, Schocken, New York, 1995. |
| 18 | Ar | B.D. Wiker, The Mystery of the Periodic Table, Bethlehem Books, New York, 2003. |
| 19 | K | H. Alderesey-Williams, Periodic Tales, Viking Press, 2011. |
| 20 | Ca | P. Strathern, Mendeleyev's Dream, Hamish-Hamilton, London, 1999. |
| 21 | Sc | D. Scott, Around the World in 18 Elements, Royal Society of Chemistry, London, 2015. |
| 22 | Ti | E. W. Collings, Gerhard Welsch, Materials Properties Handbook: Titanium Alloys, ASM International, Geauga County, Ohio, 1994. |
| 23 | V | D. Rehder, Bioinorganic Vanadium Chemistry, Wiley-Blackwell, Weinheim, 2008. |
| 24 | Cr | K. Chapman, Superheavy, Bloomsbury Sigma, New York, 2019. |
| 25 | Mn | E.R. Scerri, E. Ghibaudi (eds.), What is an Element? Oxford University Press, New York, 2020. |
| 26 | Fe | M. Soon Lee, Elemental Haiku, Ten Speed Press, New York, 2019. |
| 27 | Co | J. Emsley, Nature's Building Blocks, An A-Z Guide to the Elements, Oxford University Press, Oxford, 2001. |
| 28 | Ni | T. James, Elemental, Robinson, London, 2018. |
| 29 | Cu | E.R. Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, New York, 2007. |
| 30 | Zn | H. Rossotti, Diverse Atoms, Oxford University Press, Oxford, 1998. |
| 31 | Ga | P. Ball, A Very Short Introduction to the Elements, Oxford University Press, 2004. |
| 32 | Ge | I. Asimov, The Building Blocks of the Universe, Lancer Books, New York, 1966. |
| 33 | As | J. Browne, Seven Elements that Changed the World, Weidenfeld and Nicholson, London, 2013. |
| 34 | Se | N. Raos, Bezbroj Lica Periodnog Sustava Elemenata, Technical Museum of Zagreb, Croatia, 2010. (Croatian) |
| 35 | Br | P. Strathern, The Knowledge, The Periodic Table, Quadrille Publishing, London, 2015. |
| 36 | Kr | A. Ede, The Chemical Element, Greenwood Press, Westport, CT, 2006. |
| 37 | Rb | A. Stwertka, The Elements, Oxford University Press, Oxford, 1998. |
| 38 | Sr | E.R. Scerri, A Tale of Seven Elements, Oxford University Press, New York, 2013. |
| 39 | Y | H.-J. Quadbeck-Seeger, World of the Elements, Wiley-VCH, Weinheim, 2007. |
| 40 | Zr | M. Fontani, M. Costa, M.V. Orna (eds.), The Lost Elements, Oxford University Press, New York, 2015. |
| 41 | Nb | M. Seegers, T. Peeters (eds.), Niobium: Chemical Properties, Applications and Environmental Effects, Nova Science Publishers, New York, 2013. |
| 42 | Mo | E.R. Scerri, Selected Papers on the Periodic Table, Imperial College Press, Imperial College Press, London and Singapore, 2009. |
| 43 | Tc | A. Dingle, The Periodic Table, Elements with Style, Kingfisher, Richmond, B.C. Canada, 2007. |
| 44 | Ru | G. Rudorf, Das periodische System, seine Geschichte und Bedeutung für die chemische Sysytematik, Hamburg-Leipzig, 1904. (German) |
| 45 | Rh | I. Nechaev, G.W. Jenkins, The Chemical Elements, Tarquin Publications, Publications, Norfolk, UK, 1997. |
| 46 | Pd | P. Davern, The Periodic Table of Poems, No Starch Press, San Francisco, 2020. |
| 47 | Ag | C. Fenau, Non-ferrous metals from Ag to Zn, Unicore, Brussells, 2002. |
| 48 | Cd | J. Van Spronsen, The Periodic System of the Chemical Elements, A History of the First Hundred Years, Elsevier, Amsterdam, 1969. |
| 49 | In | M. Tweed, Essential Elements, Walker and Company, New York, 2003. |
| 50 | Sn | M.E. Weeks, Discovery of the Elements, Journal of Chemical Education, Easton PA, 1960. |
| 51 | Sb | P. Wothers, Antimony Gold Jupiter's Wolf, Oxford University Press, Oxford, 2019. |
| 52 | Te | W. Zhu, Chemical Elements in Life, World Scientific Press, Singapore, 2020. |
| 53 | I | O. Sacks, Uncle Tungsten, Vintage Books, New York, 2001. |
| 54 | Xe | E.R. Scerri, (ed.), 30-Second Elements, Icon Books, London, 2013. |
| 55 | Cs | M. Jacob (ed.), It's Elemental: The Periodic Table, Celebrating 80th Anniversary, Chemical & Engineering News, American Chemical Society, Washington D.C., 2003. |
| 56 | Ba | J. Marshall, Discovery of the Elements, Pearson Custom Publishing, New York,1998. |
| 57 | La | K. Veronense, Rare, Prometheus Books, Amherst, New York, 2015. |
| 58 | Ce | N. Holt, The Periodic Table of Football, Ebury Publishing, London, 2016. |
| 59 | Pr | S. Alvarez, C. Mans, 150 Ans de Taules Périodiques a la Universitat de Barcelona, Edicions de la Universitat de Barcelona, Barcelona, 2019. (Catalan) |
| 60 | Nd | L. Garzon Ruiperez, De Mendeleiev a Los Superelementos, Universidad de Oviedo, Oviedo, 1988. (Spanish) |
| 61 | Pm | P. Ball, A Guided Tour of the Ingredients, Oxford University Press, Oxford, 2002. |
| 62 | Sm | S. Esteban Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009. (Spanish). |
| 63 | Eu | A. E. Garrett, The Periodic Law, D. Appleton & Co., New York, 1909. |
| 64 | Gd | M.S. Sethi, M. Satake, Periodic Tables and Periodic Properties, Discovery Publishing House, Delhi, India, 1992. |
| 65 | Tb | M. Eesa, The cosmic history of the elements: A brief journey through the creation of the chemical elements and the history of the periodic table, Createspace Independent Publishing Platform, 2012. |
| 66 | Dy | P. Depovere, La Classification périodique des éléments, De Boeck, Bruxelles, 2002. (French). |
| 67 | Ho | F. Habashi, The Periodic Table & Mendeleev, Laval University Press, Quebec, 2017. |
| 68 | Er | W.J. Nuttall, R. Clarke, B. Glowacki, The Future of Helium as a Natural Resource, Routledge, London, 2014. |
| 69 | Tm | R.D. Osorio Giraldo, M.V. Alzate Cano, La Tabla Periodica, Bogota, Colombia, 2010. (Spanish). |
| 70 | Yb | P.R. Polo, El Profeta del Orden Quimico, Mendeleiev, Nivola, Spain, 2008. (Spanish). |
| 71 | Lu | E.R. Scerri, A Very Short Introduction to the Periodic Table, 2nd edition, Oxford University Press, Oxford, 2019. |
| 72 | Hf | D.H. Rouvray, R.B. King, The Mathematics of the Periodic Table, Nova Scientific Publishers, New York, 2006. |
| 73 | Ta | P. Thyssen, A. Ceulemans, Shattered Symmetry, Oxford University Press, New York, 2017. |
| 74 | W | P.W. Atkins, The Periodic Kingdom, Basic Books, New York, NY, 1995. |
| 75 | Re | D.G. Cooper, The Periodic Table, 3rd edition. Butterworths, London, 1964. |
| 76 | Os | E. Lassner, W.-D. Schubert, Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds, Springer, Berlin, 1999. |
| 77 | Ir | J.C.A. Boeyens, D.C. Levendis, Number Theory and the Periodicity of Matter, Springer, Berlin, 2008. |
| 78 | Pt | R. Hefferlin, Periodic Systems and their Relation to the Systematic Analysis of Molecular Data, Edwin Mellen Press, Lewiston, NY, 1989. |
| 79 | Au | R.J. Puddephatt, The Chemistry of Gold, Elsevier, Amsterdam, 1978. |
| 80 | Hg | D.H. Rouvray, R.B. King, The Periodic Table Into the 21st Century, Research Studies Press, Baldock, UK, 2004. |
| 81 | Tl | R.E. Krebs, The History and Use of Our Earth's Chemical Elements, Greenwood Publishing Group, Santa Barbara, CA, 2006. |
| 82 | Pb | E. Torgsen, Genier, sjarlataner og 50 bøtter med urin - Historien om det periodiske system, Spartacus, 2018. (Norwegian). |
| 83 | Bi | K. Buchanan, D. Roller, Memorize the Periodic Table, Memory Worldwide Pty Limited, 2013. |
| 84 | Po | D. Morris, The Last Sorcerers, The Path from Alchemy to the Periodic Table, Joseph Henry Press, New York, 2003. |
| 85 | At | T. Jackson, The Elements, Shelter Harbor Press, New York, 2012. |
| 86 | Rn | R.J.P. Williams, J.J.R. Frausto da Silva, The Natural Selection of the Chemical Elements: The Environment and Life's Chemistry, Clarendon Press, Oxford, 1997. |
| 87 | Fr | G. Rudorf, The Periodic Classification and the Problem of Chemical Evolution, Whittaker & Co., London, New York, 1900. |
| 88 | Ra | L. Van Gorp, Elements, Compass Point Books, Manakato, MN, 2008. |
| 89 | Ac | G.T. Seaborg, J.J. Katz, L.R. Morss, Chemistry of the Actinide Elements, Springer, Berlin, 1986. |
| 90 | Th | G. Münzenberg, Superheavy Elements - Searching for the End of the Periodic Table, Manipal Universal Press, India, 2018. |
| 91 | Pa | A. Castillejos Salazar, La Tabla Periòdica: Abecedario de la Quimica, Universidad Autonoma de Mexico, D.F. Mexico, 2005. (Spanish). |
| 92 | U | T. Zoellner, Uranium, Penguin Books, London, 2009. |
| 93 | Np | J. Barrett, Atomic Structure and Periodicity, Royal Society of Chemistry, London, 2002. |
| 94 | Pu | J. Bernstein, Plutonium, Joseph Henry, Washington DC, 2007. |
| 95 | Am | S. Hofmann, Beyond Uranium, Taylor & Francis, London, 2002. |
| 96 | Cm | H.M. Davis, The Chemical Elements, Ballantine Books, New York, 1961. |
| 97 | Bk | P.González Duarte, Les Mils Cares de la Taula Periòdica, Universitat Autonoma de Barcelona, Bellaterra Barcelona, 2005 (Catalan). |
| 98 | Cf | R. Rich, Periodic Correlations, Benjamin, New York, 1965. |
| 99 | Es | E. Rabinowitsch, E. Thilo, Periodisches System, Geschichte und Theorie, Stuttgart, 1930. (German). |
| 100 | Fm | P.K. Kuroda, The Origin of the Chemical Elements, and the Oklo Phenomenon, Springer-Verlag, Berlin, 1982. |
| 101 | Md | G. Villani, Mendeleev, La Tavola Periodica Degli Elementi, Grandangolo, Milan, 2016. (Italian). |
| 102 | No | J. Russell, Elementary: The Periodic Table Explained, Michael O'Mara, London, 2020. |
| 103 | Lr | P. Enghag, Encyclopedia of the Elements, Wiley-VCH, Weinheim, 2004. |
| 104 | Rf | R.J. Puddephatt, The Periodic Table of the Elements, Oxford University Press, Oxford, 1972. |
| 105 | Db | L. Ohrström, The Last Alchemist in Paris, Oxford University Press, New York, 2013. |
| 106 | Sg | N.N. Greenwood, E. Earnshaw, Chemistry of the Elements, 2nd edition, Elsevier, Amsterdam, 1997. |
| 107 | Bh | R. Luft, Dictionnaire des Corps Simples de la Chimie, Association Cultures et Techniques, Nantes, 1997. (French) |
| 108 | Hs | Science Foundation Course Team, The Periodic Table and Chemical Bonding, The Open University, Milton Keynes, 1971. |
| 109 | Mt | W.W. Schulz, J. Navratil, Transplutonium Elements, American Chemical Society, Washington D.C., 1981. |
| 110 | Ds | I. Nechaev, Chemical Elements, Lindsay Drummond, 1946. |
| 111 | Rg | F. Hund, Linienspektren und Periodisches System Der Elemente, Springer, Berlin, 1927. |
| 112 | Cn | F.P. Venable, The Development of the Periodic Law, Chemical Publishing Co., Easton, PA, 1896. |
| 113 | Nh | O. Baca Mendoza, Leyes Geneticas de los Elementos Quimicos. Nuevo Sistema Periodico, Universidad Nacional de Cuzco, Cuzco, Peru, 1953 (Spanish). |
| 114 | Fl | B. Yorifuji, Wonderful Life with the Elements, No Starch Press, San Francisco, 2012. |
| 115 | Mc | D.I. Mendeléeff, The Principles of Chemistry, American Home Library, New York, 1902. |
| 116 | Lv | A. Lima-de-Faria, Periodic Tables Unifying Living Organisms at the Molecular Level: The Predictive Power of the Law of Periodicity, World Scientific Press, Singapore, 2018. |
| 117 | Ts | H.B. Gray, J.D. Simon, W.C. Trogler, Braving the Elements, University Science Books, Sausalito, CA, 1995. |
| 118 | Og | E.R. Scerri, G. Restrepo, Mendeleev to Oganesson, Oxford University Press, New York, 2018. |
| Year: 2022 | PT id = 1239, Type = formulation |
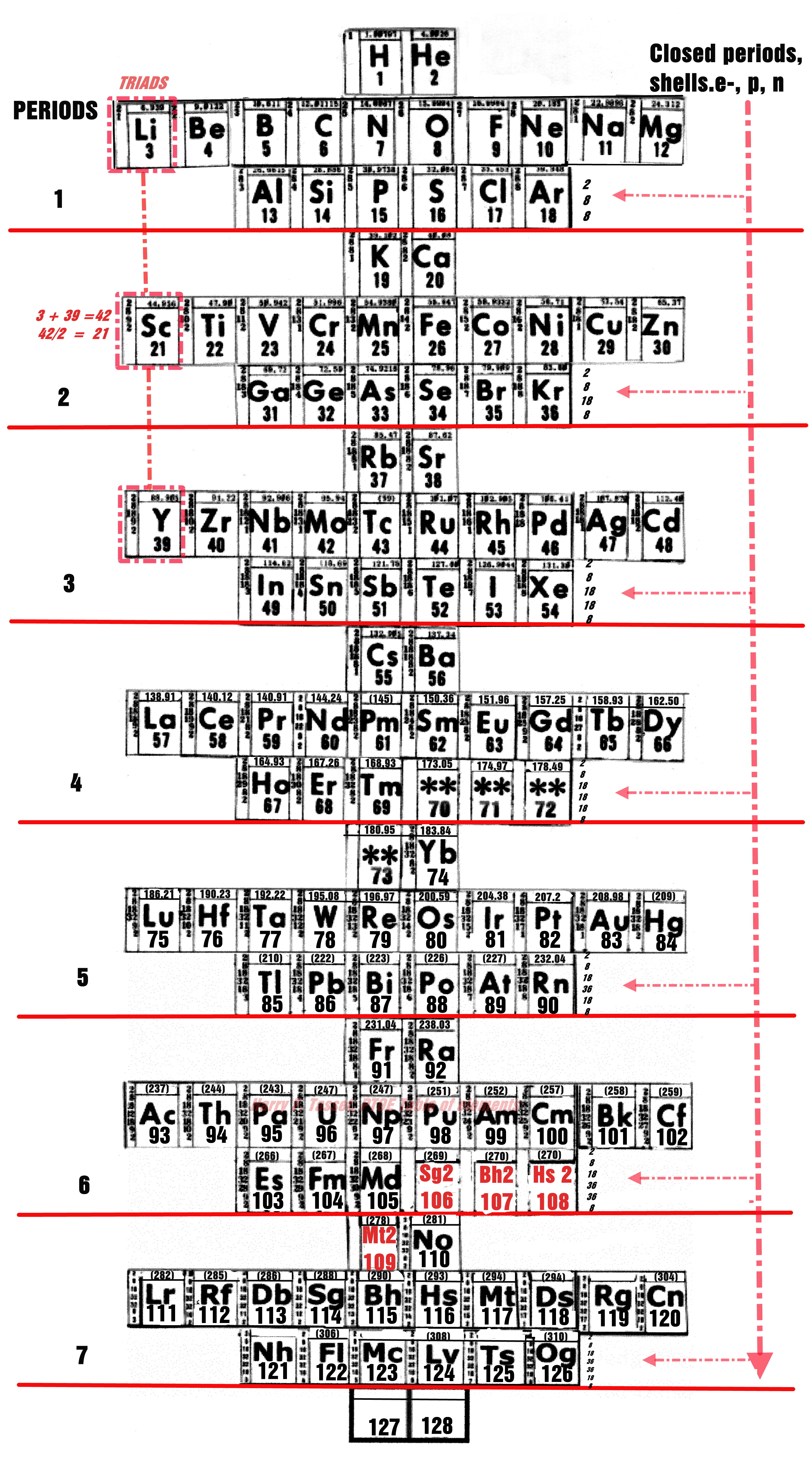
Tassitus' Periodic Table
By Harry Tassitus who wites:
"Here is my narrative; After 50 years of study I have released this periodic table which is a synthesis of the work of Dr Isaac Asimov, Dr Ida Noddack, and Edward g. Mazurs, the latter of which I found on the Internet Database of Periodic Tables web site."
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.

 dic System of Chemical Elements
dic System of Chemical Elements