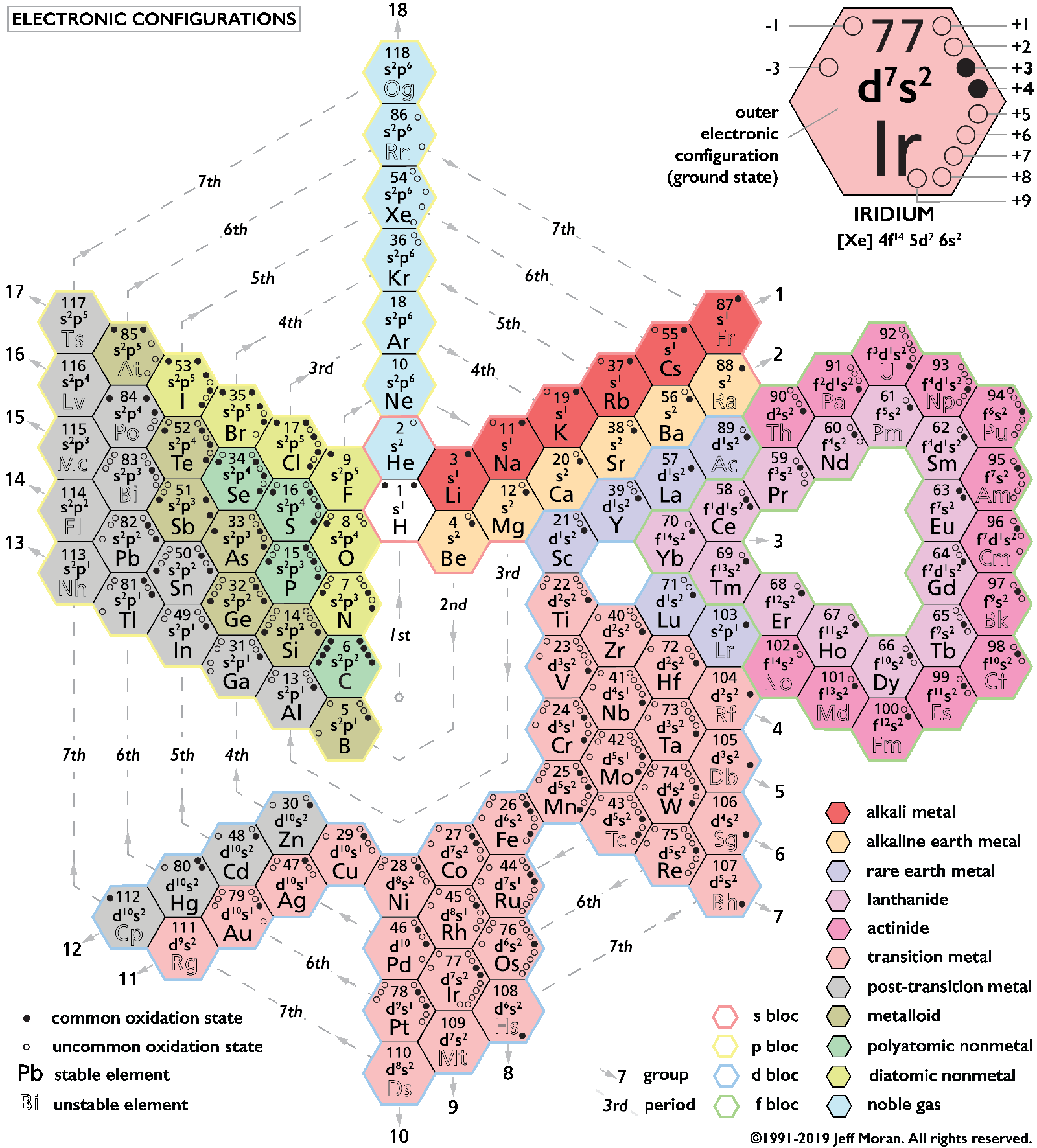
Periodic Table |
 |
 |
 |
 |
 |
 |
 |
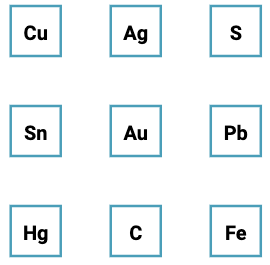
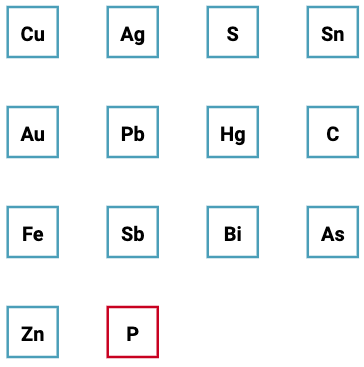
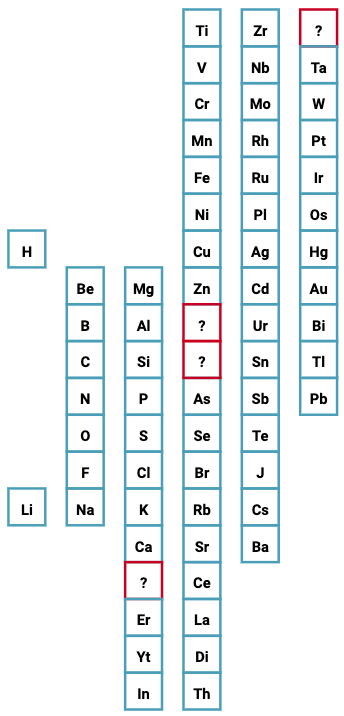
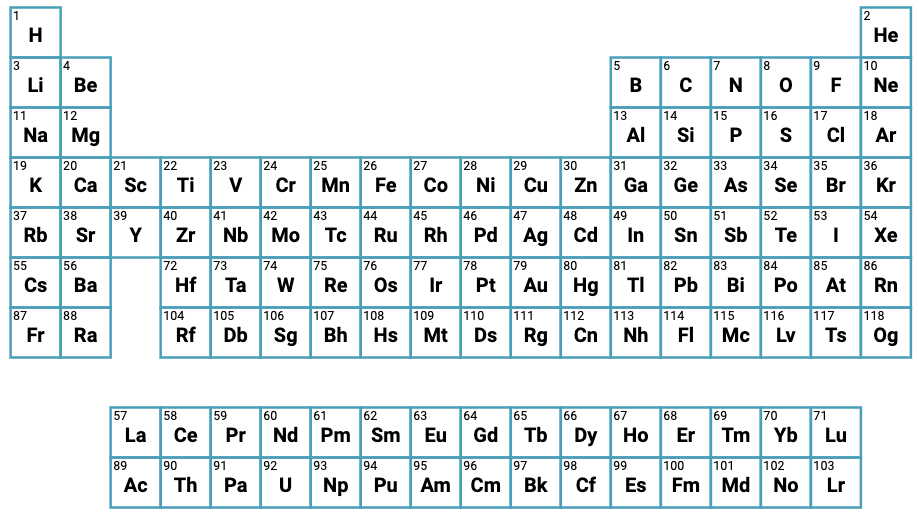
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables from the year 2019:
| Year: 2019 | PT id = 958, Type = formulation data |
Leach's Empirical Periodic Table
The common/conventional/standard 'medium form' periodic table is based on the 1945 Seaborg formulation, and it is interesting to explore where this formulation – and its 1939 predecessor – come from. (Interestingly, the Werner formulation of 1905 is not cited as a source and there are no other similar formulations in the (this) Periodic Table Database.)
However, it is possible to get to the common/conventional/standard periodic table directly from two readily available data-sets: (1) first ionisation energy of the gas phase atoms, and (2) atomic radius.
The procedure involved plotting the data, rotating 90°, squeezing vertically and smoothing. The points need a little tidying up, and then they can be mapped directly onto the Seaborg formulation periodic table.
The only element which does no obviously 'line-up' with the periodic table is hydrogen, but many modern periodic tables have H floating as it is not obvious if it should be considered to be a Group 1 alkali metal or a Group 17 halogen.
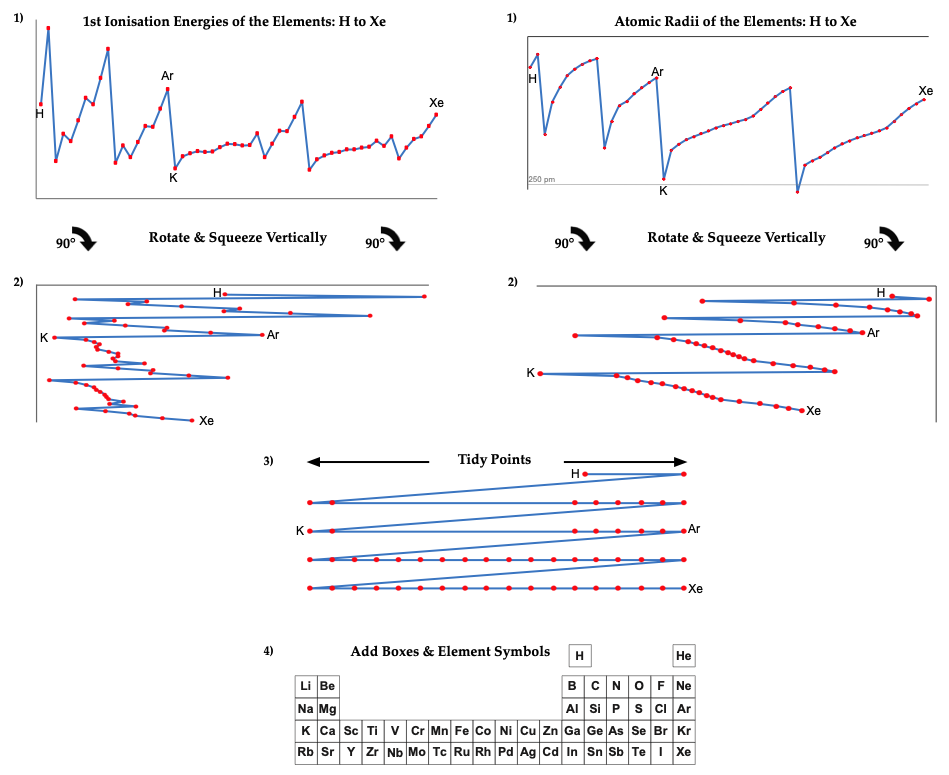
Note:
There are advantages and disadvantages to each data set. The 1st ionisation energy data from NIST is known with up to seven significant figures of precision, but the data jumps about at times due to the presence of the s & p-orbitals, which appears to make the data a little noisy. (Actually, this 'noise' is embedded information about the electronic structure of the atoms.) The atomic radius gives smoother data, but as gas phase atoms do not have hard edges calculated (Clementi 1967) rather than experimental values, must be used.
| Year: 2019 | PT id = 959, Type = formulation |
UCLA Periodic Table (Proposed)
Eric Scerri writes:
During an office hour here at UCLA with a couple of students – Annelise Gazale & Chidinma Onyeonwu – we came up with a 'new periodic table'.
The basis of it is related to a point you frequently make against the Left Step formulation, namely that it messes up trends in atomic radius etc.
So how about this: Traditionally on the right side of the table elements become less reactive as we move down, but on the right side of the table elements become more reactive as we move down. Consequently, the noble gases are anomalous in the way they usually sit since they become more reactive as we move down the table.
Ergo: Move the noble gases to the left edge of the table. (Yes, this has been done before of course but not for this reason.)
Thanks to Eric Scerri for the tip! See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 961, Type = data misc |
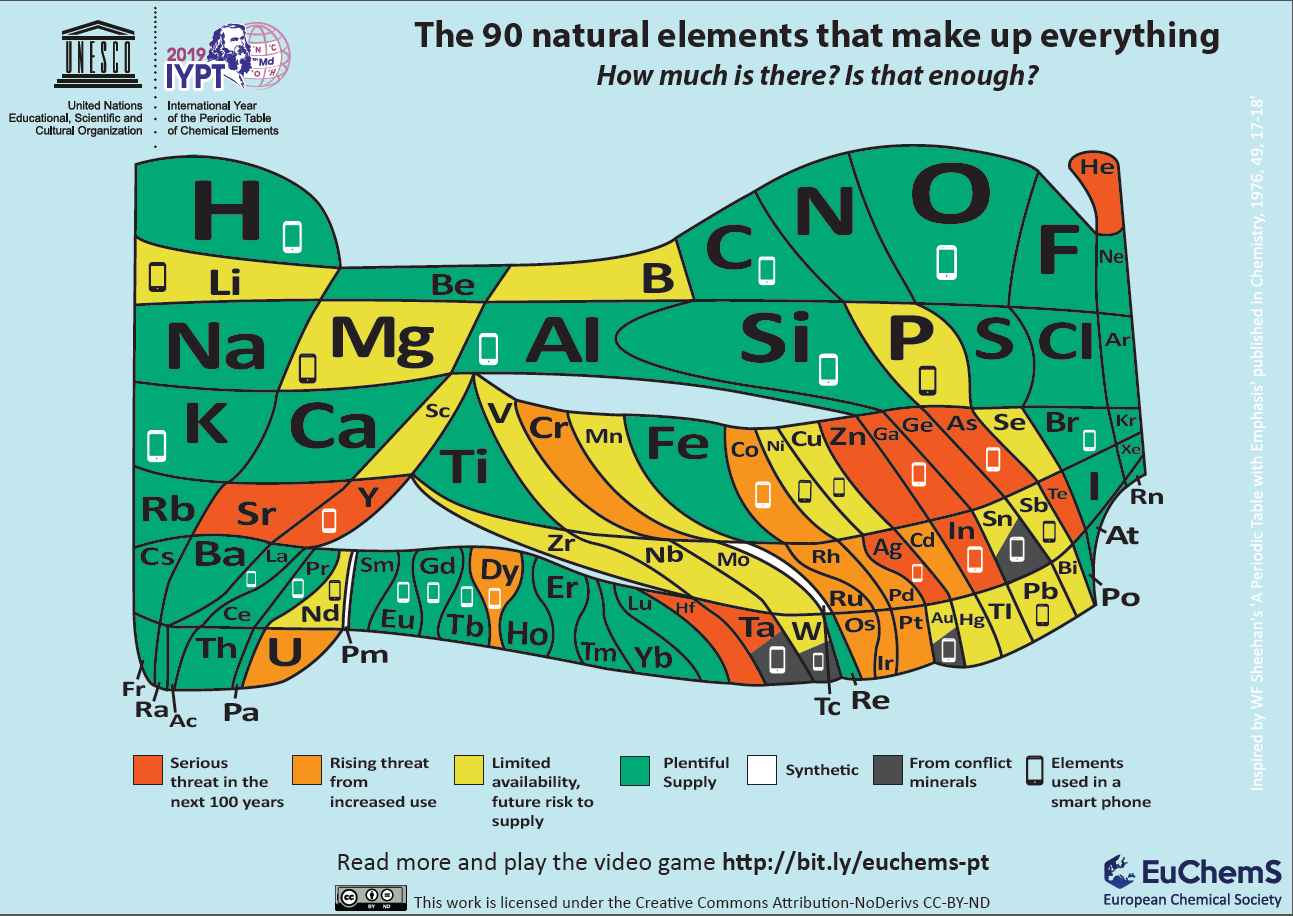
Element Scarcity, Periodic Table of
The European Chemical Society Periodic Table depicting element scarcity was unveiled and discussed at a EuChemS event in the European Parliament on Tuesday 22nd January 2019.
The event, chaired by MEPs Catherine Stihler and Clare Moody, presented an encompassing overview of what element scarcity means for us: both on a scientific level, but also economically and politically. A presentation from speaker Natalia Tarasova, IUPAC Past President, contextualised EuChemS' work within the celebrations of the International Year of the Periodic Table, whilst M Pilar Gil, from the University of St Andrews, delivered a remarkable and exhilarating talk on how the recently discovered oldest known wallchart of the Periodic Table was uncovered and dated.
An article in The Conversation, by David Cole-Hamilton of the University of St Andrews, uses this periodic table to look at elements that are overexploited in the modern world.
"Red indicates that dissipation will make the elements much less readily available in 100 years or less: helium (He), silver (Ag), tellurium (Te), gallium (Ga), germanium (Ge), strontium (Sr), yttrium (Y), zinc (Zn), indium (In), arsenic (As), hafnium (Hf) and tantalum (Ta).
"Helium is used to cool the magnets in MRI scanners and to dilute oxygen for deep sea diving. Vital rods in nuclear reactors use hafnium. Strontium salts are added to fireworks and flares to produce vivid red colours. Yttrium is a component of camera lenses to make them shock and heat resistant. It is also used in lasers and alloys. Gallium, meanwhile, is used to make very high-quality mirrors, light-emitting diodes and solar cells."
| Year: 2019 | PT id = 962, Type = formulation review misc |
Möbius-Escher Periodic Table
A comment article in Nature by Prof. Eric Scerri about quantum mechanics and the periodic table:
"Can quantum ideas explain chemistry's greatest icon? Simplistic assumptions about the periodic table lead us astray.
"Such has been the scientific and cultural impact of Dmitri Mendeleev's periodic table of the elements that many people assume it is essentially complete. [But] in its 150th year, can researchers simply raise a toast to the table's many dividends, and occasionally incorporate another heavy synthetic element?
"No – this invaluable compilation is still not settled. The placements of certain elements, even hydrogen and helium, are debated."
The article is accompanied by a fantastic illustration by Señor Salme with ideas from the Möbius strip and M.C. Escher:
| Year: 2019 | PT id = 967, Type = formulation 3D |
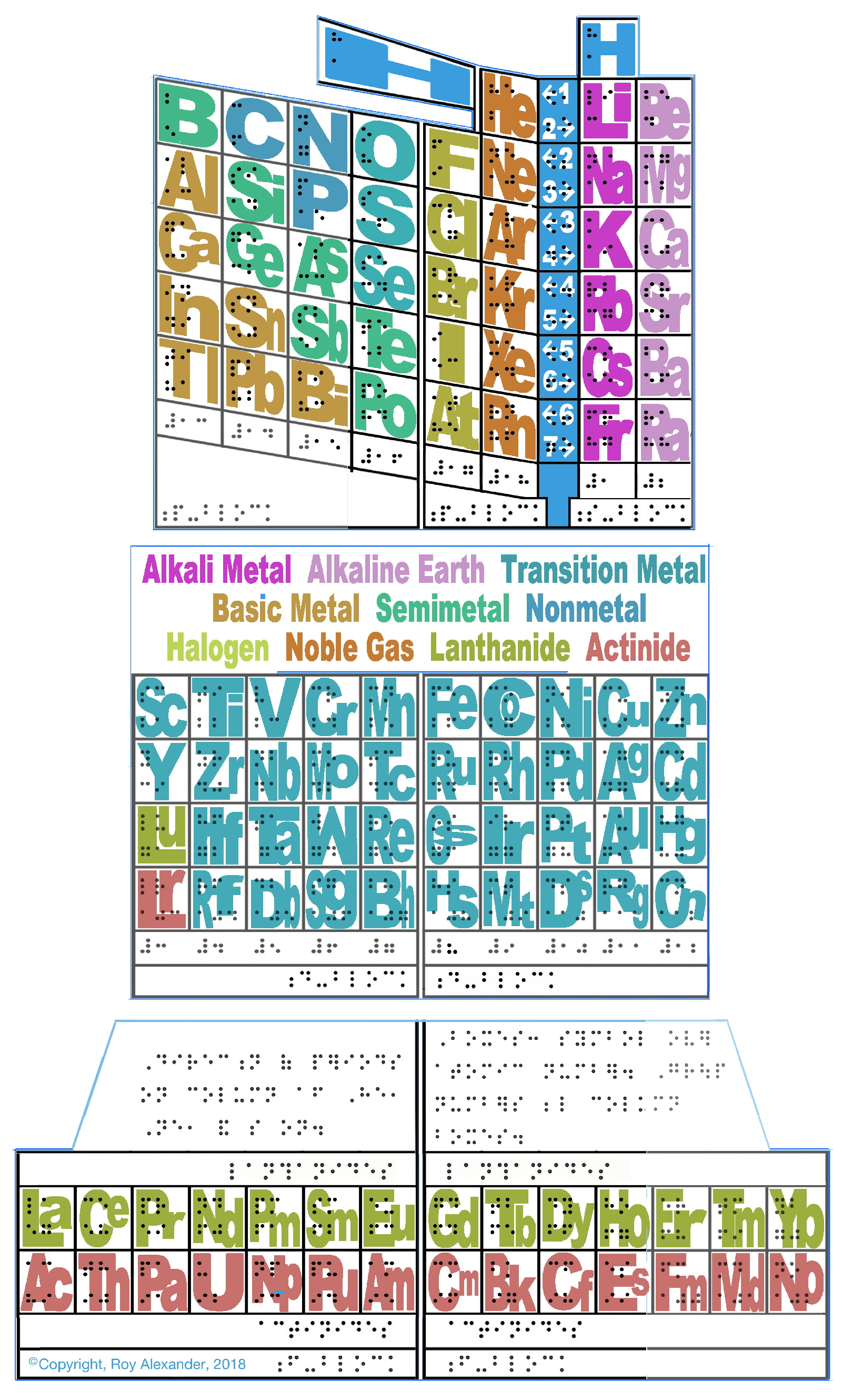
ElementBook Braille version of the AAE
From Roy Alexander of the AAE (Alexander Arrangement of the Elements):
"My [first] contribution to celebrate this Year of the Periodic Table is to reach out to folks who have yet to see what everyone's talking about, so they can get the feel of it: a 3D periodic table in Braille."
For the premise for this PT, here.
For your own model (with Braille) of the ElementBook: print*, cut out each part at the outside blue lines, etc.
The ElementBook Braille version of the AAE is in the beta-test phase for the rest of the year, so if any of you know of an aspiring chemist who would be willing to use it while learning (and keep me informed of that experience all year) send them to www.chemicalelementsystem.com/braille/

| Year: 2019 | PT id = 969, Type = formulation misc |
Kid's Periodic Table
From Cognitive Classroom, a Kid's 'cut-down' Periodic Table:
| Year: 2019 | PT id = 979, Type = formulation |
Döbereiner Revisited
Gordon Marks has developed a "Döbereiner Revisited" periodic table. Read all about it here.
| Year: 2019 | PT id = 982, Type = formulation misc |
Archetypes of Periodic Law
Archetypes of Periodic Law by Dmitry Weise, read more on the website.
One of the creators of quantum mechanics Wolfgang Ernst Pauli wrote in his work The Influence of Archetypal Ideas on the Scientific Theories of Kepler (1948):
"The process of understanding nature as well as the happiness that man feels in understanding – that is, in the conscious realization or new knowledge – seems thus to be based on a correspondence, a 'matching' of inner images pre-existent in the human psyche with external objects and their behavior. This interpretation of scientific knowledge, of course, goes back to Plato and is, as we shall see, advocated very clearly by Kepler. These primary images, which the soul can perceive with the aid of an innate 'instinct', are called by Kepler archetypal. Their agreement with the 'primordial images' or archetypes introduced into modern psychology by C. G. Jung and functioning as 'instincts of imagination' is very extensive. A true spiritual descendant of the Pythagoreans, he attached the utmost importance to geometric claiming that its theorems 'have been in the spirit of God since eternity'. His basic principle was: 'Geometria est archetypus pulchritudinis mundi' (Geometry is the archetype of the beauty of the world)."
Dmitry writes:
"The key archetype, in our opinion, is the concept of the square and its gnomon. This is due to the well-known fact that the electron filled shell contains 2n2 electrons, and the number of electrons on the subshell is twice the odd number; the gnomon of the square. Triangle, tetrahedron, square pyramid, octahedron, pyramid-like figures composed of square layers are also considered. The methodical concept for these constructions is the figurate numbers, actively studied by the Pythagoreans. The tables of the periodic law built on the motifs of ancient folk and modern ornaments take a special place. They include not only geometric archetypes, but also magic-symbolic, cultural and religious archetypes of the collective unconscious. Note that the periodic law table, built on the basis of the Native American ornament, surpasses the modern Mendeleev table in the parameter reflecting quantum numbers in its structure."
Note the final photograph below shows Prof. Martyn Poliakoff of The University on Nottingham and Periodic Videos:

| Year: 2019 | PT id = 983, Type = misc review |
International Year of the Periodic Table (in Paris and Moscow)
Prof. Martyn Poliakoff of The University on Nottingham and Periodic Videos at the opening of the International Year of the Periodic Table:
| Year: 2019 | PT id = 984, Type = formulation 3D spiral |
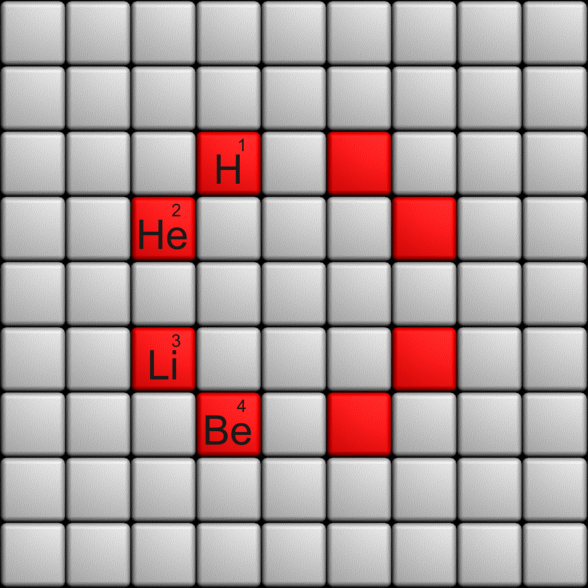
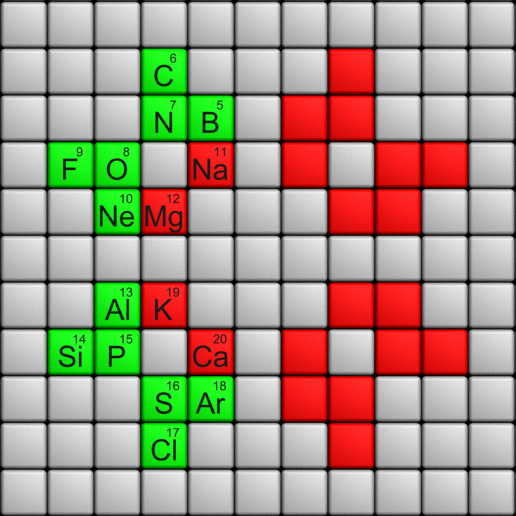
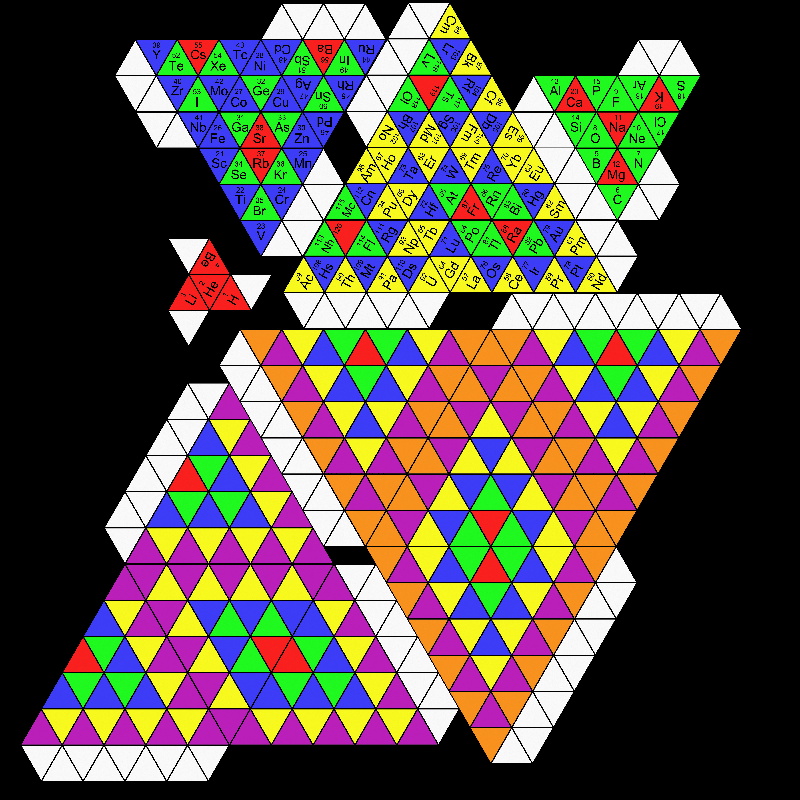
Telluric Remix in Colour
Philip Stewart writes (this is the same text that accompanies the 2018 B/W version):
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version (pdf).
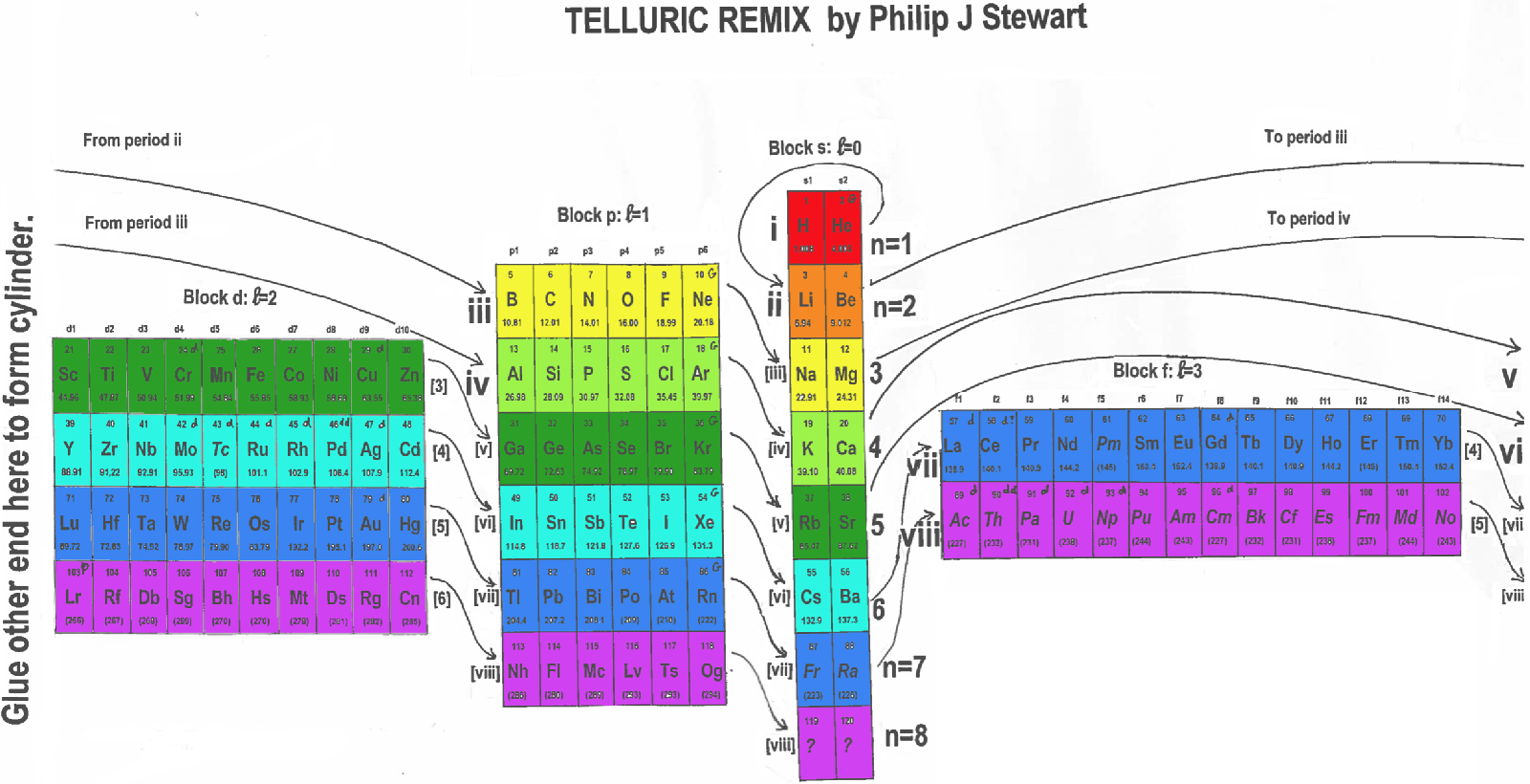
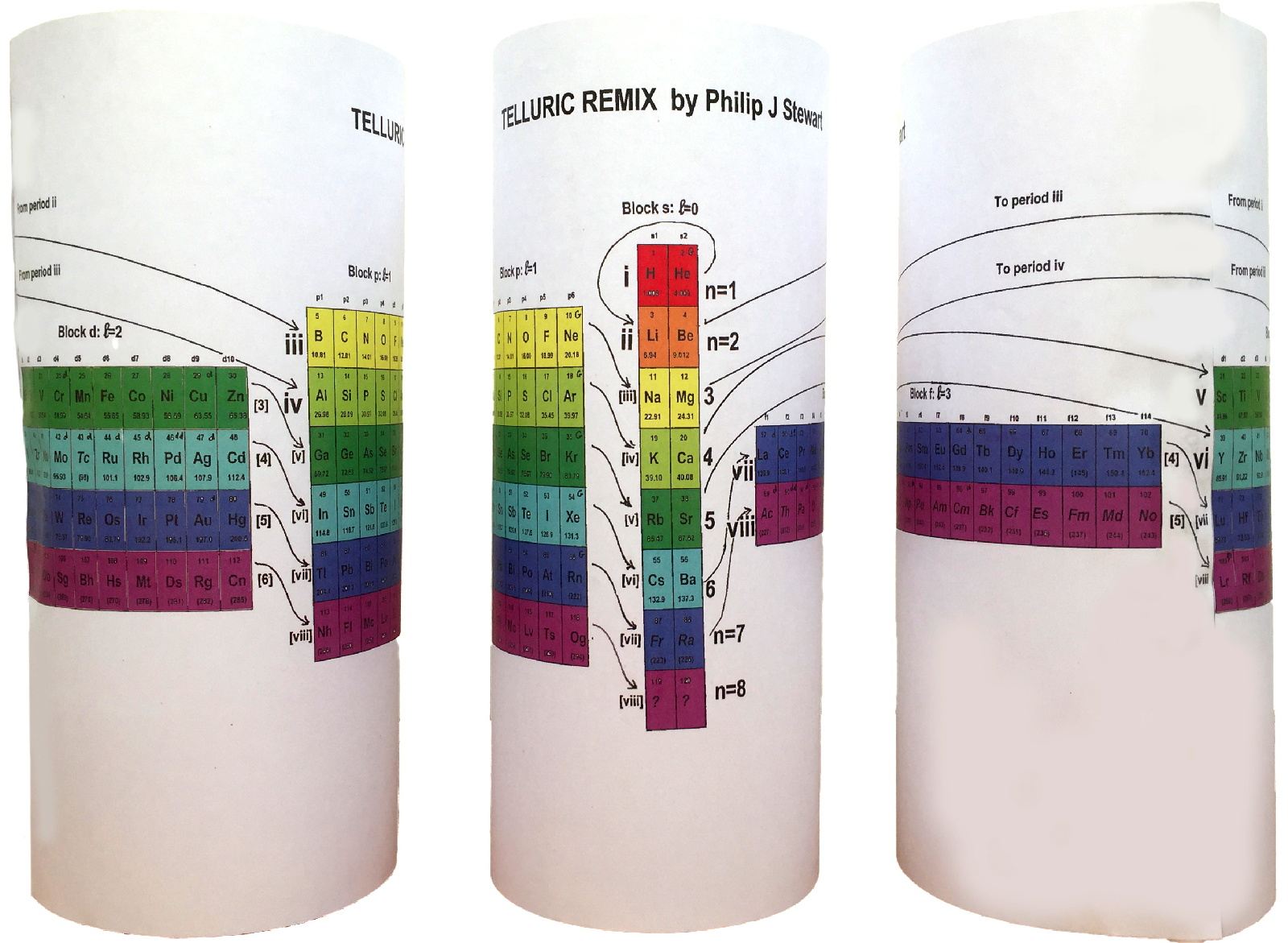
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
| Year: 2019 | PT id = 987, Type = misc |
Homage to The Elements
Eulalia Bosch writes:
"As a curator of the Eugènia Balcells Foundation, I would like to share with you the project to celebrate the 2019 UN decreed International Year of the Periodic Table (IYPT).
"Eugènia Balcells included the mural Homage to The Elements in her exhibition FREQUENCIES at the Santa Monica Art Center in Barcelona in 2009. The exhibit incorporates the spectrum of light that identifies each element. The result is not just another presentation of the periodic table, but a tribute to the set of elements that, in their intertwining, make up the material world and to those spectra that, as Eugènia Balcells like to say are: 'the voice of matter'.
"Over the last few years, the mural Homage to The Elements has also been incorporated at the Pascual Vila Research and Development Center of the CSIC in Barcelona, at the Science Museum, CosmoCaixa, in Barcelona and we are finishing the formalities for its installation in the Universities of Tarragona and Girona. It has also been acquired by the Technische Universität Berlin, and by the Friedrich-Alexander-Universität Erlangen-Nürnberg, both in Germany. In the city of NY, where Eugènia lived for more than thirty years, the mural has found its place at the Maxine Greene High School for Imaginative Inquiry, located at the Martin Luther King Educational Campus in New York, in front of the Lincoln Center, the great ally artistic ally of the School.
"The Eugènia Balcells Foundation wants to actively collaborate in the celebration proposed by the United Nations offering to the educational world the mural Homage to The Elements, this sign that represents universal unity, and records the human knowledge acquired to this day.

| Year: 2019 | PT id = 996, Type = formulation spiral 3D |
Grainger's Elemental Periodicity with "Concentric Spheres Intersecting Orthogonal Planes" Formulation
From Tony Grainger, an Elemental Periodicity formulation with concentric spheres intersecting orthogonal planes.
Tony writes:
"I hand sketched this periodic table about a decade ago and placed it on my cubicle window at UTAS, with minimal comments from work mates. It bears some similarity to other formulations in the database, especially when cut along the left axis and laid flat. The concept of all elements of a period being aligned along orthogonal planes cutting a sphere was inherent in the original sketch. When I began using SVG about five years ago I realised I could draw this as a real projection of the 3D model. It was on the back burner, until I found the original sketch during a tidy up."
There are two images of this 3D formulation: an "inside_corner_below/outside_corner_above" (top image) and an "outside_corner_below/inside_corner_above" lower image.
- The "inside corner below" is like looking at the junction of a floor and two walls in the corner of a room.
- The "outside corner above" is like looking up at the underside of an overhanging corner of a building.
- The "outside corner below" is like looking down on the corner of a large box.
- The "inside corner above" is like looking at the junction of walls and a ceiling in a room.
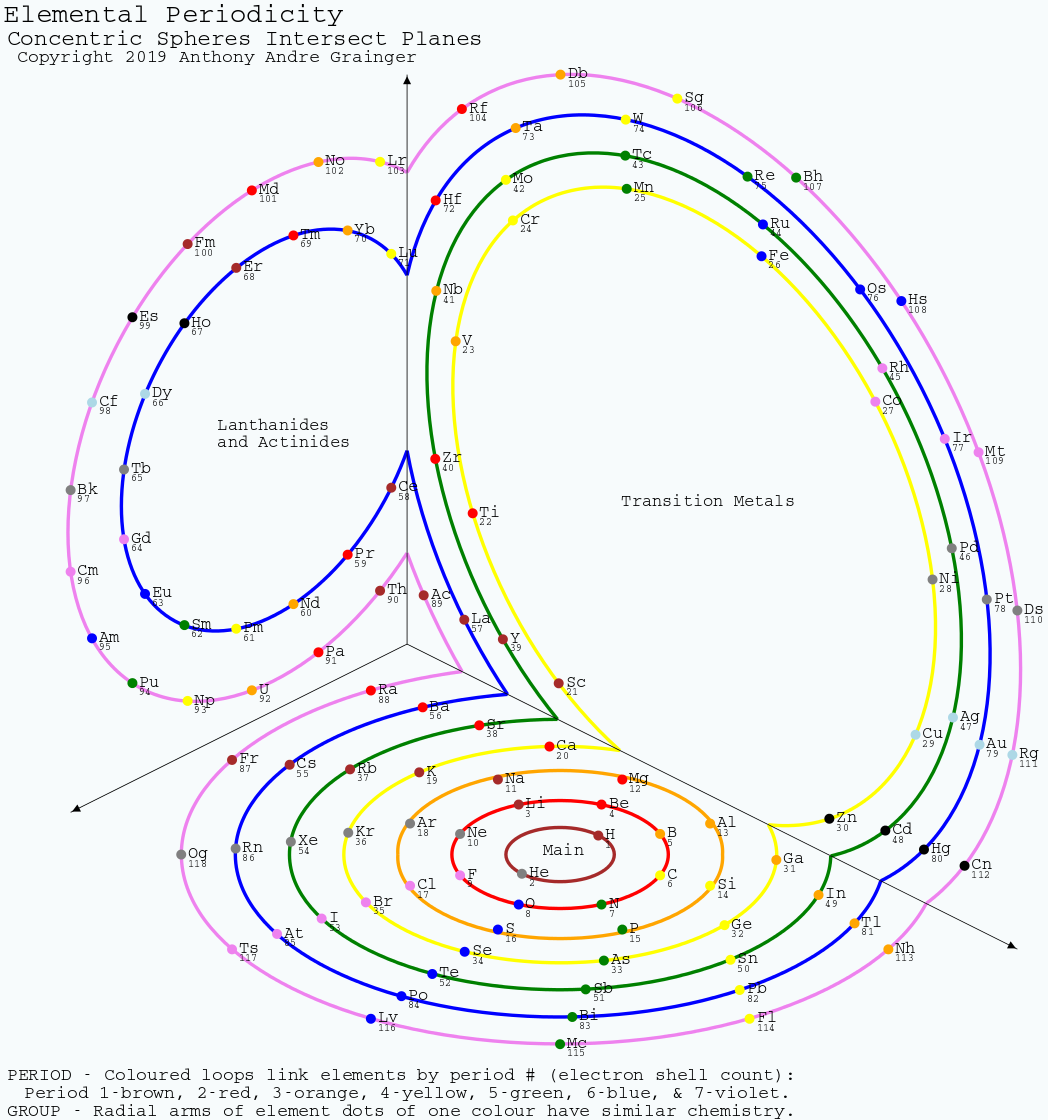
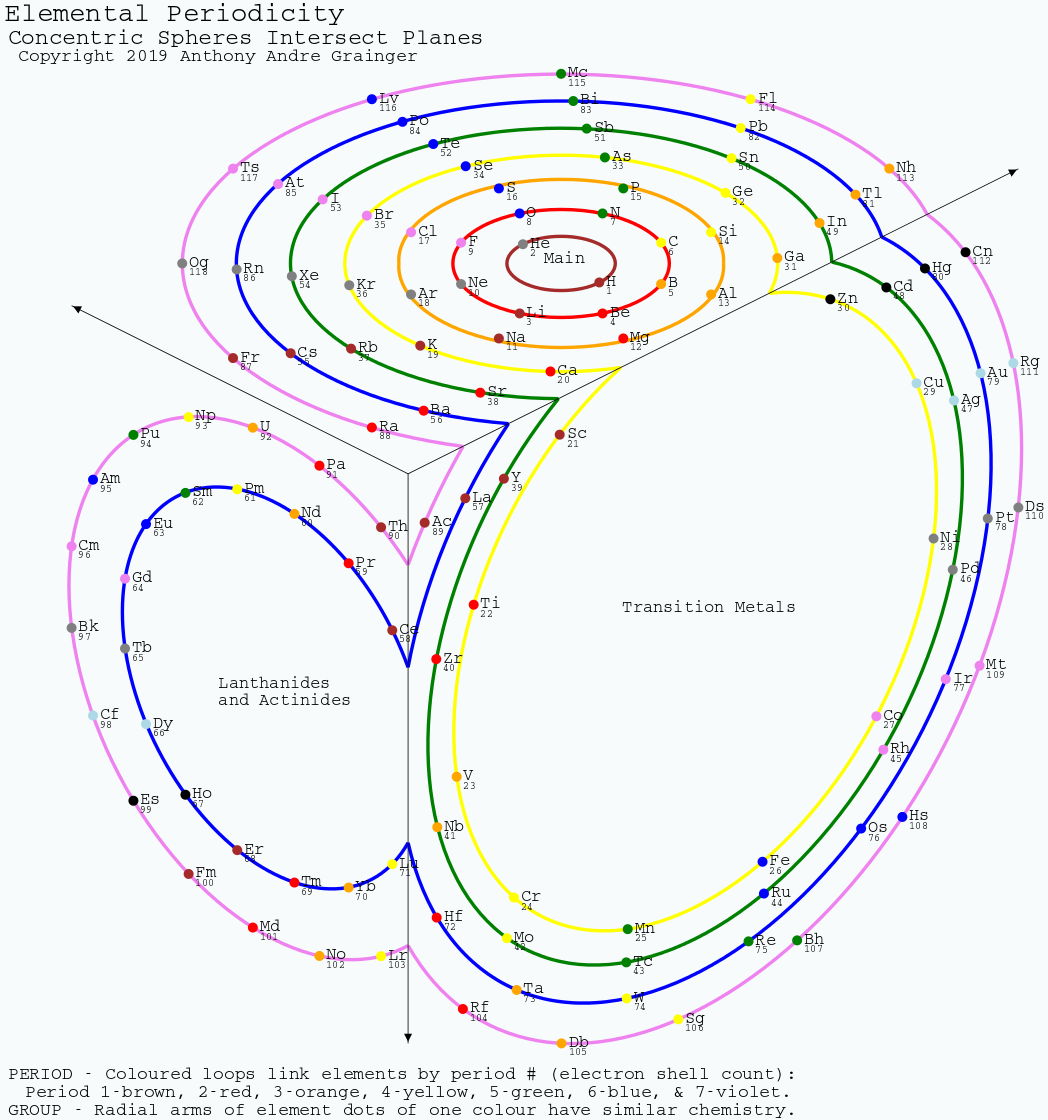
| Year: 2019 | PT id = 1001, Type = review |
Kultovoy's Periodic Table Book
Nicolay Kultovoy, website, as sent me a copy of his Periodic Table book, entitled [Google Translate]: Book 5. Part 11-08. A single quantum mechanical model of the structure of the atomic nucleus and the periodic table of chemical elements of D.I. Mendeleev.
In a mixture of Russian & English, the PDF of the book can be viewed here.
Chapter 1. Triune (electrons, nucleons, chemical elements) quantum mechanical model of Colt. Three
1.1 the Rules of filling of the orbits of electrons.
1.2 Pyramidal lattice.
1.3 models with cubic sieve.
1.4 models with face-centered lattice.
1.5 quantum Mechanical form of the periodic table of chemical elements.
1.6 Stowe-Janet-Scerri Periodic Table.
Chapter 2. A lattice model of the nucleus. Model 62
2.1 Berezovsky G. N.
2.2 I. Boldov
2.4 Konovalov.
2.5 Manturov V.
2.6 Semikov S. A.
2.7 alpha-partial model of the atomic nucleus.
2.8 Burtaev V.
Chapter 3. Various lattice (crystal) model of the nucleus of an atom. One hundred five
3.0 Luis Pauling.
3.1 Valery Tsimmerman. ADOMAH Periodic Table. Model 3-2.
3.2 Klishev B. V. Model 3-1.
3.3 Garai J. Model 3-1.
3.4 Winger E Model 4-2.
3.5 Norman D. Cook. Model 4-1.
3.6 Gamal A. Nasser. Model 4-1.
3.7 D. Asanbaeva Model 4-1.
3.8 Datsuk V. K.
3.9 Bolotov B.
3.10 Djibladze M. I.
3.11 Dyukin S. V.
3.12 A. N. Mishin.
3.13 M. M. Protodyakonov
3.14 Dry I. N.
3.15 Ulf-G. Meißner.
3.16 Foreign works.
Chapter 4. Long-period periodic table. One hundred eighty one
4.1 long-Period representation of the periodic table.
4.2 Artamonov, G. N.
4.3 Galiulin R. V.
4.4 E. K. Spirin
4.5. Khoroshavin L.
4.6 Step form proposed by Thomsen and Bohr.
4.7 Symmetrical shape of the periodic table.
Chapter 5. Construction of a periodic table based on the structure of orbitals. Two hundred twenty one
5.1 construction of the periodic table on the basis of orbitals.
5.2 Short V. M.
5.3 Kulakov, the Novosibirsk table of multiplets.
Chapter 6. Atomic structure. Two hundred forty eight
6.1 Table of isotopes.
6.2 the structure of the orbitals.
| Year: 2019 | PT id = 1004, Type = review |
St Catharine's College: Celebrating the Periodic Table
The United Nations have proclaimed 2019 to be the International Year of the Periodic Table of Chemical Elements since it is the 150th anniversary of the publication of Dmitri Mendeleev's first Periodic Table. But was it really the first?
St Catharine's College, Cambridge, in the UK, is proud to exhibit its fine collection of material relating to the early development of the Periodic Table. Starting from the first list of elements which emerged around the time of the French Revolution in the late 1780s, and the first list of atomic masses drawn up by Manchester chemist John Dalton, we explore why six different chemists from around the world each came up with their own versions of the iconic table in the 1860s.

From the RSC Website:
"Curated by periodic table superfan Peter Wothers, the main body of the exhibition is a staggering collection of historic books that trace the creation of chemistry's roadmap.
"This is an unprecedented record of the periodic table's origins, from early alchemical texts through to original copies of Antoine Lavoisier's 1789 Elementary Treatise of Chemistry – the first true list of elements – and notes on the discoveries of (among others) John Newlands, Julius Lothar Meyer through to Dmitri Mendeleev".
Celebrating the periodic table – the first edition of Mendeleev's textbook from Chemistry World on Vimeo.
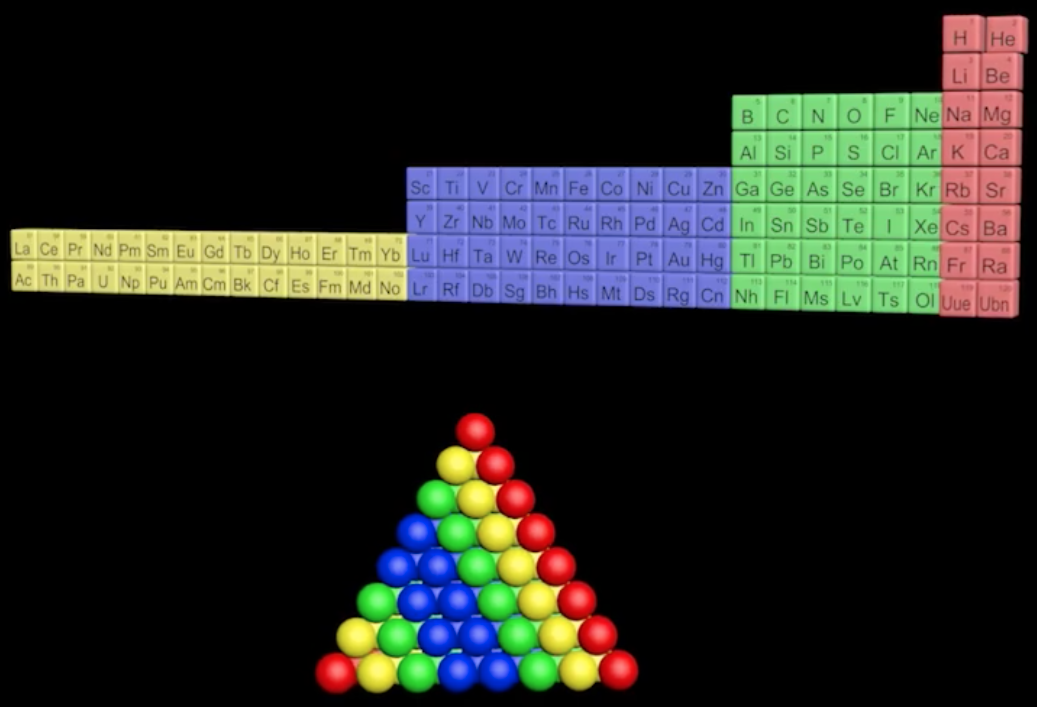
| Year: 2019 | PT id = 1005, Type = formulation misc 3D spiral |
Schaltenbrand's Helical Gathering of the Elements
From the RSC Website:
"A glistering, shining spiral made of silver, gold, platinum, palladium and a diamond forms the show-stopping apex of the tribute from the University of Cambridge's St Catharine's college to the International Year of the Periodic Table.
"Commissioned to match George Schaltenbrand's 1920 design for a helical gathering of the elements – albeit extended to all 118 current elements – and signed by Yuri Oganessian, it is almost certainly the most expensive periodic table in the world."


Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1008, Type = formulation |
Poliakoff's Inverted Periodic Table
From Nature Chemistry. Martyn Poliakoff et al write:
"The inverted periodic table is obtained by rotating the conventional one by 180° about a horizontal axis.
"a, The lighter elements are now at the bottom and the filling of the electron shells occurs upwards. Just like the traditional representation, many properties (for example, atomic number) increase across the table as one proceeds from left to right, but in the inverted version, the same properties now increase as one moves from the bottom to the top, which is the way that most graphs are plotted. Also like the conventional table, the lanthanides and actinides still sit uncomfortably in an isolated block.
"b, In principle, this could be overcome by inverting the 'long form' of the table but, like the conventional long form, it is probably too elongated to be very useful to most chemists."
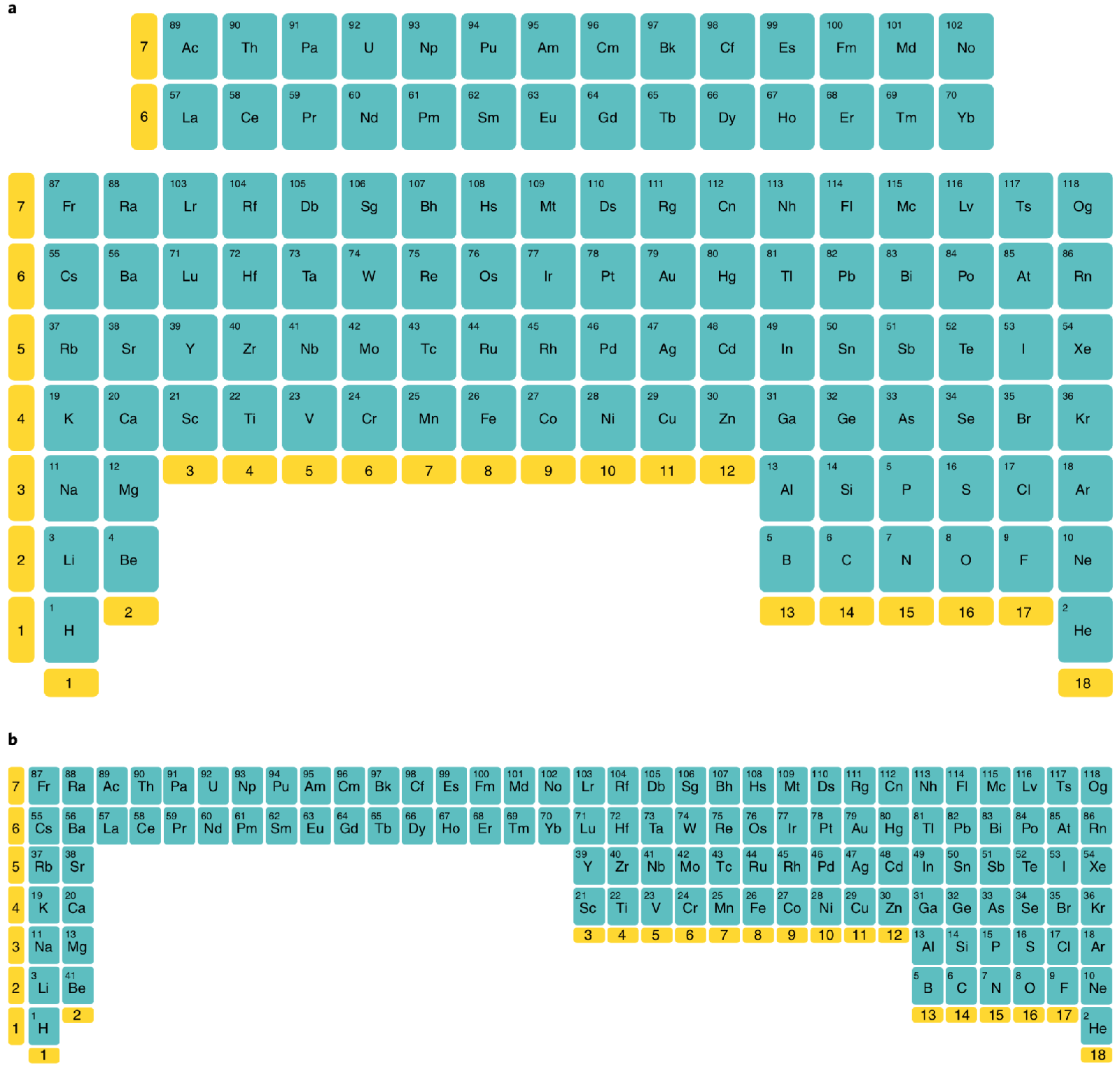
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1013, Type = formulation spiral |
Moran's Periodic Spiral (2019)
Jeff Moran has been working on his Periodic Spiral for more than twenty years. Here is the latest iteration, click to enlarge:
| Year: 2019 | PT id = 1014, Type = formulation spiral |
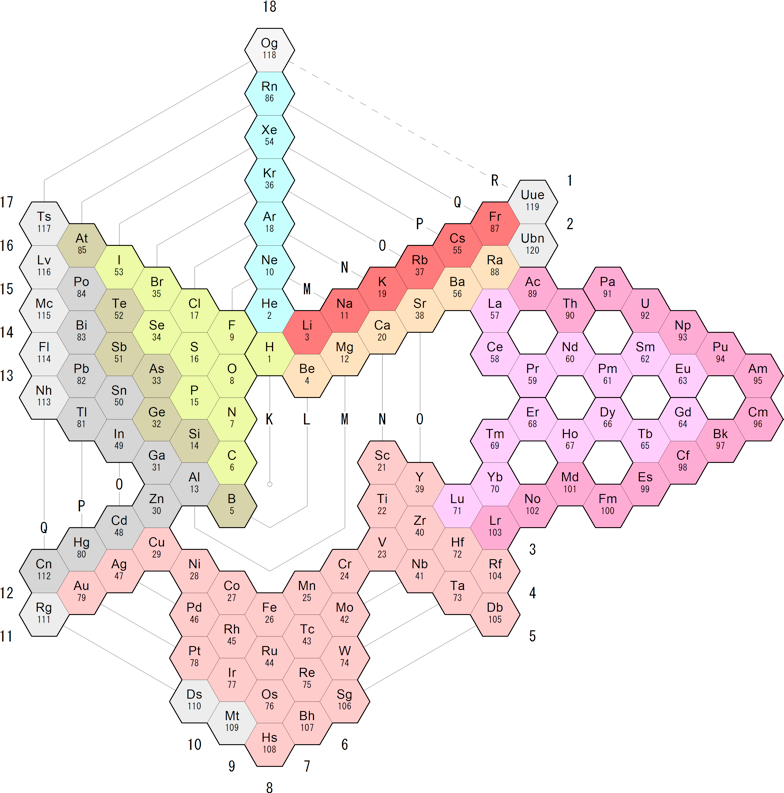
NAWA's Version of Moran's Periodic Spiral
Periodic table designer Nagayasu Nawa has put his spin on Moran's Periodic Spiral:
| Year: 2019 | PT id = 1019, Type = formulation 3D |
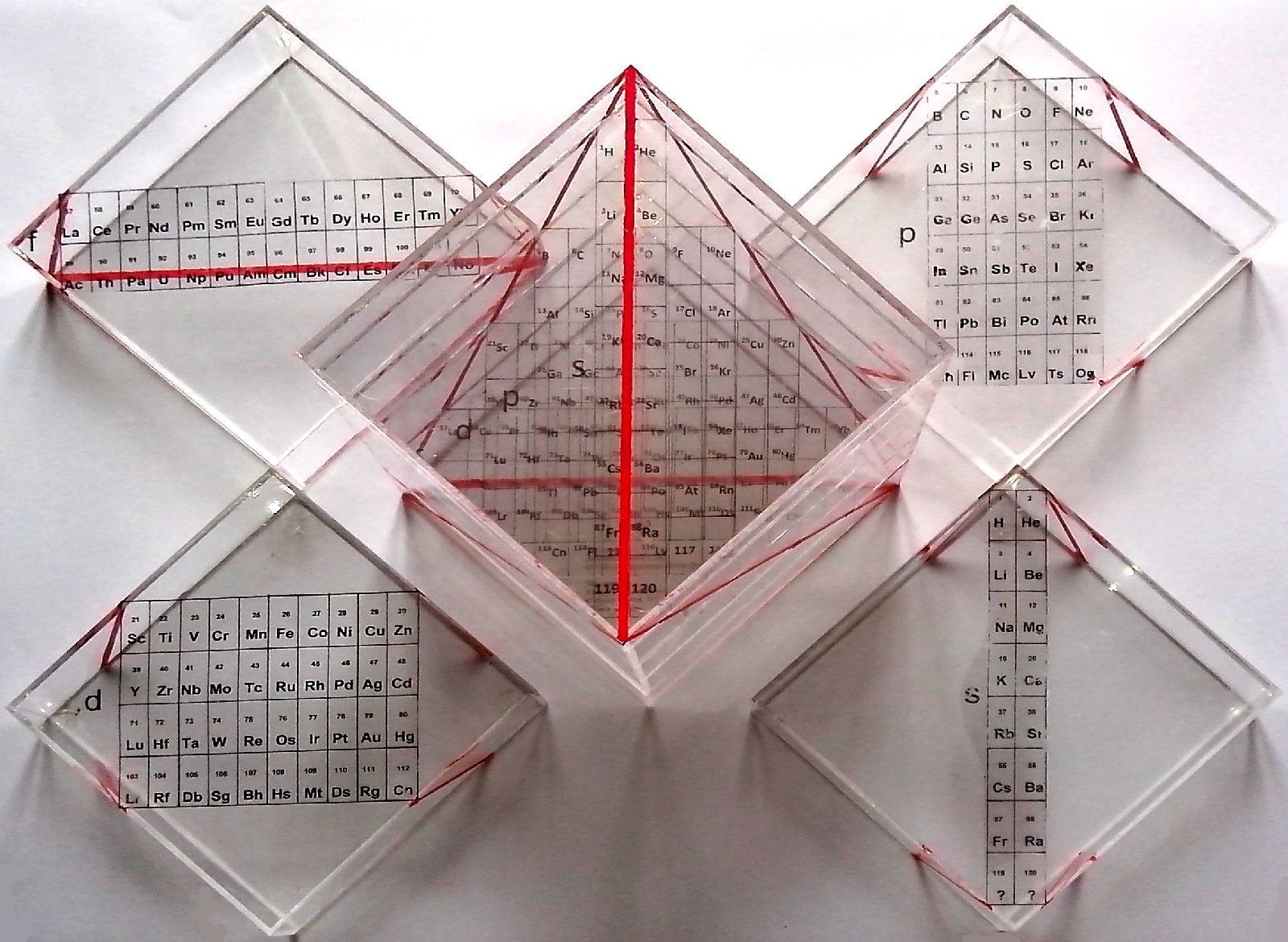
Stewart's Quantahedron Formulation
From Philip Stewart, here & here, comes a three dimensional Quantahedron Formulation.
Philip writes:
"The Quantahedron is based on Tsimmerman's Adomah cube, realised in transparent plastic, in the usual order in which Z values are read, printed on separable blocks so that it can be assembled."
| Year: 2019 | PT id = 1022, Type = review |
Evolution and Understanding of the d-Block Elements in the Periodic Table
Edwin C. Constable's review, Evolution and understanding of the d-block elements in the periodic table:
| Year: 2019 | PT id = 1023, Type = data review |
Nucleosynthesis of the Heavy Elements
A PBS video explaining how neutron star mergers lead to the formation of heavy elements, and how a merger only 80 million years before the formation of the solar system, 4.5 billions years ago, seeded the Earth wth the heavy elements of the periodic table:
| Year: 2019 | PT id = 1024, Type = formulation review |
5 Periodic Tables We Don't Use (And One We Do)
SciShow says:
"From Mendeleev's original design to physicist-favorite "left-step" rendition, the periodic table of elements has gone through many iterations since it was first used to organize elements 150 years ago - each with its own useful insights into the patterns of the elements":
| Year: 2019 | PT id = 1026, Type = formulation review |
Papers of Mendeleev, Odlings, Newlands & Chancourtois from the 1860s
Peter Wothers from the University of Cambridge with Sir Martyn Poliakoff, of the University of Nottingham discuss the discovery/development of the periodic table in the 1860s with the original publications.
Links to some of the formulations discussed in the video:
- Mendeleeve Table I
- Mendeleeve Table II
- Odlings' Formulation
- Newlands Octaves
- Chancourtois' Teluric Helix
| Year: 2019 | PT id = 1027, Type = formulation data |
Chemical Bonds, Periodic Table of
The Max Planck Society (M-P-G, Max-Planck-Gesellschaft) has an article about the hidden structure of the periodic system.
Guillermo Restrepo, MPI for Mathematics in the Sciences:
"A periodic table of chemical bonds: Each of the 94 circles with chemical element symbols represents the bond that the respective element forms with an organic residue. The bonds are ordered according to how strongly they are polarized. Where there is a direct arrow connection, the order is clear: Bonds of hydrogen, for example, are more polarized than bonds of boron, phosphorus, and palladium. The same applies to rubidium in comparison to caesium, which has particularly low polarized bonds and is therefore at the bottom of the new periodic table. If there is no direct arrow between two elements, they may still be comparable – if there is a chain of arrows between them. For example, the bonds of oxygen are more polarized than the bonds of bromine. Bonds represented by the same colour have the same binding behaviour and belong to one of the 44 classes.":

Thanks to René for the tip!
| Year: 2019 | PT id = 1028, Type = formulation |
Slightly Different Periodic Table
The Max Planck Society (M-P-G, Max-Planck-Gesellschaft) has an article about the hidden structure of the periodic system.
Guillermo Restrepo, MPI for Mathematics in the Sciences:
"A slightly different periodic table: The table of chemical elements, which goes back to Dmitri Mendeleev and Lothar Meyer, is just one example of how objects – in this case the chemical elements – can be organized in such a system. The researchers from Leipzig illustrate the general structure of a periodic table with this example: The black dots represent the objects ordered by the green arrows. Using a suitable criterion, the objects can be classified into groups (dashed lines) in which the red arrows create a sub-order":
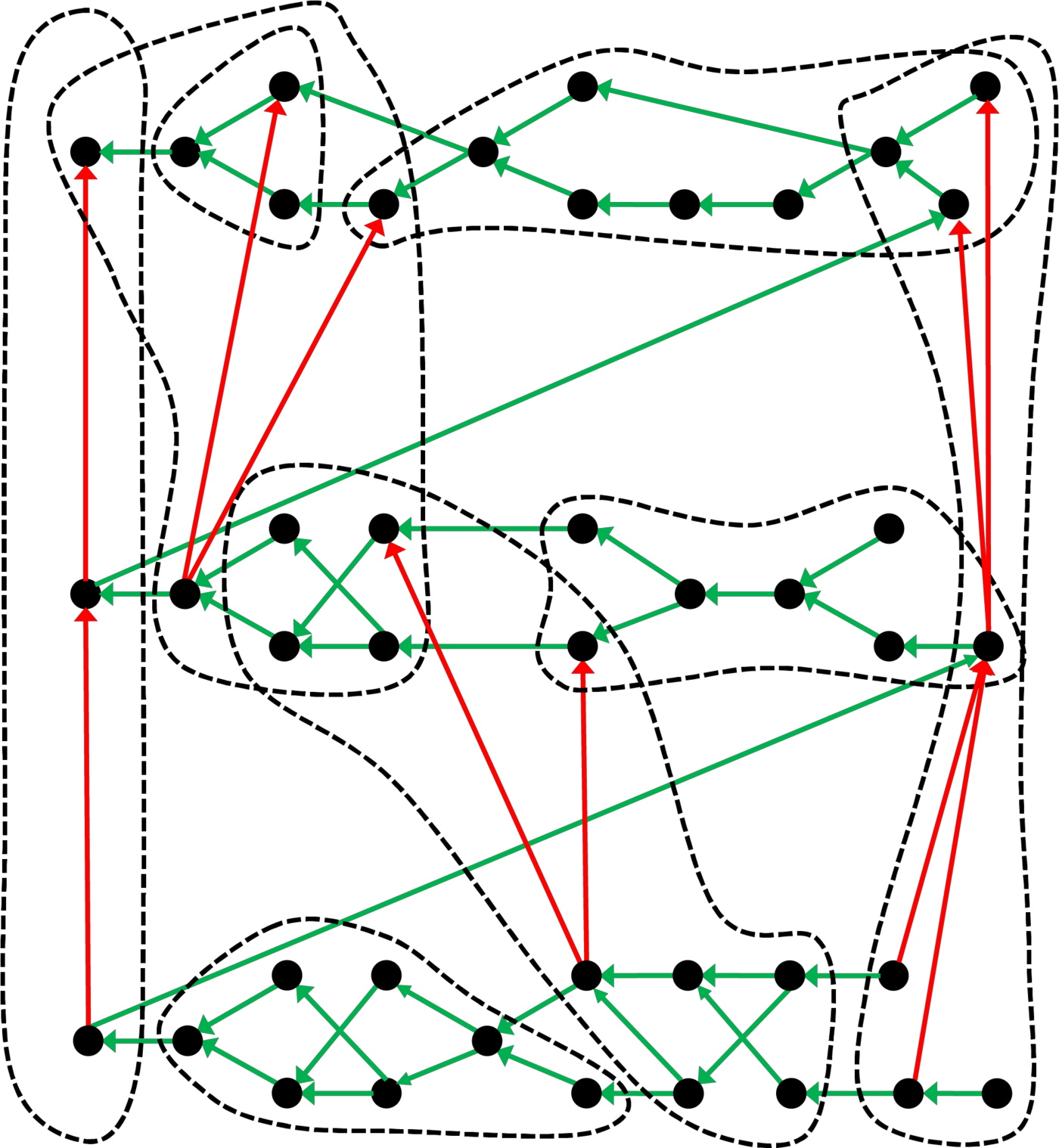
Thanks to René for the tip!
| Year: 2019 | PT id = 1029, Type = data misc |
Heritage Periodic Table Display
By Engineered Labs, the Heritage Periodic Table Display.
"Introducing the world's first and only miniature Periodic Table with the actual elements in it.
"Over the last year, we have successfully collected each and every stable element. After considerable R&D, we have finally developed a method of embedding each element in acrylic and we have to say, the result is awesome!
"The Heritage Periodic Table pretty much speaks for itself. The collection looks great on a desk, in your hands, and anywhere else it can be displayed."

| Year: 2019 | PT id = 1030, Type = formulation |
Béguyer de Chancourtois' Vis Tellurique: A Better View
The content of Béguyer de Chancourtois' Vis Tellurique decanted into a flat table.
The flattened version – prepared by Conal Boyce – shows important aspects that cannot be 'read' from the helix itself.
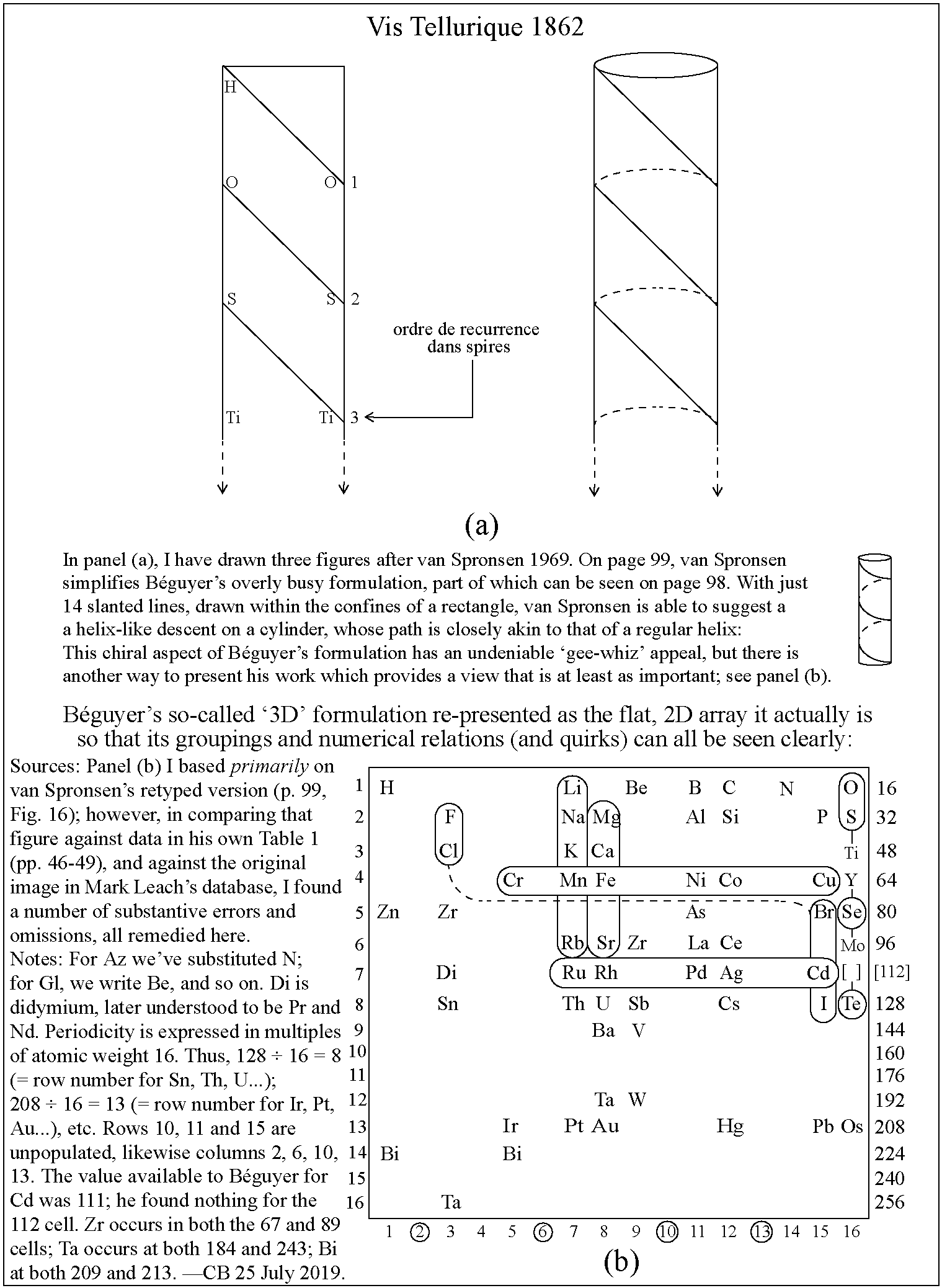
| Year: 2019 | PT id = 1031, Type = misc |
Setting The Table
The journal Science gives "a visual brief history" of the periodic table, with some neat graphics showing the PT grew and changed with time. (You will need to visit the webpage to see the cool graphics inaction):
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 2019 | PT id = 1036, Type = misc |
Celebrate 150 Years Of The Periodic Table By Tying 200,000 Tiny Knots
Jane Stewart decided to Celebrate 150 Years Of The Periodic Table By Tying 200,000 Tiny Knots:


| Year: 2019 | PT id = 1037, Type = misc |
Knitted Blanket Periodic Table, In Time to Celebrate 150th Anniversary
Trish Bosco thought we might be interested in her periodic table blanket.
"It took me almost 4 years to make it, but I finished in time to celebrate the 150th anniversary! You can see my progression here":
| Year: 2019 | PT id = 1040, Type = formulation 3D misc |
Frog Periodic Table
One of the frogs from Stockport's (UK) Giant Leap Frog Art Trail. This frog is Chemit.

Thanks to Helen P for the tip!
| Year: 2019 | PT id = 1041, Type = misc |
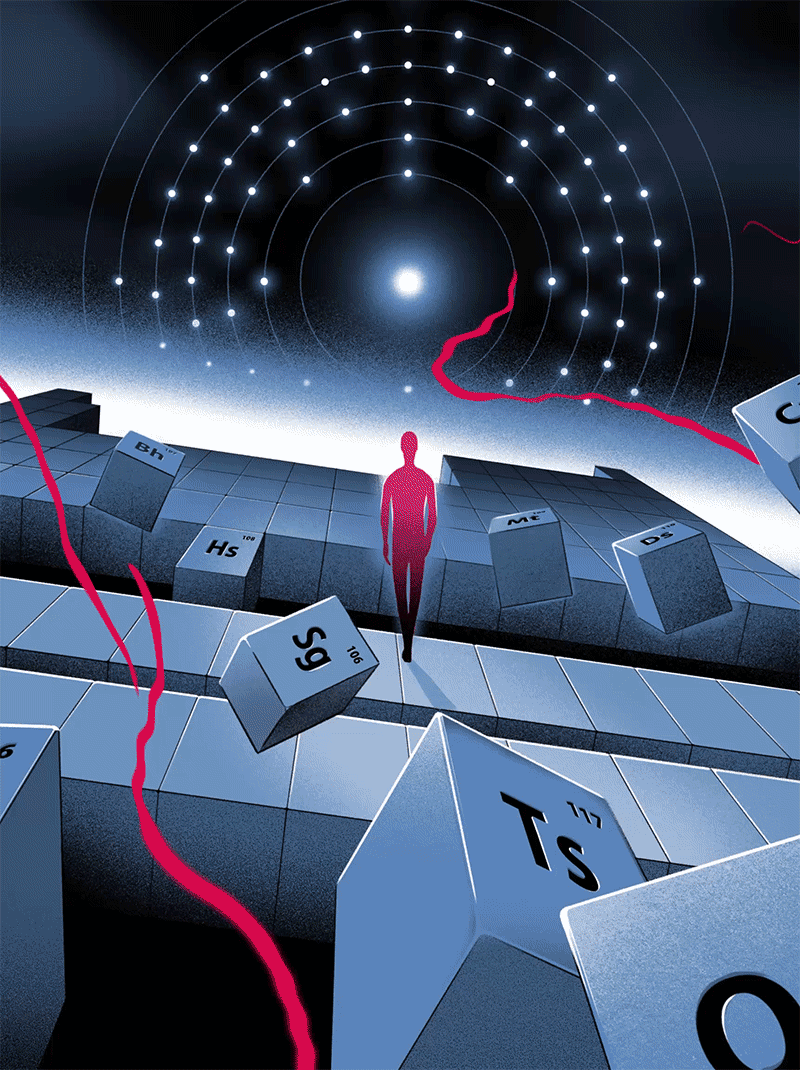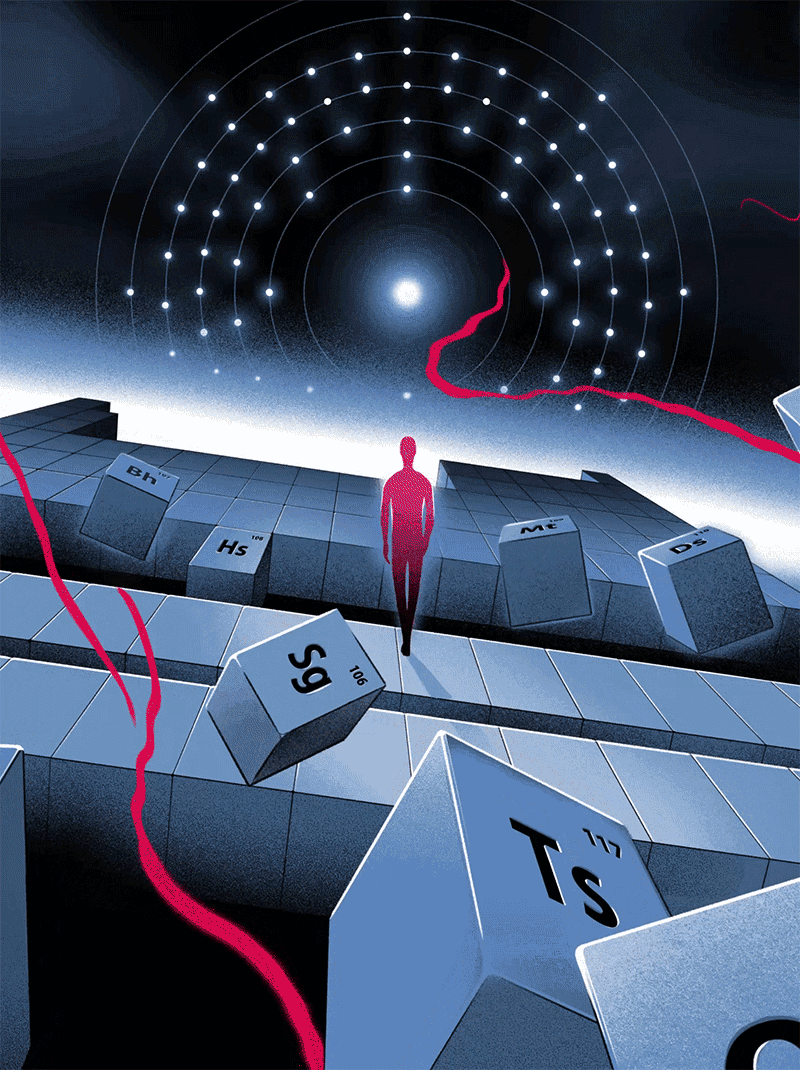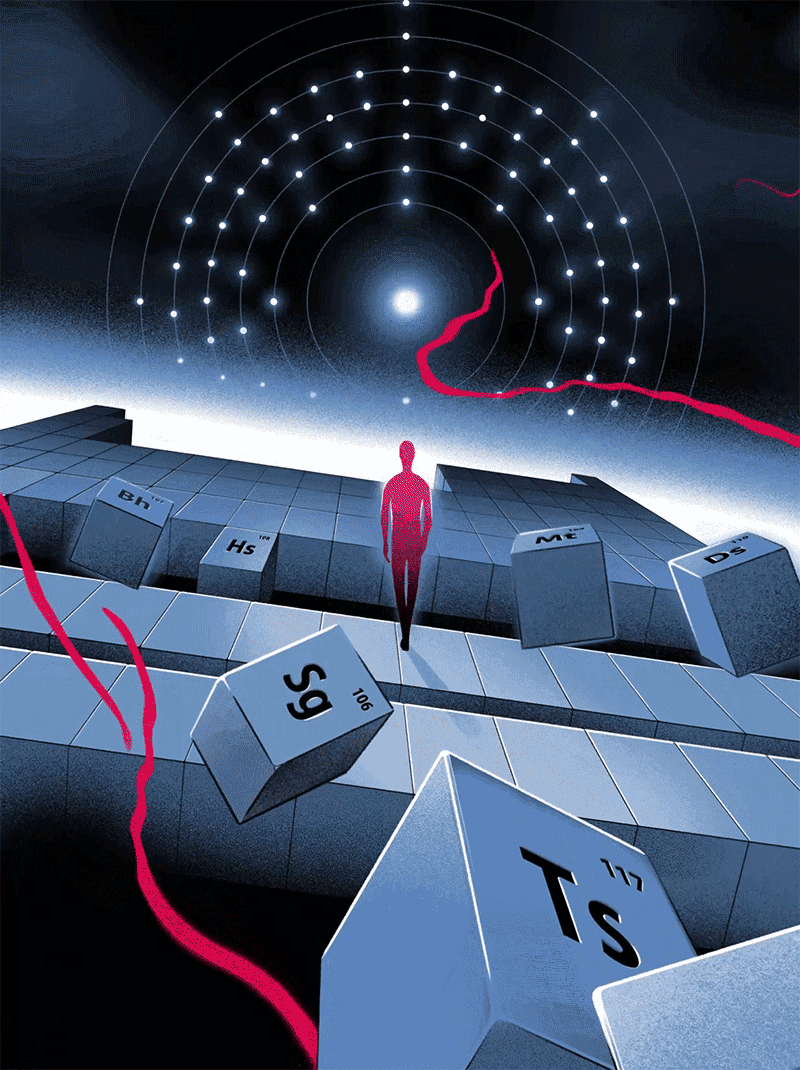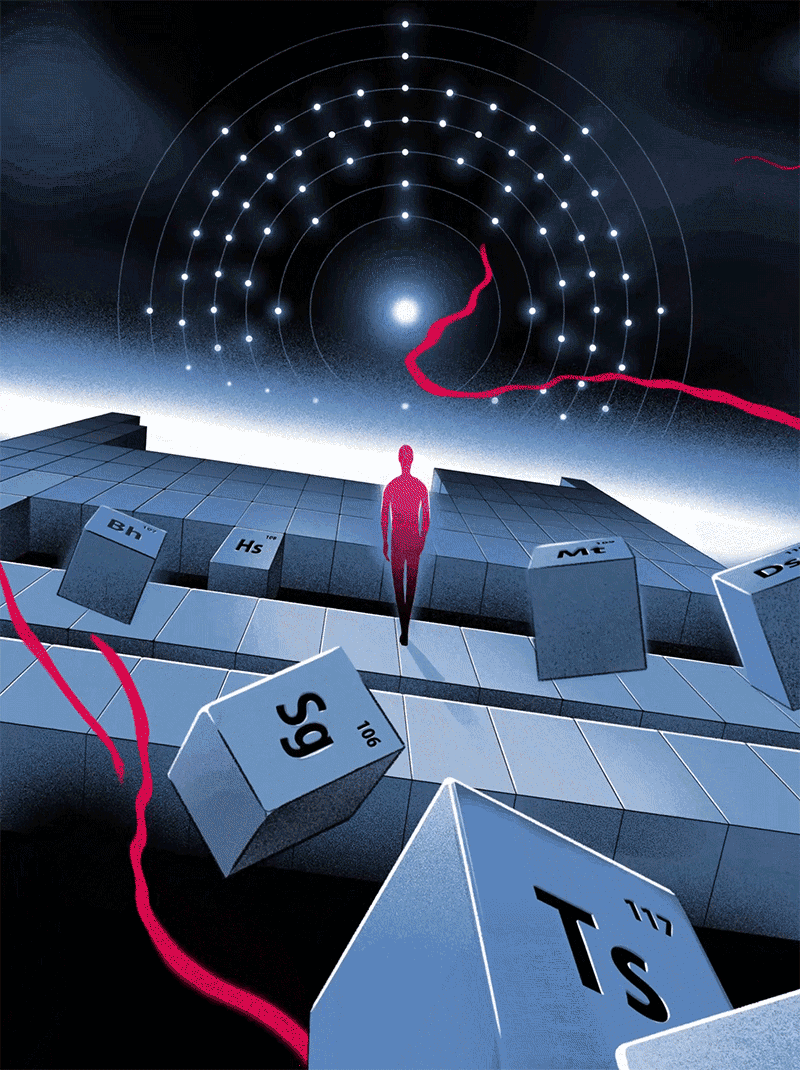
Quantum Victoria Periodic Art
In 2019 Quantum Victoria, an Australian specialist STEM education centre for primary and secondary students and teachers, commissioned a series of 51 images illustrating the birth of the Universe through elements of significance from the Periodic Table.
These will be permanently installed at Quantum Victoria and launched in July to celebrate IYPT2019 - the 150th anniversary of the discovery of the Periodic Table in 1869.
All designs are available as limited edition prints in sizes from A3 to one metre across, printed with archival inks on 100% cotton paper.
Click here or on the image below to access the website and larger versions of the illustrations.
| Year: 2019 | PT id = 1042, Type = data misc |
Ultimate Periodic Table by Goodfellow
While the 'ultimate' periodic table by Goodfellow may not appear to be very ultimate, it does actually a possess a very rare property: Goodfellow is a materials company that supplies most of the chemical elements for industrial and research use.
By clicking on the element palladium, various facts about, and properties of, Pd are shown. Additionally, Goodfellow can supply:
| Year: 2019 | PT id = 1046, Type = formulation data |
Group 3 of The Periodic Table
There are several ways in which the 'common/modern medium form' periodic table are shown with respect to the Group 3 elements and how the f-block is shown. Indeed, there is even some dispute about which elements constitute Group 3. There are three general approaches to showing Group 3:
- Sc, Y, La, Ac
- Sc, Y than a gap for the lanthanides & a gap for the actinides
- Sc, Y, Lu, Lr
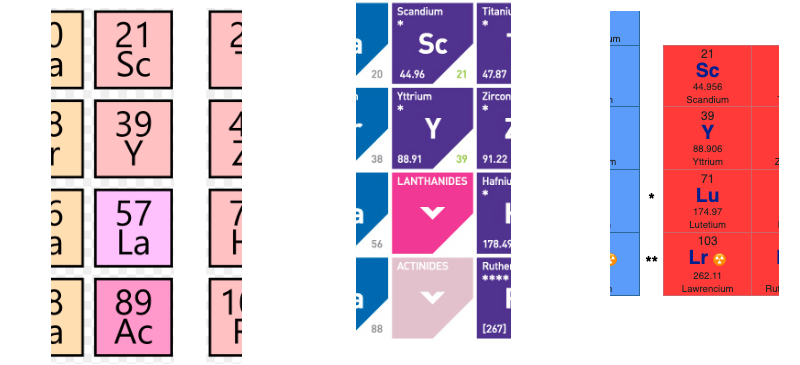
(See Scerri's take and Thyssen's view on this matter.)
So, which one of the three options is 'better'?
The general feeling amongst the knowledgeable is that leaving a gap is not an option, so it comes down to:
Sc, Y, La, Ac vs. Sc, Y, Lu, Lr
René Vernon has looked as the properties of the potential Group 3 elements, including: densities, 1st ionisation energies, ionic radii, 3rd ionisation energies, melting points & electron affinity:
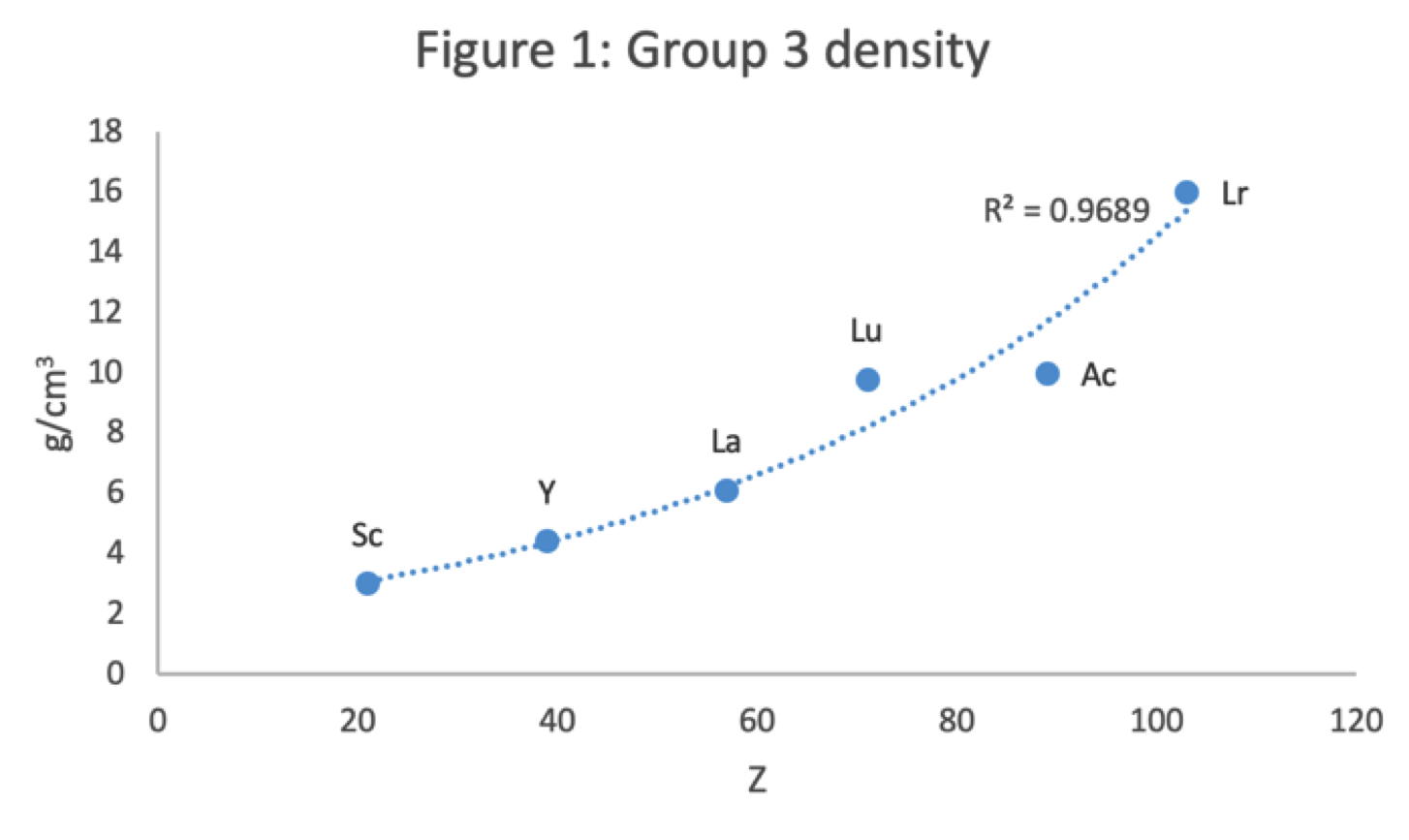
Figure 1 shows that a Z plot of the density values for Sc, Y, La, Lu Ac and Lr follows a smooth trendline.
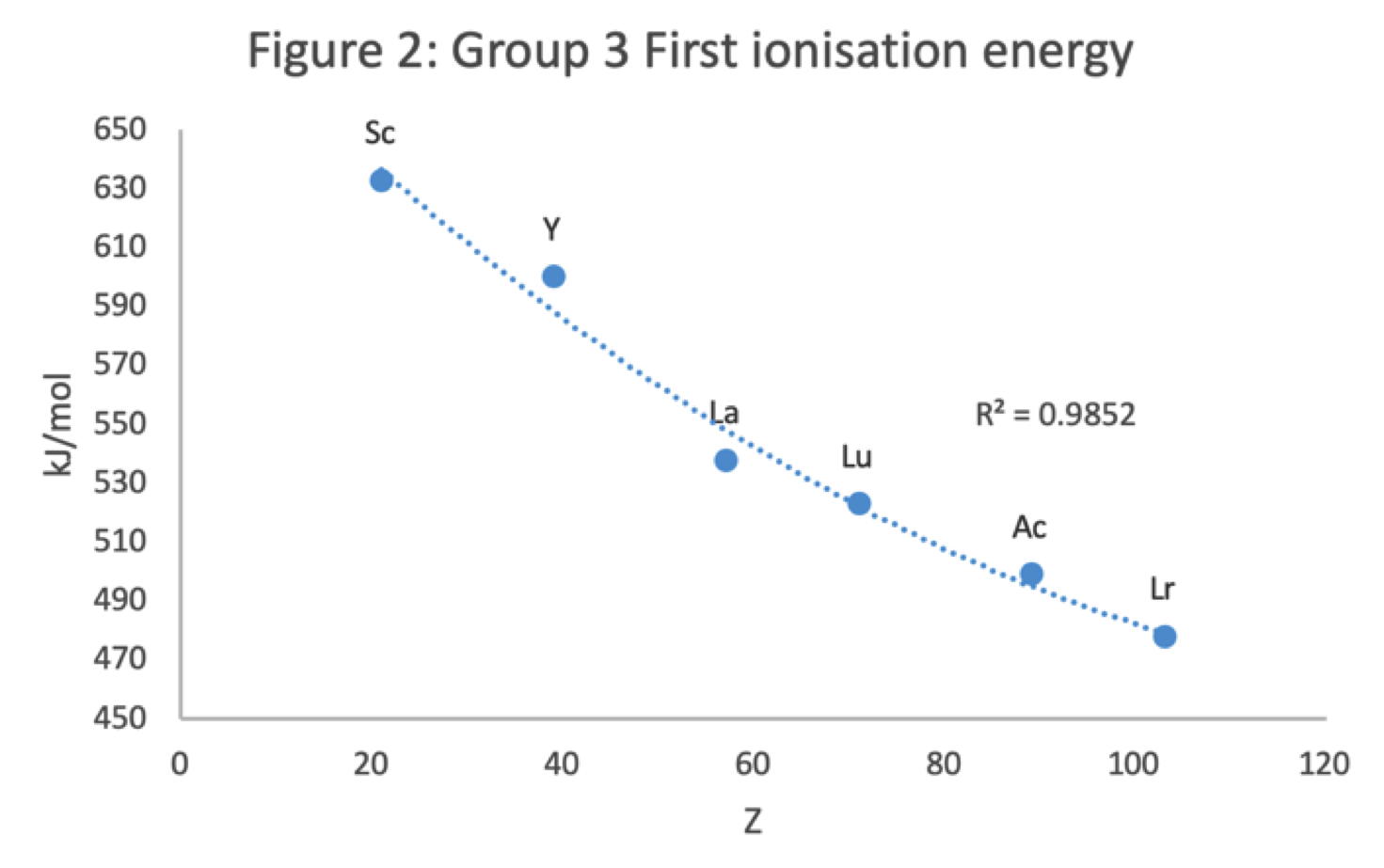
Figure 2 shows that a Z plot of the first ionization energy values follows a smooth trendline.
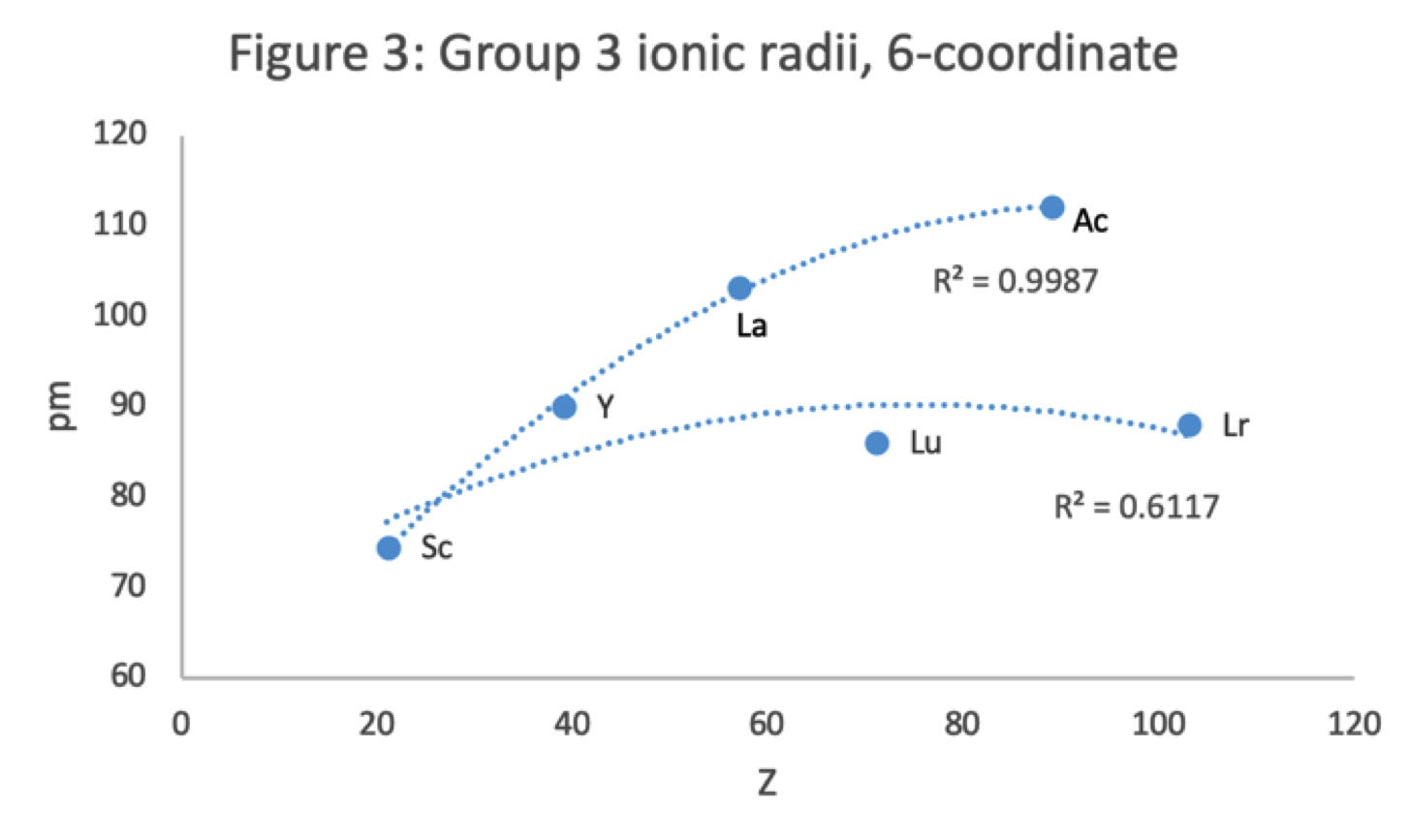
Figure 3 shows that a Z plot of the 6-coordinate ionic radii for the subject elements bifurcates after Y into an -La-Ac tranche (R2 = 0.99) and a -Lu-Lr branch (0.61). The trendline for -La-Ac is smoother.
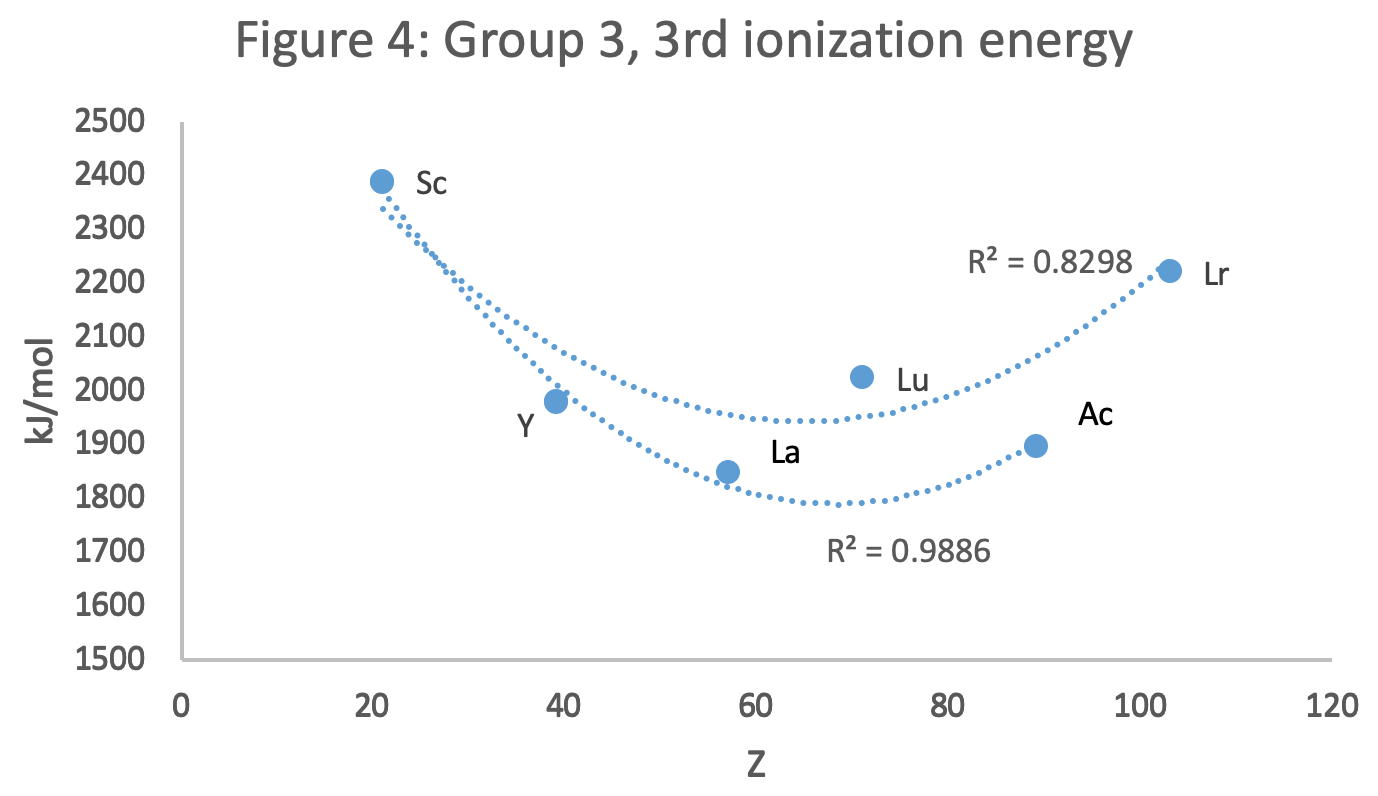
Figure 4 shows a Z plot of 3rd ionisation energy values bifurcating after Y into a -Lu-Lr tranche (R2 = 0.83) and a -La-Ac branch (0.98). The trendline for -La-Ac is smoother.
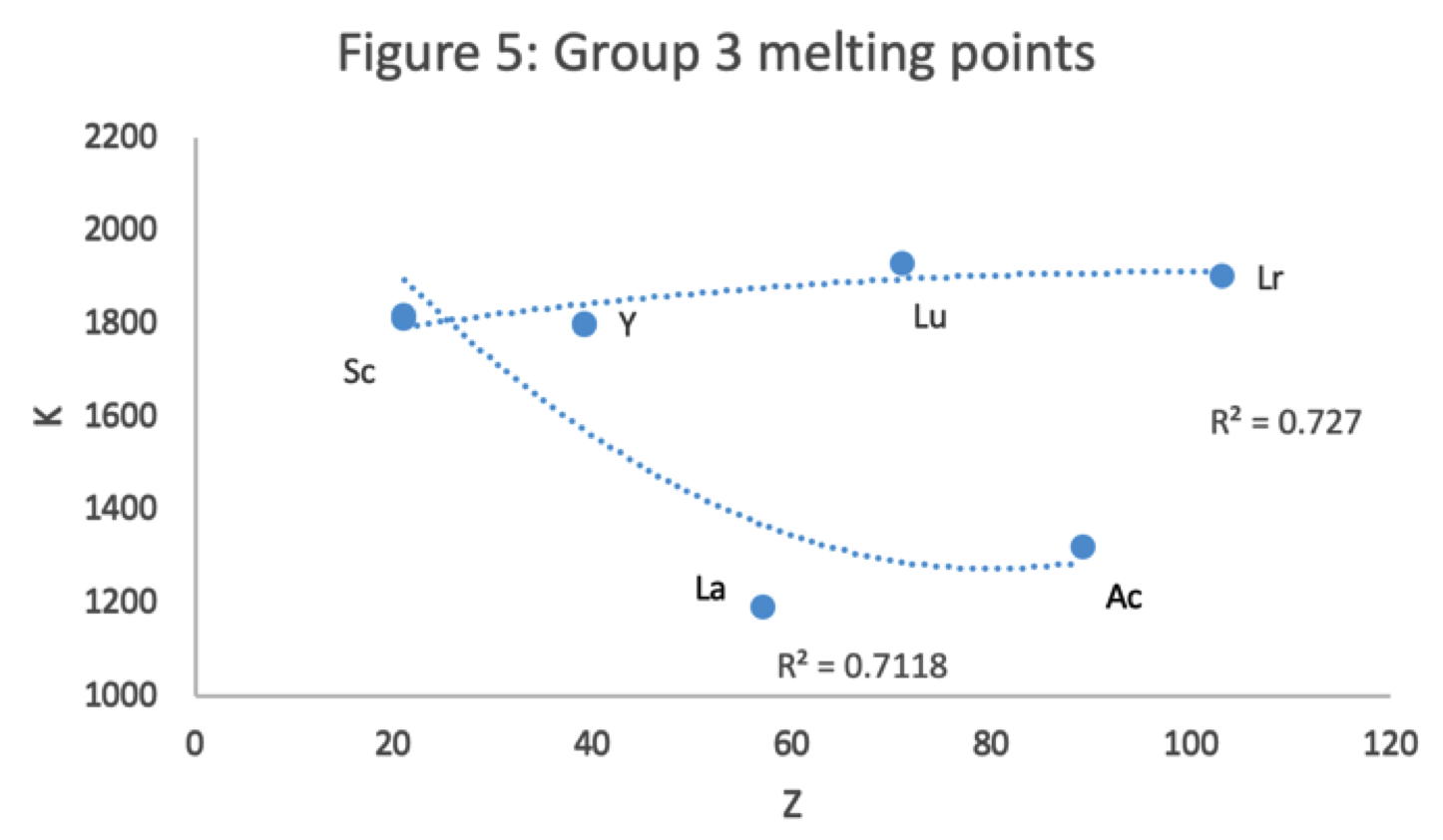
Figure 5 shows that a Z plot of the melting points bifurcates after Y into an -Lu-Lr (R2 = 0.72) tranche and a -La-Ac (0.71) branch. While the fit values for the two options are comparable, -Lu-Lr is preferred since Y and La show a greater departure from trend.
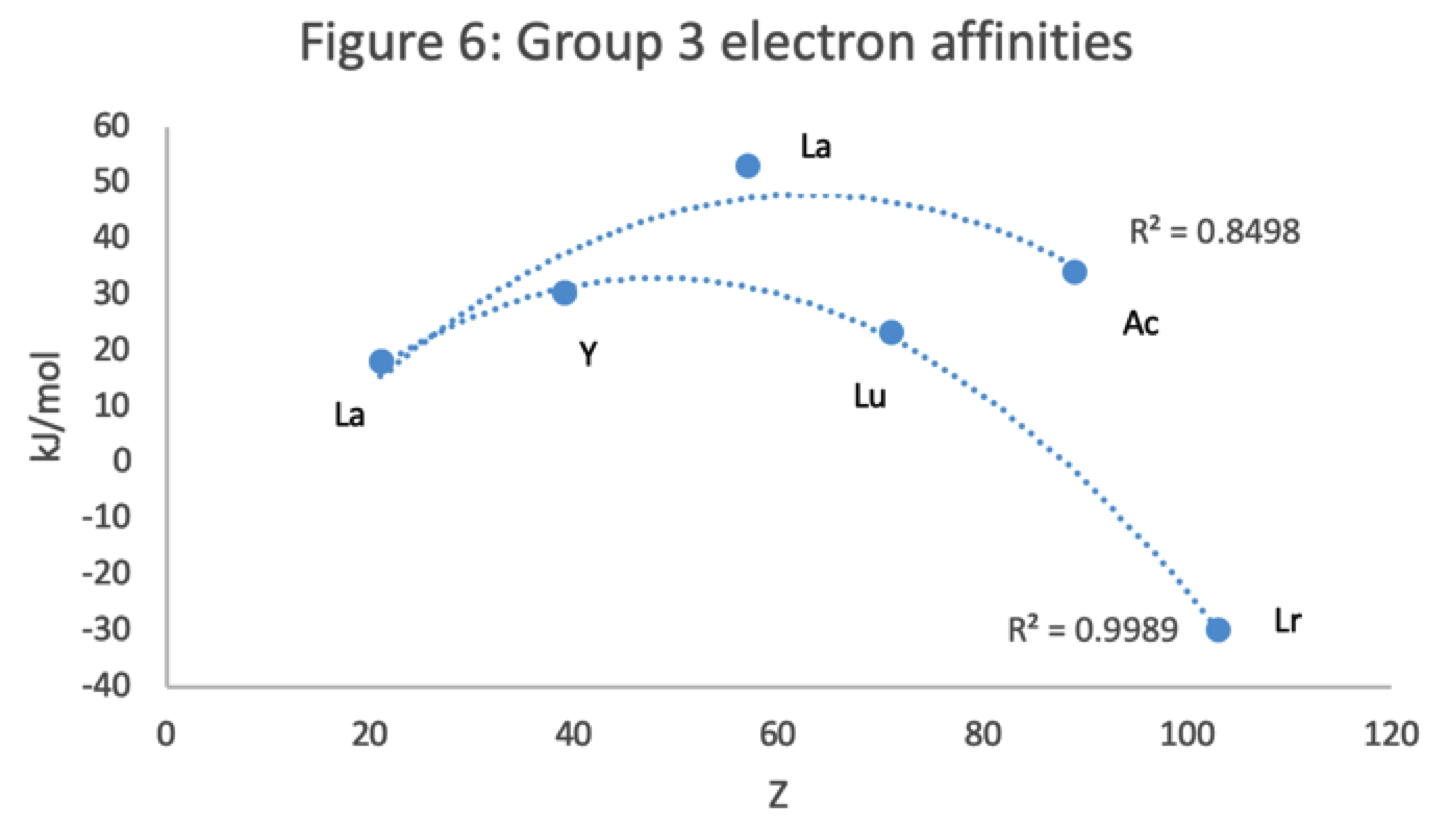
Figure 6 has a Z plot of electron affinity values bifurcating after Y into an -La-Ac tranche (R2 = 0.85) and a -Lu-Lr branch (0.99).[iii] The trendline supports Lu-Lr. The trend-lines by themselves are inconclusive: two show no difference; two support -La-Ac; two support -Lu-Lr.
Upon reviewing the data, René's comment is that: "The net result is that the two options seem inseparable" and he proposes that IUPAC adopt the following periodic table numbering system:
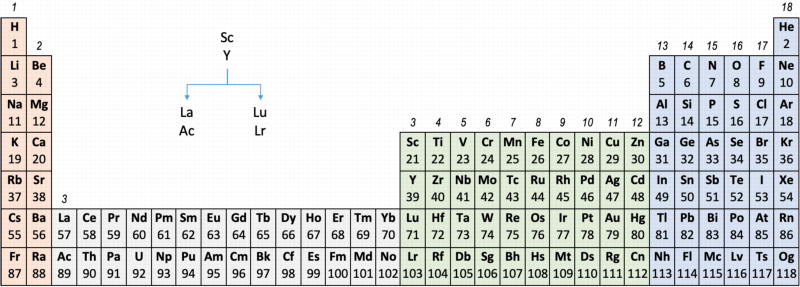
Professor Sir Martyn Poliakoff's [of the Periodic Videos YouTube channel & Nottiningham University] take on this matter:
| Year: 2019 | PT id = 1050, Type = formulation |
Janet Rejuvenated: Stewart-Tsimmerman-Nawa
An updated version of Philip Stewart's Janet Rejuvenated by Valery Tsimmerman redrawn by Nawa.
| Year: 2019 | PT id = 1053, Type = formulation |
Chavhan's Third Generation Periodic Table of the Elements
Randhir Bhavial Chavhan's Third Generation Periodic Table of the Elements poster, as presented 4th International Conference on Periodic Table at St. Petersburg, Russia.
Click here, or on the image, to enlarge:
| Year: 2019 | PT id = 1057, Type = misc review formulation |
Meyer's NYT Graphic
A nice graphic by Alex Eben Meyer in the New York Times accompanying an article about the periodic table and some of Sir Martyn Poliakoff ideas.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1059, Type = formulation review |
Where Mendeleev Was Wrong
A paper by Gábor Lente, Where Mendeleev was wrong: predicted elements that have never been found, from ChemTexts https://doi.org/10.1007/s40828-019-0092-5.
As is well known, Mendeleev sucessfully predicted the existance of several elements, but he was not always correct.
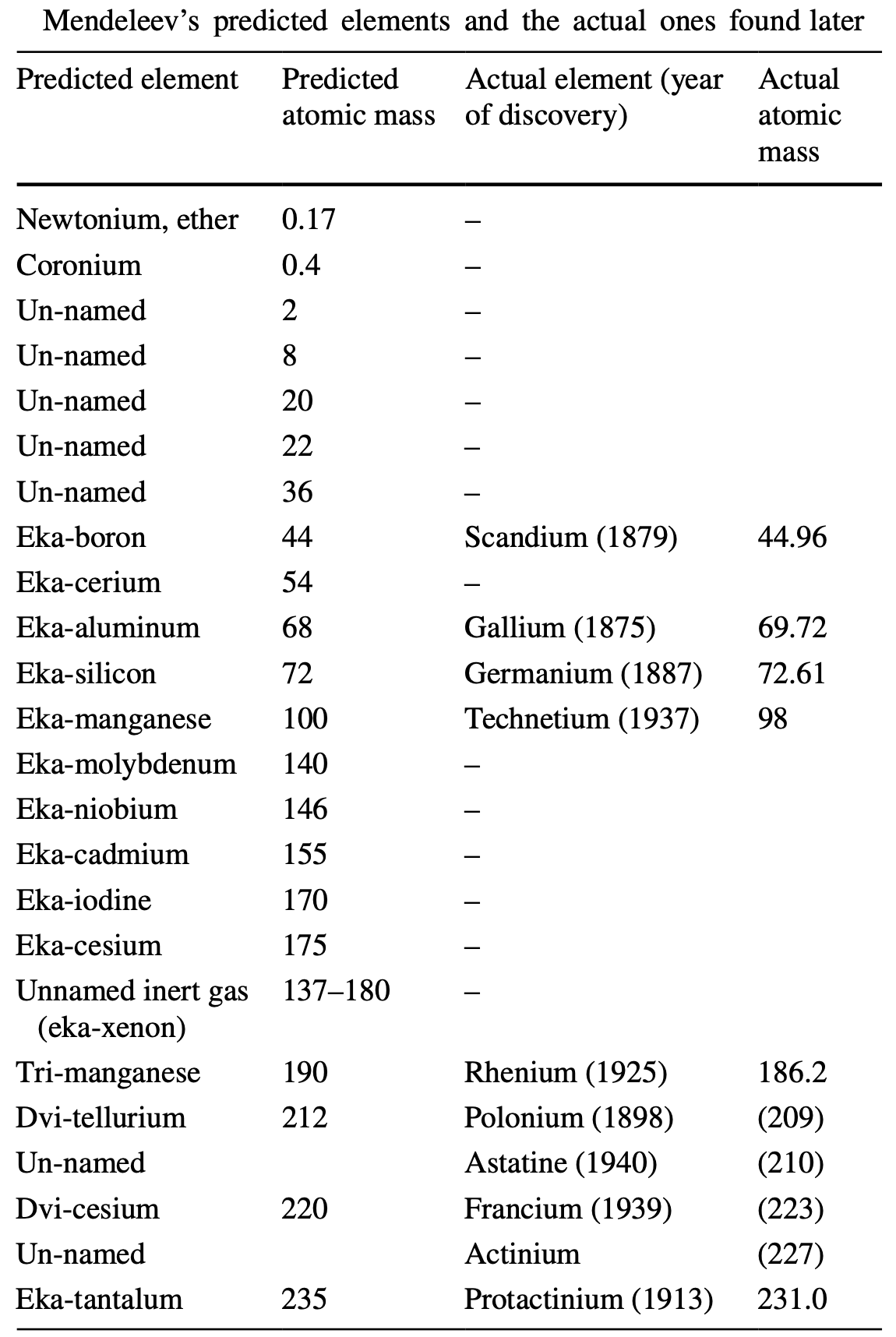
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1060, Type = data misc review |
Bloomberg Businessweek Special Issue: The Elements
A Bloomberg Businessweek Special Issue on The Elements.
Using state of the art [2019] web graphics, and packed with interesting business stories:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1061, Type = data review |
Bloomberg Businessweek: Why the Periodic Table of Elements Is More Important Than Ever
A Bloomberg Businessweek article on the chemical elements: Mendeleev's 150-year-old periodic table has become the menu for a world hungry for material benefits. (This story is from Bloomberg Businessweek's special issue The Elements.)
Thanks to Roy Alexander for the tip!
| Year: 2019 | PT id = 1064, Type = formulation |
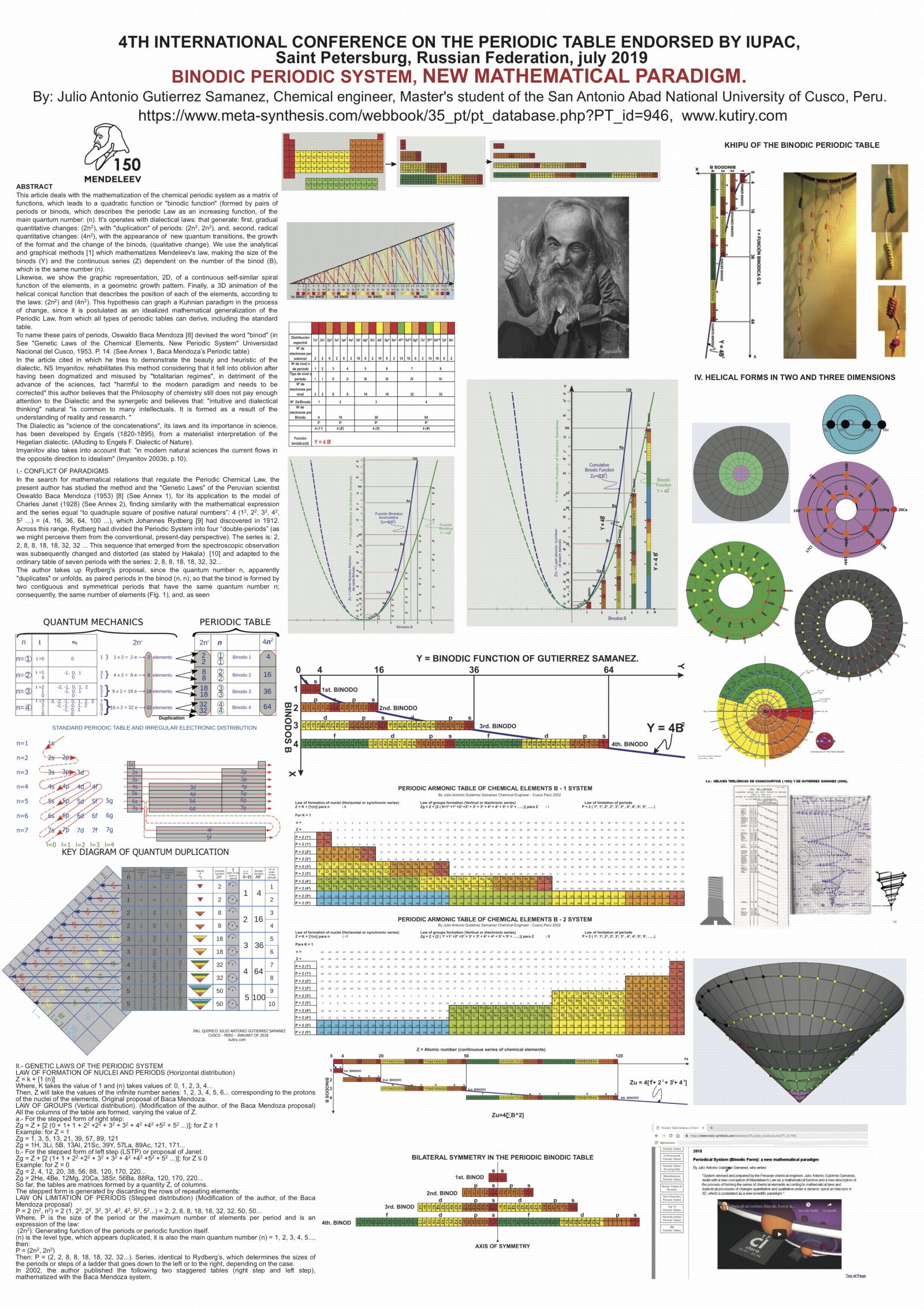
Samanez's Binodic Periodic System, New Mathematical Paradigm Poster
Julio Antonio Gutierrez Samanez (Master's student in chemical engineering at the San Antonio Abad National University of Cusco, Peru) presented a poster at the 4th International Conference on the Periodic Table arranged by IUPAC, Saint Petersburg, Russian Federation, July 2019. See the high resolution .PDF file.
More here and at kutiry.com
| Year: 2019 | PT id = 1065, Type = formulation review misc |
Mendeleev 150
Mendeleev 150 is the 4th International Conference on the Periodic Table. The event welcomed nearly 300 guests from over 30 countries and has become one of the key events of IUPAC's International Year of the Periodic Table.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1066, Type = formulation misc |
International Year of the Periodic Table with Eric Scerri
A YouTube video about IUPAC's International Year of the Periodic Table:
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1067, Type = formulation 3D spiral misc |
Scott Van Note Periodic Table Sculpture
On the Saatchi Art website, a 3D periodic table Sculpture by Scott Van Note.
Sculpture: Metal (Bronze). Ten made for the local ASM international chapter.
Loops and changes of direction show electron shell filling. S,P,D,F with S just a change of direction. Continuous spiral from top to bottom. New loops introduce as the electron shell would. Does not show the out-of-order shell filling.
Keywords: periodic, science, sculpture, functional, nerd
Thanks to Roy Alexander for the tip!
| Year: 2019 | PT id = 1068, Type = formulation review |
C&EN No Agreement
From C&EN: The periodic table is an icon. But chemists still can't agree on how to arrange it:
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1069, Type = formulation 3D |
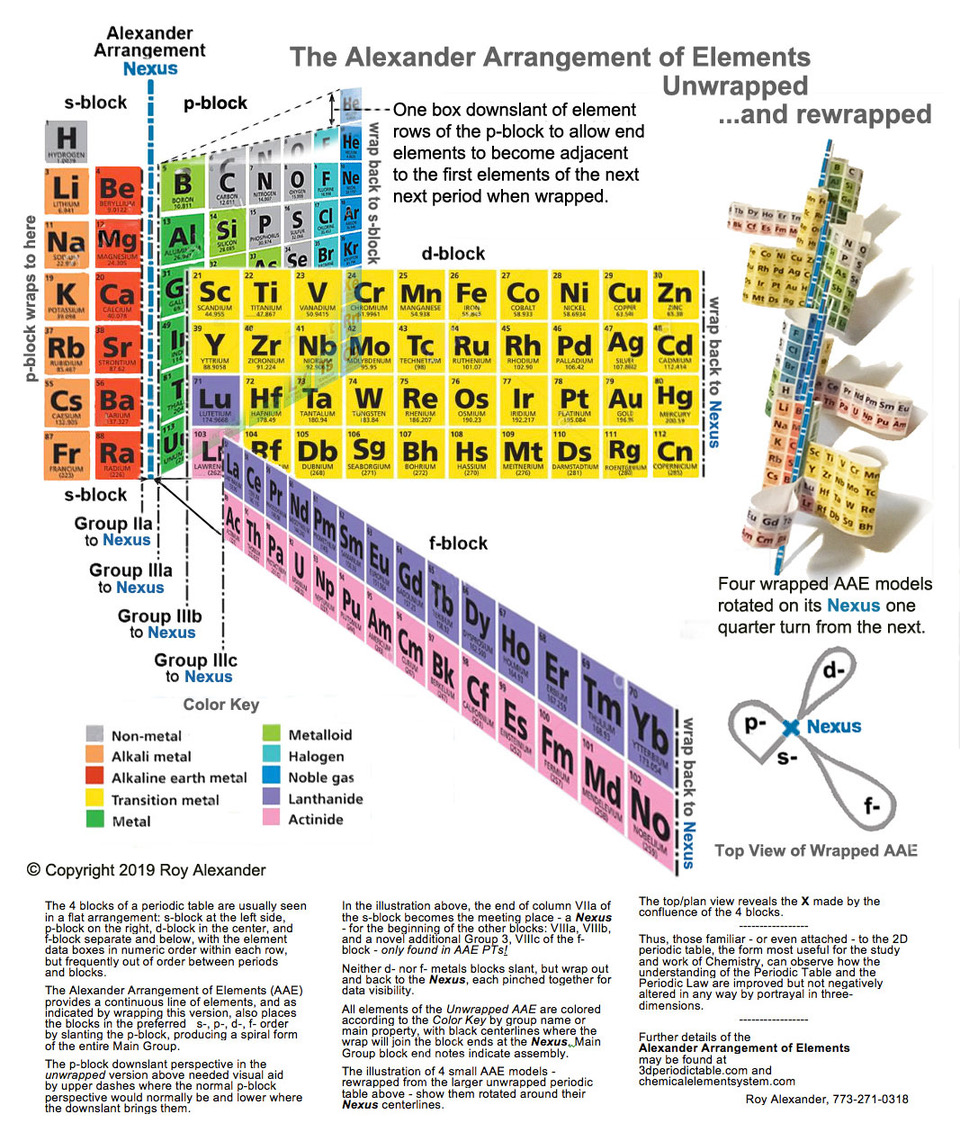
Alexander Arrangement Unwrapped... and Rewrapped
In mid-2019 Roy Alexander – of the Alexander Arrangement – produced an intriguing new formulation in sketch form that shows the p, d & f blocks moving away from the s block in three dimensional space:
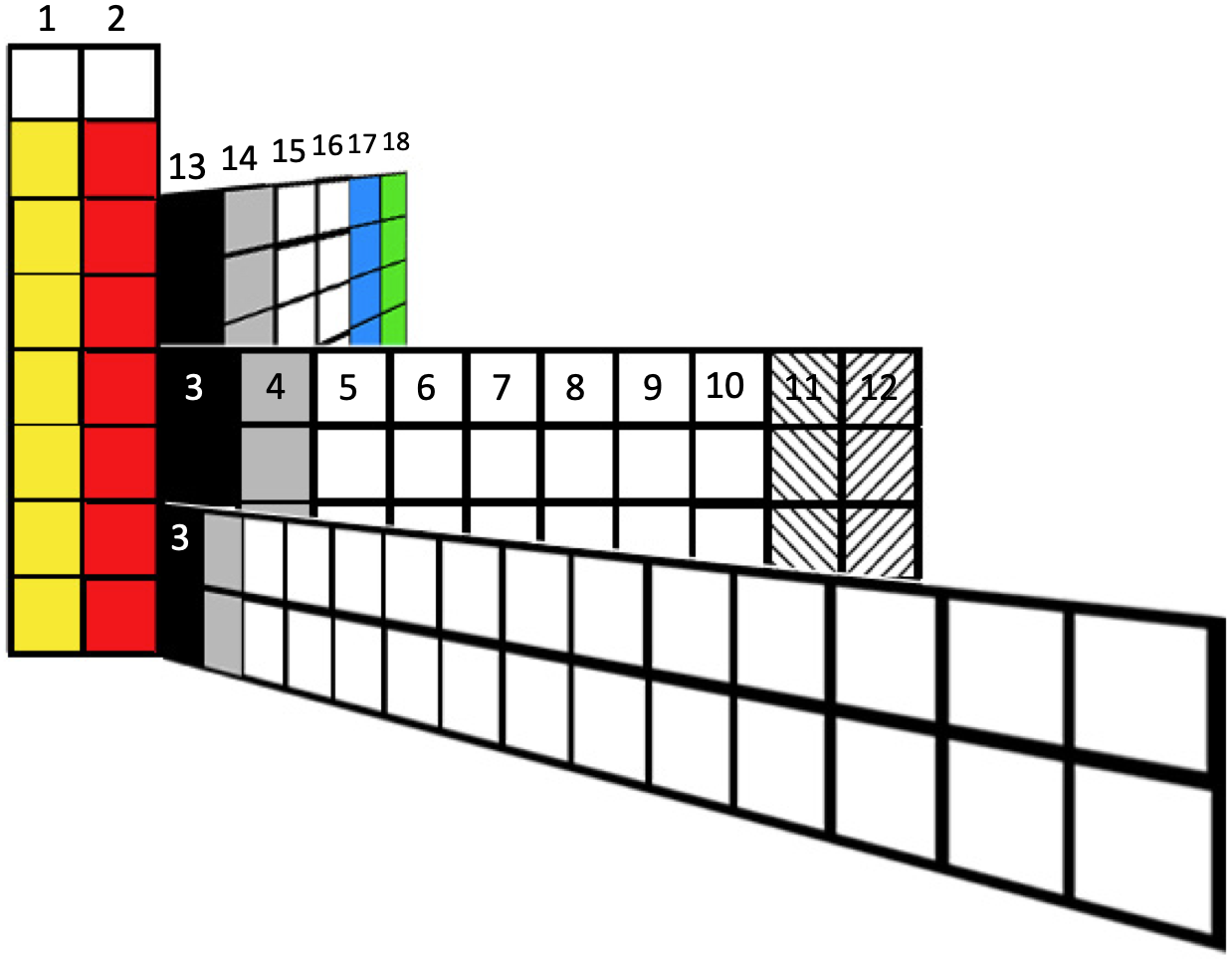
Roy has now expanded this into a full blown new formulation. (Click image to enlarge):
| Year: 2019 | PT id = 1071, Type = data |
Toma's Periodic Tables
Henrique E. Toma writes:
"I will be delighted if I could have a chance to contribute for the fantastic moment expressed by 2019 IYPT.
"I am senior Professor of Chemistry at the University of São Paulo, and great Periodic Table enthusiast since the beginning of my career about 50 years ago. This interest actually came from my supervisor and mentor, Professor Henry Taube (Nobel Prize, 1983), who taught me the beauty of the elements."
"As an inorganic chemist, I have been collecting the elements and minerals for a long time, and I built up the Periodic Table with the real elements shown below. It is one of the attractions of the campus, and has been reported in many publications1. It was visited by colleagues from IUPAC, including the President. I wouldn't be surprised if it inspired IUPAC the similar Table exposed in Paris, this year. The difference is that our table is that it also places the typical minerals together with the elements, and I believe that this is very important aspect for teaching and discussing the history behind them:

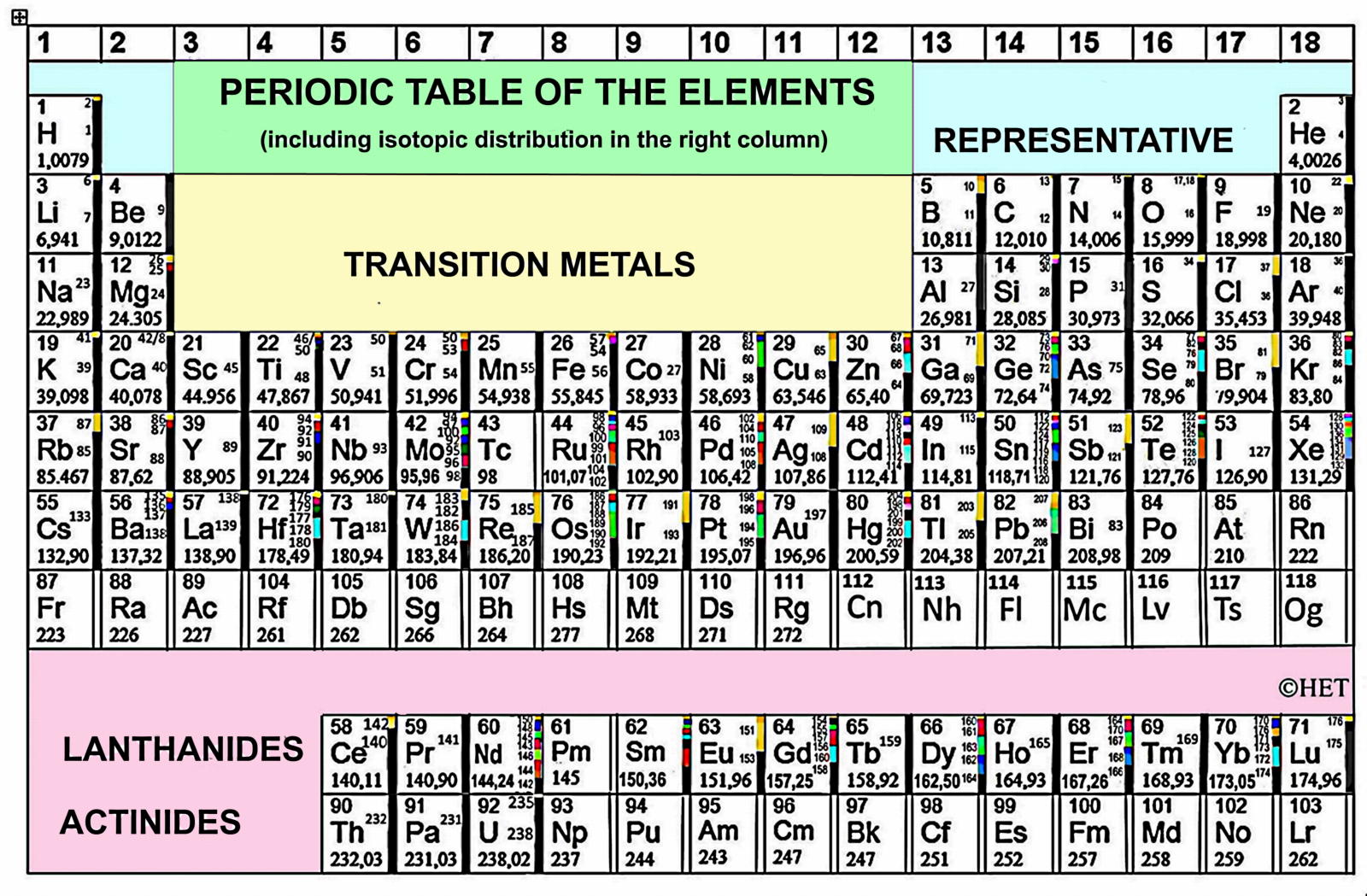
"Next, is my personal version of the IUPAC Periodic Table, shown in Figure 2, with the isotopes distributed in a column right to the element symbol. This Table is very practical, and particularly useful when you are dealing with mass spectrometry or isotopes. It is in my book of Elements2.
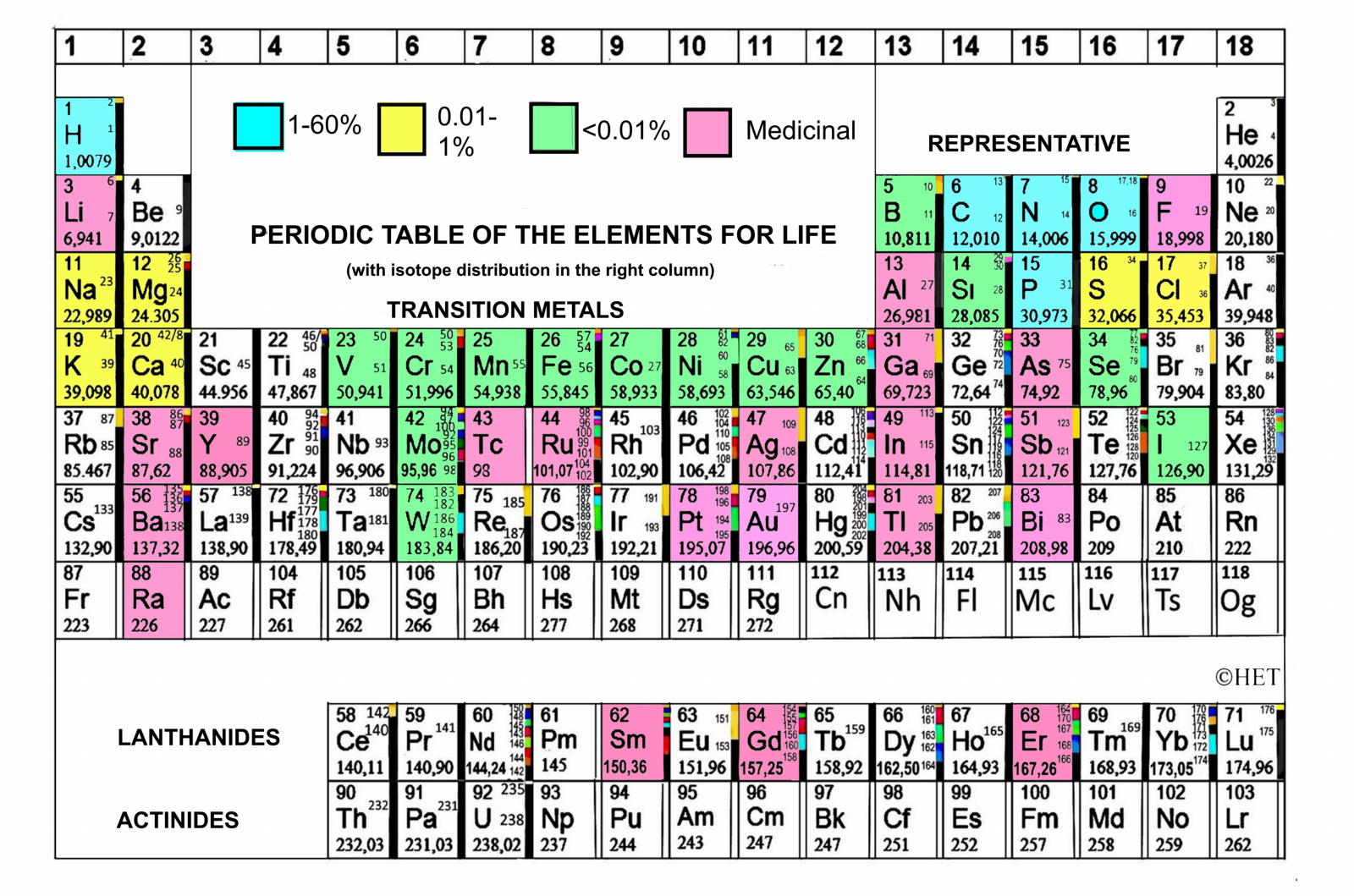
"Another is the Periodic Table of the Elements for Life, with the essential elements and abundance expressed by colors, including those used in medicine. This Table will be changing with the progress of Bioinorganic Chemistry, and is in my book of Bioinorganic Chemistry3.
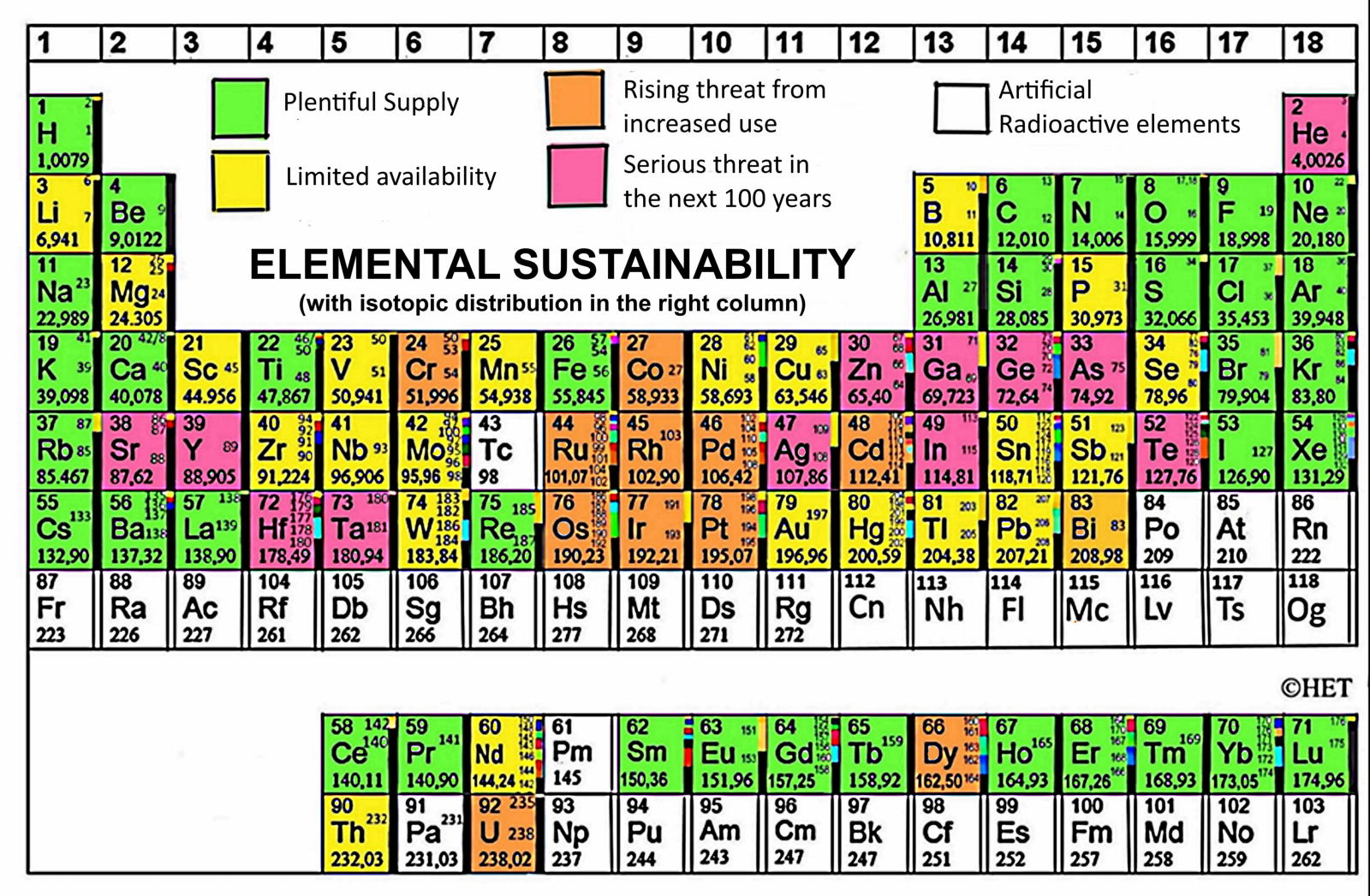
"Finally, I have adapted the periodic table of elemental sustainability, using the colors to call attention for this issue. In this form, it is can be more easily understood by the public. Elemental Sustainability is a very important issue, as discussed in Green Chemistry Journal4.
References:
- Toma, H. E. IYPT 2019 International Year of the Periodic Table of the Chemical Elements. Quimica Nova 42, 468–472 (2019).
- Toma, H. Estrutura atômica, ligações e estereoquímica. (Edgard Blucher, 2018).
- Toma, H. Química bioinorganica e ambiental. (Edgard Blucher, 2015).
- Toma, H. Green Processing of Strategic Elements Based on Magnetic Nanohydrometallurgy. Green Chem. 29, 948–959 (2015).
| Year: 2019 | PT id = 1073, Type = data |
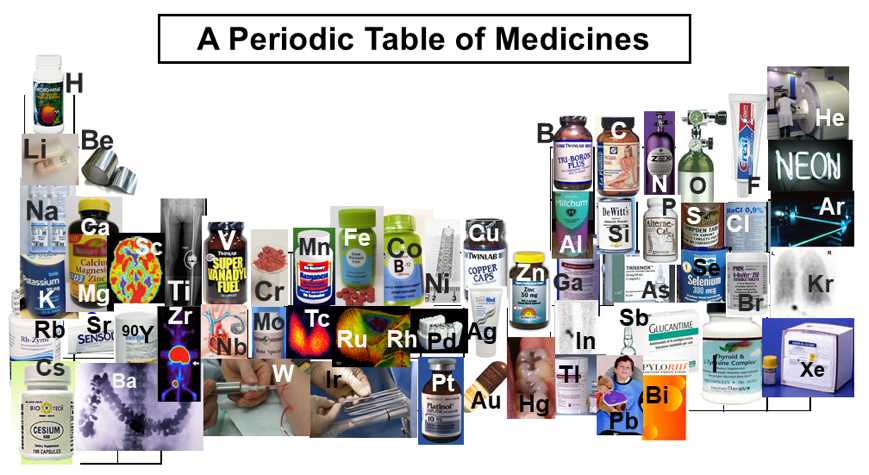
Medicines, Periodic Table of
From C. Van Cleave 1 and D. C. Crans, The First-Row Transition Metals in the Periodic Table of Medicine, Inorganics 2019, 7, 111 (Inorganics 2019, 7, 111; doi:10.3390/inorganics7090111, www.mdpi.com/journal/inorganics).
From the paper, specifically the text associated with the figure:
The periodic table with known medicinal uses of each main group or transition metal element when available. In the following, we list the use of each element.
- Hydrogen (H), boron (B), carbon (C), calcium (Ca), phosphorous (P), potassium (K), magnesium (Mg), vanadium (V), manganese (Mn), iron (Fe), cobalt (Co), copper (Cu), zinc (Zn), selenium (Se), rubidium (Ru), molybdenum (Mo), and cesium (Cs) are commonly found in supplements readily available to the public and are illustrated as such. Helium (He) is crucial in the operation of MRI machines.
- Lithium (Li) as lithium carbonate is the most common treatment of bipolar disorder.
- Beryllium (Be) foil is used as shielding in radiographic instruments.
- Nitrogen (N), as nitrous oxide, is a common anesthetic.
- Oxygen (O) has many medical uses, including anesthetics and resuscitation, and is illustrated here for use in ventilation.
- Fluorine (F) and tin (Sn) as stannous fluoride are a common ingredient in toothpaste.
- Sodium (Na) and chlorine (Cl) are used as NaCl in saline solutions.
- Aluminum (Al) compounds are a common active ingredient in antiperspirant deodorants.
- Silicon (Si) is used in antacid products.
- Sulfur (S) is illustrated as campden tablets, which are used for sterilization in beer fermentation.
- Argon (Ar) lasers are used in eye surgery.
- Zirconium (Zr) is used in immuno-positron emission tomography (PET) imaging while scandium (Sc) is a candidate for the same technique.
- Titanium (Ti), palladium (Pd), niobium (Nb), nickel (Ni), and tantalum (Ta) are used in medical implants.
- Chromium (Cr) is shown as Cr(III) picolinate, which is a controversial supplement used in lowering insulin resistance.
- Gallium (Ga), yttrium (Y), technetium (Tc), lanthanum (La), astatine (At), and actinium (Ac) are all used in nuclear medicine.
- Arsenic (As), as As(III) trioxide, is used to treat certain forms of leukemia.
- Bromine (Br) as KBr is an active ingredient in canine seizure medication.
- Krypton (Kr) was used in lung ventilation studies but has since been phased out.
- Strontium (Sr) is used in Sensodyne® toothpaste.
- Rhodium (Rh), ruthenium (Ru), and rhenium (Re) complexes are used as anticancer agents.
- Silver (Ag) is used in antibacterial ointments.
- Indium (In) is used in white blood cell scans.
- Antimony (Sb) is used in leishmania medicine.
- Barium (Ba) is used in X-ray imaging of the gastrointestinal tract.
- Tungsten (W) is used in shielded syringes.
- Iridium (Ir) is used in brachytherapy.
- Gold (Au) was used as a treatment for rheumatoid arthritis but has been phased out.
- Mercury (Hg) is used in dental amalgams.
- Lead (Pb) is used in X-ray aprons.
- Bismuth (Bi) is used in stomach ulcer medicine.
- Neon (Ne), germanium (Ge) cadmium (Cd), tellurium (Tl), hafnium (Hf), osmium (Os), polonium (Po), francium (Fr), radon (Rn), and radium (Ra) although most of these are toxic elements for human life, some of these elements are under development as potential agents for disease treatment but to our knowledge they are not currently used for beneficial applications in medicine.
| Year: 2019 | PT id = 1077, Type = formulation 3D spiral |
Weise's Tetrahedral Periodic Table
A Facebook video by Dmitry Weise showing how the conventional periodic table can be morphed into a tetrahedral formulation via the Janet Left Step:
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1083, Type = formulation 3D |
Cylindrical Periodic Table of Elements
Two YouTube videos by Takehiko Ishiguro (original & updated): Cylindrical Periodic Table of Elements.
Three types of the cylindrical periodic table of elements are demonstrated with a rotating table. Comments on them are given at the end of the video (in English).
| Year: 2019 | PT id = 1084, Type = review |
Scerri's The Periodic Table: Its Story & Its Significance 2nd Edition
The 2nd Edition of Eric Scerri's well regarded book, The Periodic Table: Its Story & Its Significance has been published by Oxford University Press and is available at all good bookshops, including online.
See Eric's website EricScerri.com and Twitter Feed.


| Year: 2019 | PT id = 1085, Type = data |
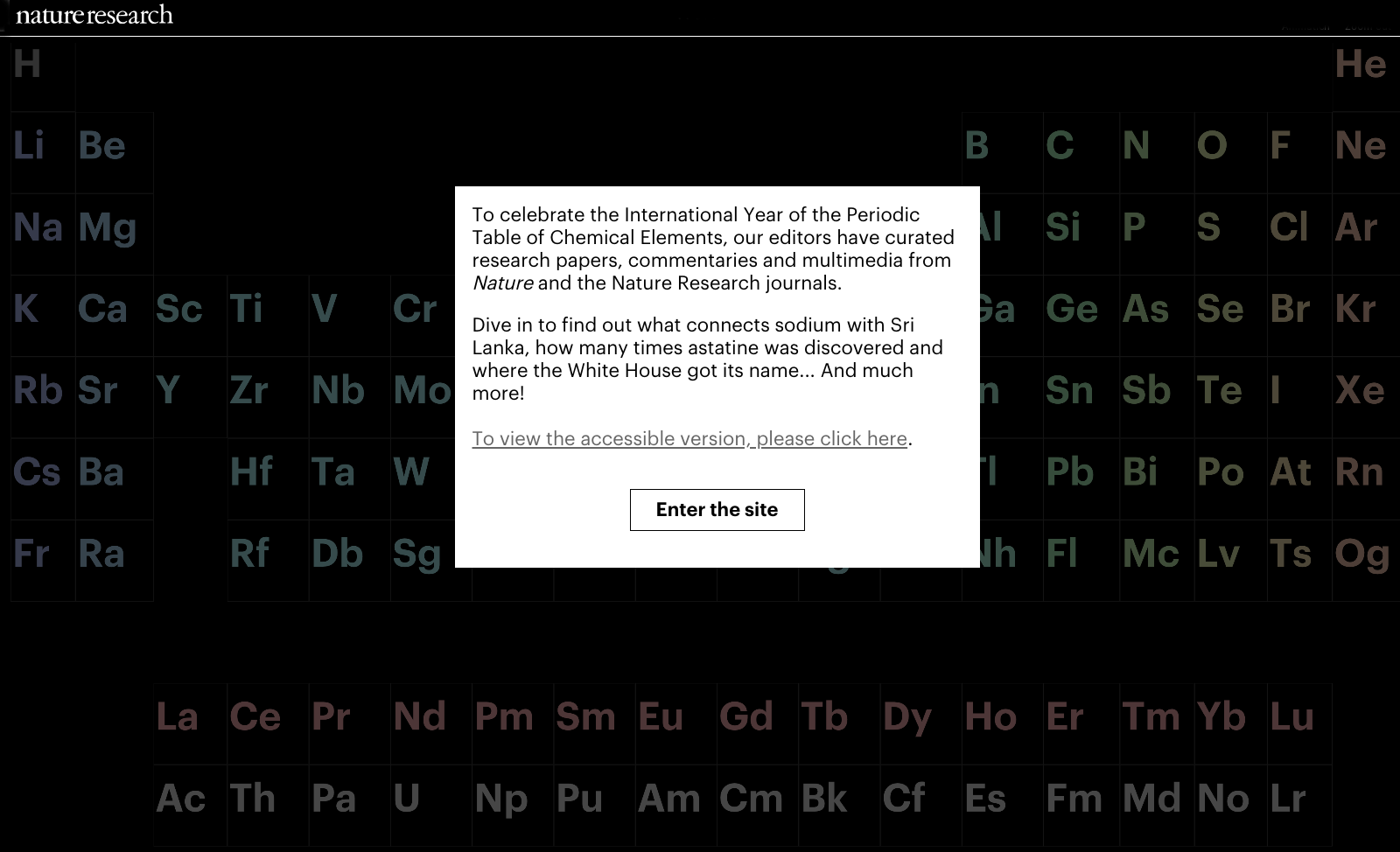
Nature's IYPT Interactive Periodic Table
Nature's IYPT Interactive Periodic Table.
"To celebrate the International Year of the Periodic Table of Chemical Elements, our editors have curated research papers, commentaries and multimedia from Nature and the Nature Research journals. Dive in to find out what connects sodium with Sri Lanka, how many times astatine was discovered and where the White House got its name... And much more!"
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1089, Type = data misc |
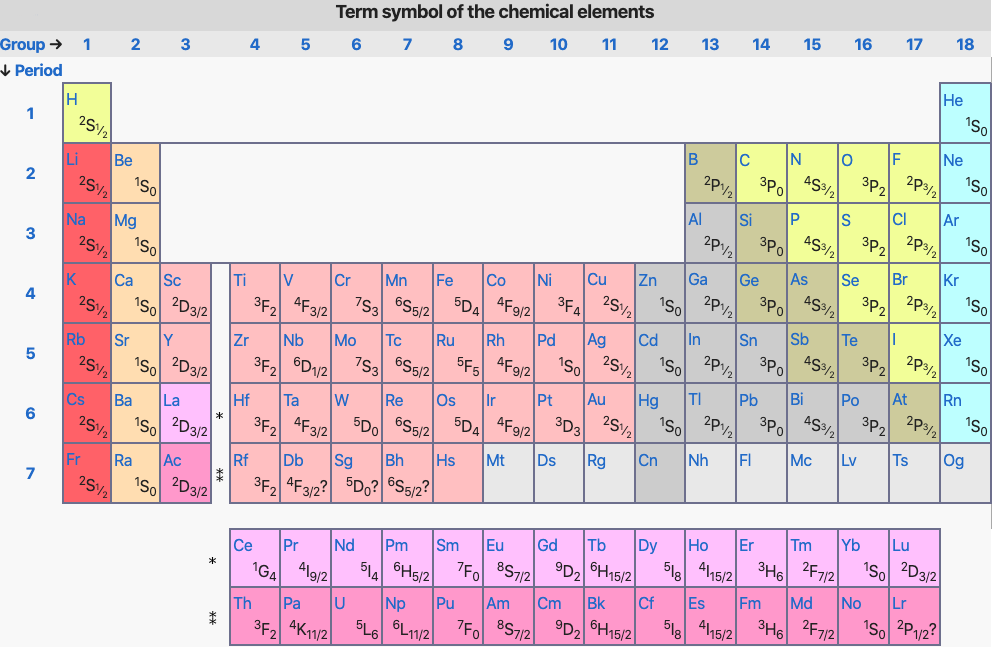
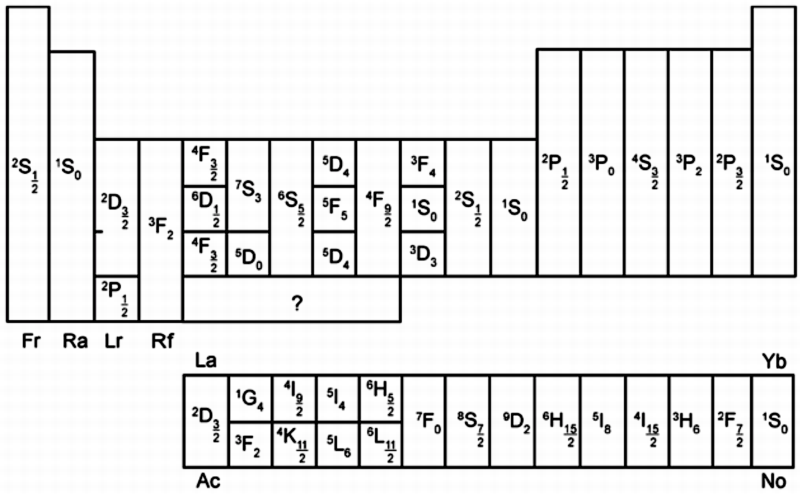
Term Symbol of the Chemical Elements
From Wikipedia (edited):
In quantum mechanics, the term symbol is an abbreviated description of the (total) angular momentum quantum numbers in a multi-electron atom. Each energy level of an atom with a given electron configuration is described by not only the electron configuration but also its own term symbol, as the energy level also depends on the total angular momentum including spin. The usual atomic term symbols assume LS coupling (also known as Russell-Saunders coupling or spin-orbit coupling). The ground state term symbol is predicted by Hund's rules.
Neutral atoms of the chemical elements have the same term symbol for each column in the s-block and p-block elements, but may differ in d-block and f-block elements, if the ground state electron configuration changes within a column.
For (far more) information refer to the Wikipedia Term Symbol page.
And from T.F. Yen, Chemistry for engineers, Imperial College Press, London, p. 53 (2008)
| Year: 2019 | PT id = 1090, Type = misc |
Elements Song 2019
From the Chemistry World YouTube channel: The Elements Song 2019
Join Helen Arney, the Waterbeach Brass Band and chemists from around the world in an updated version of Tom Lehrer's Elements Song:
| Year: 2019 | PT id = 1094, Type = formulation data review |
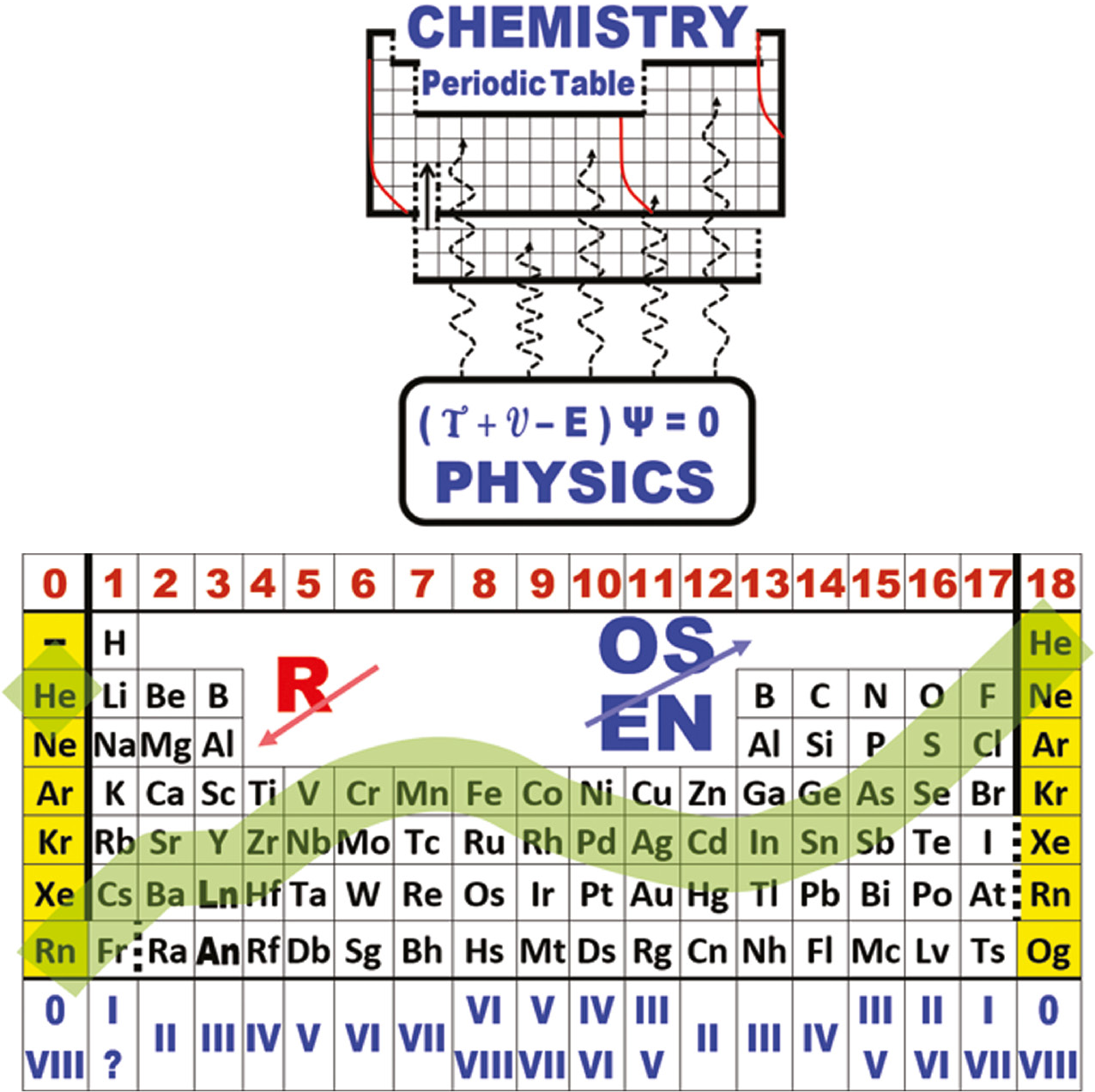
Physical Origin of Chemical Periodicities in the System of Elements
From de Gruyter: Physical origin of chemical periodicities in the system of elements, Chang-Su Cao, Han-Shi Hu, Jun Li* and W. H. Eugen Schwarz*, Pure Appl. Chem. 2019; 91(12).
Published Online: 2019-11-30 | DOI: https://doi.org/10.1515/pac-2019-0901 (open access)
Abstract:
The Periodic Law, one of the great discoveries in human history, is magnificent in the art of chemistry. Different arrangements of chemical elements in differently shaped Periodic Tables serve for different purposes. "Can this Periodic Table be derived from quantum chemistry or physics?" can only be answered positively, if the internal structure of the Periodic Table is explicitly connected to facts and data from chemistry.
Quantum chemical rationalization of such a Periodic Tables is achieved by explaining the details of energies and radii of atomic core and valence orbitals in the leading electron configurations of chemically bonded atoms. The coarse horizontal pseudo-periodicity in seven rows of 2, 8, 8, 18, 18, 32, 32 members is triggered by the low energy of and large gap above the 1s and nsp valence shells (2 ≤ n ≤ 6 !). The pseudo-periodicity, in particular the wavy variation of the elemental properties in the four longer rows, is due to the different behaviors of the s and p vs. d and f pairs of atomic valence shells along the ordered array of elements. The so-called secondary or vertical periodicity is related to pseudo-periodic changes of the atomic core shells.
The Periodic Law of the naturally given System of Elements describes the trends of the many chemical properties displayed inside the Chemical Periodic Tables. While the general physical laws of quantum mechanics form a simple network, their application to the unlimited field of chemical materials under ambient 'human' conditions results in a complex and somewhat accidental structure inside the Table that fits to some more or less symmetric outer shape. Periodic Tables designed after some creative concept for the overall appearance are of interest in non-chemical fields of wisdom and art.
| Year: 2019 | PT id = 1095, Type = formulation data |
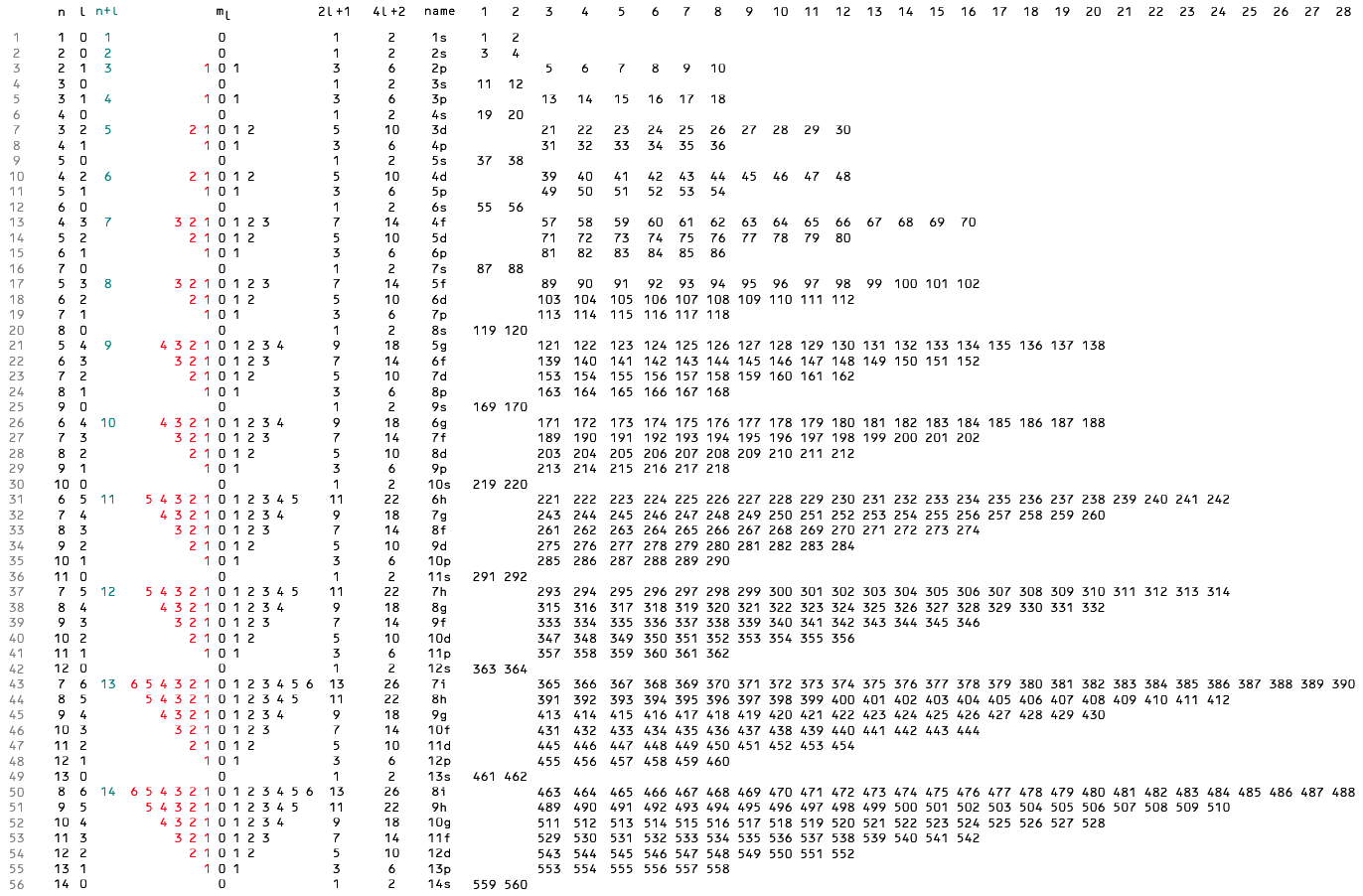
Global Periodic Table
Brian Gregory of keytochemicstry.com presents a Global Periodic Table.
The figure below shows the subshell strings aligned in columns based on the total pool of valence electrons as described above. This is the global periodic table. Each column constitutes a global group. Each term in column 1 launches a global period. The global periodic table is a purely mathematical matrix that assigns precise row and column coordinates to all positive integers but makes no predictions as to the order in which subshells are filled. Read more here.
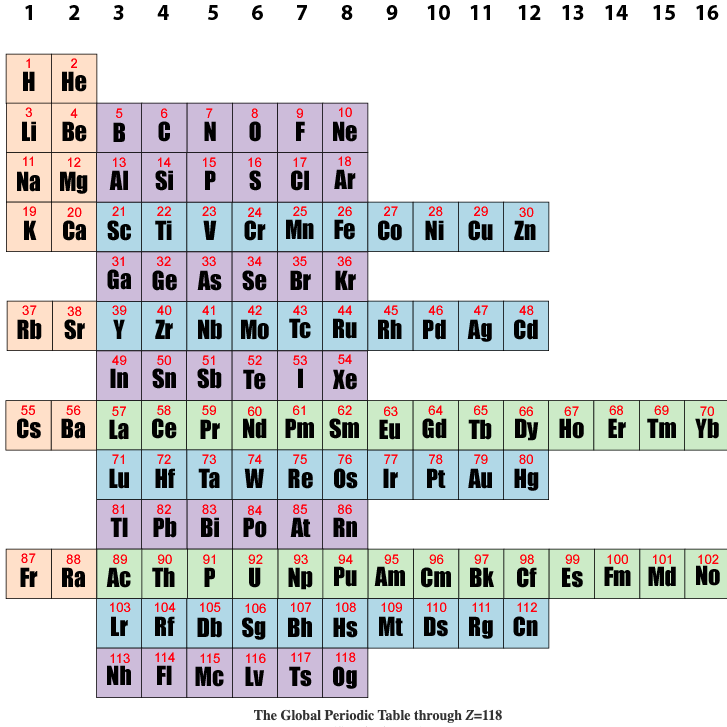
| Year: 2019 | PT id = 1099, Type = formulation |
Colburn's 2019 Periodic Table of The Elements
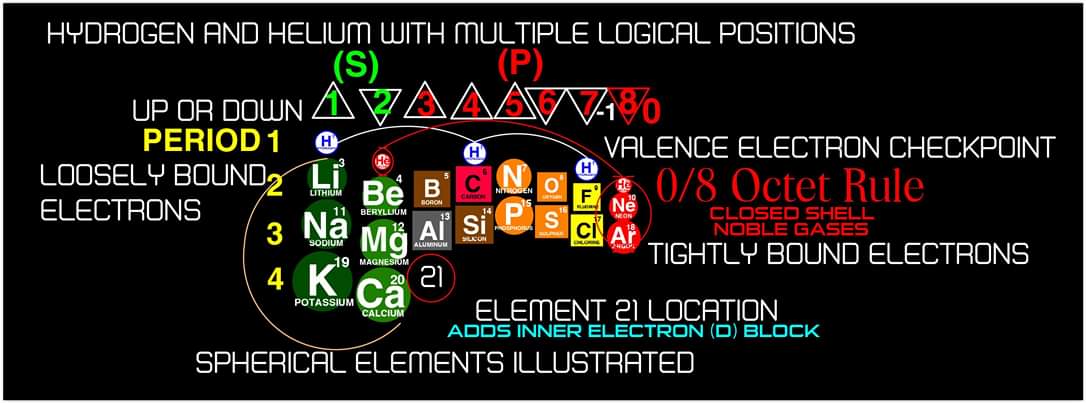
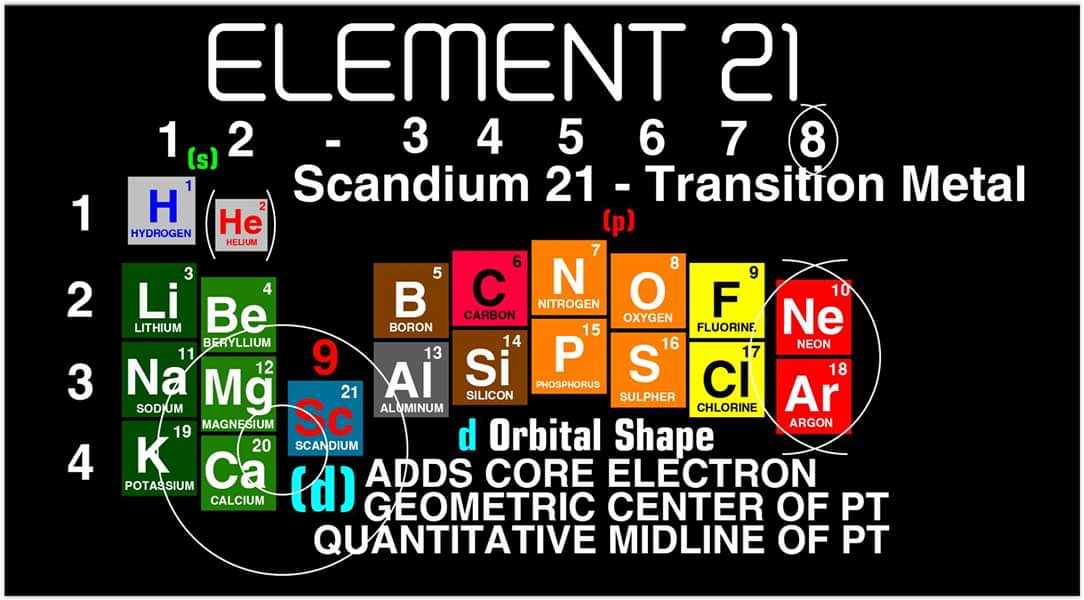
Justin Lee Colburn writes:
"What is unique to my Periodic Table is the fact that any Elements Electron Spin can be identified for Orbital Diagrams using a technique I have called 'Element Shifting'.
"Elements with an Up spin Valence Electron are shifted up and Elements with a down spin are shifted down. Also, the Hund's rule Exceptions are Highlighted in the Transition Metals so their orbital diagrams can also be identified easily.
"In addition, an accurate numbering system can be applied to all the elements with Helium placed in Group 2 instead of Group 18. I believe that quantitative data should take priority when giving elements their position, but this system is meant to be dynamic rather than static. In my Periodic Table System, (1-8) corresponds to Valence Electrons in the s and p orbitals and then the 9-18 and 19-32 corresponds to core electron in d and f orbitals .
I believe that it is important to begin by showing students the first 20 Elements FIRST because they all add Outer Valence Electrons which makes the Periodic Table logic easy to follow. Also explaining that Hydrogen and Helium are anomalies with more than one logical position, can really help clear up confusion for new students.
"After element 20, the Transition Elements such as scandium 21 begin adding core electrons in the d orbital the current standard (1-18) numbering system does not reflect this. One of the reasons why I prefer keeping the s and p block elements on the outside of the table and the f and d block elements on the inside is because of how they add electrons to their orbitals.
"I have been developing a curriculum based on this system that I believe will help students learn and understand the logic and trends of The Periodic Table more efficiently than the standard. Rather than memorizing Element information, Students will truly be able to follow the logic based on the location of the Elements, simple counting and using the numbering system."
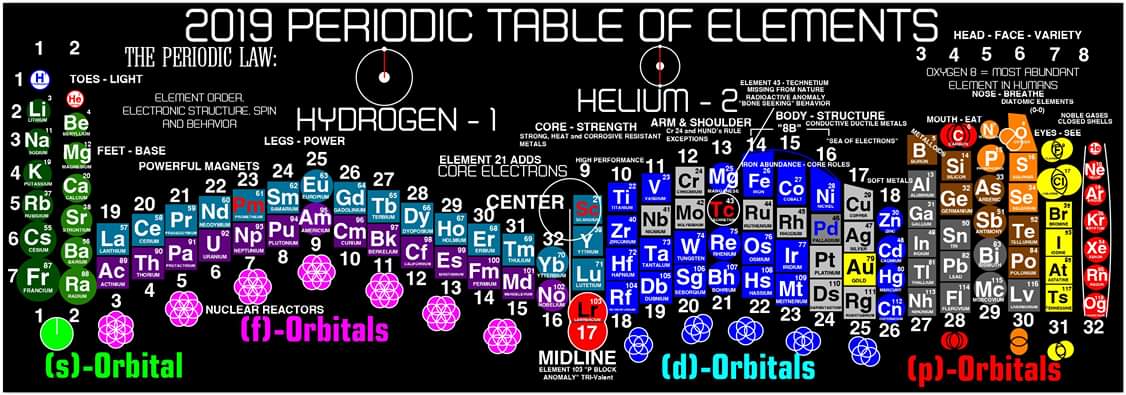
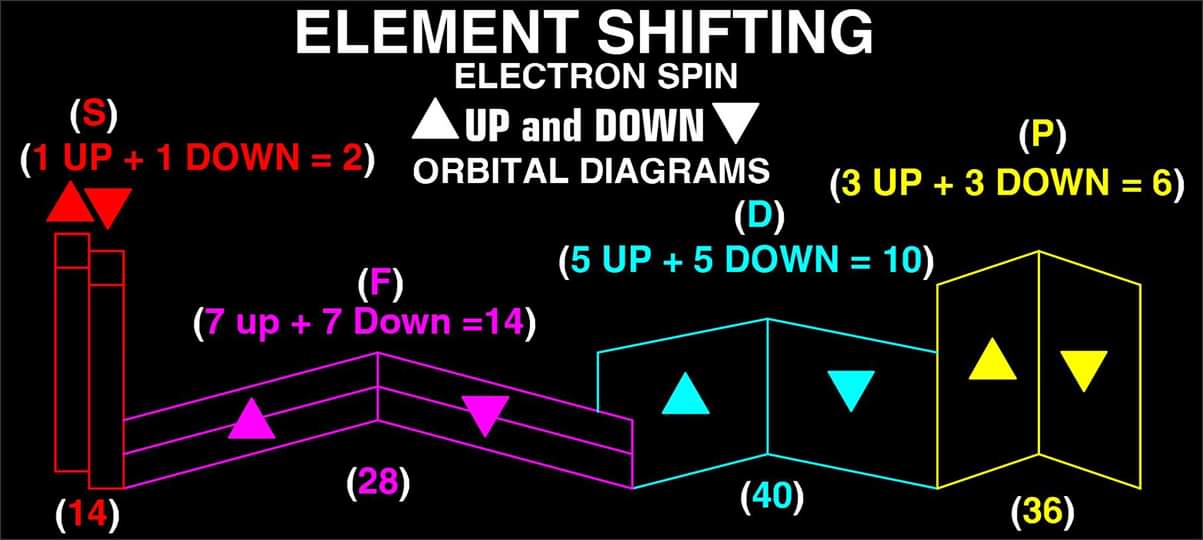
| Year: 2019 | PT id = 1101, Type = review formulation |
International Year of the Periodic Table with Eric Scerri
Interview with leading expert on the periodic table and UCLA professor, Eric Scerri, to celebrate the International Year of the Periodic Table.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1109, Type = misc |
Students, Periodic Table of
Translated from Catalan: Representation of the periodic table made by the students of Baccalaureate of the INS Santa Coloma de Farners in commemoration of the international year of the Periodic Table (2019). Photo: Emma Masó

Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1124, Type = formulation misc |
Elements & Anti-Elements (Atom-to-Adam)
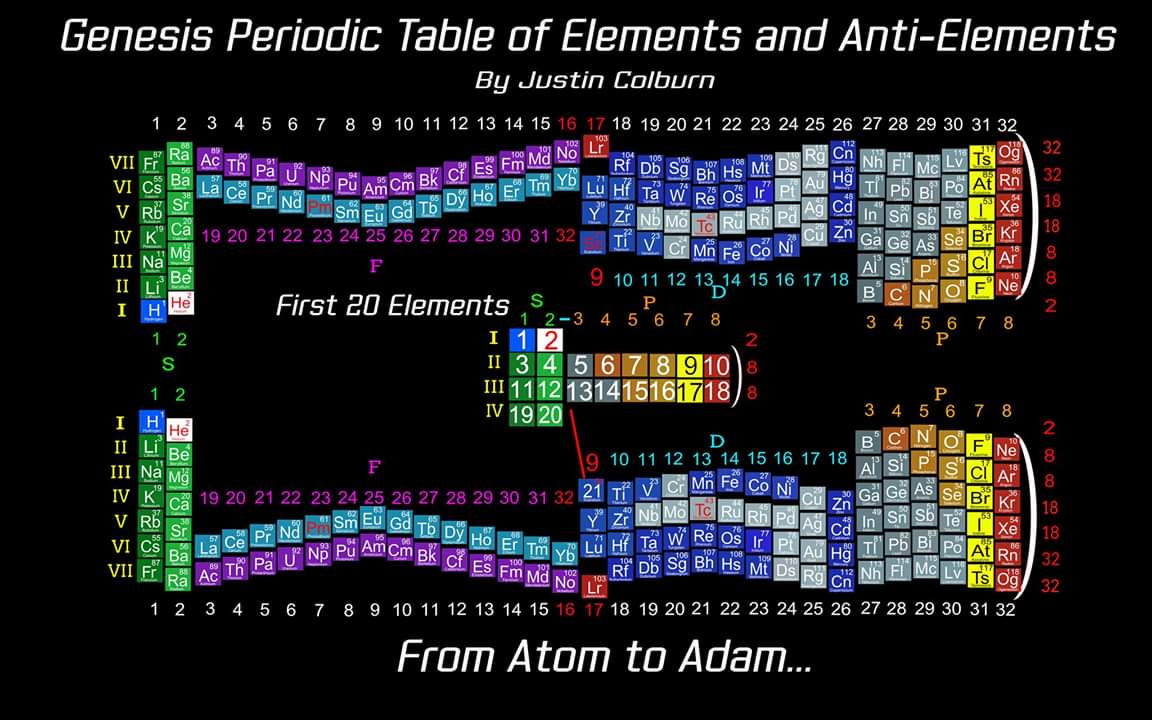
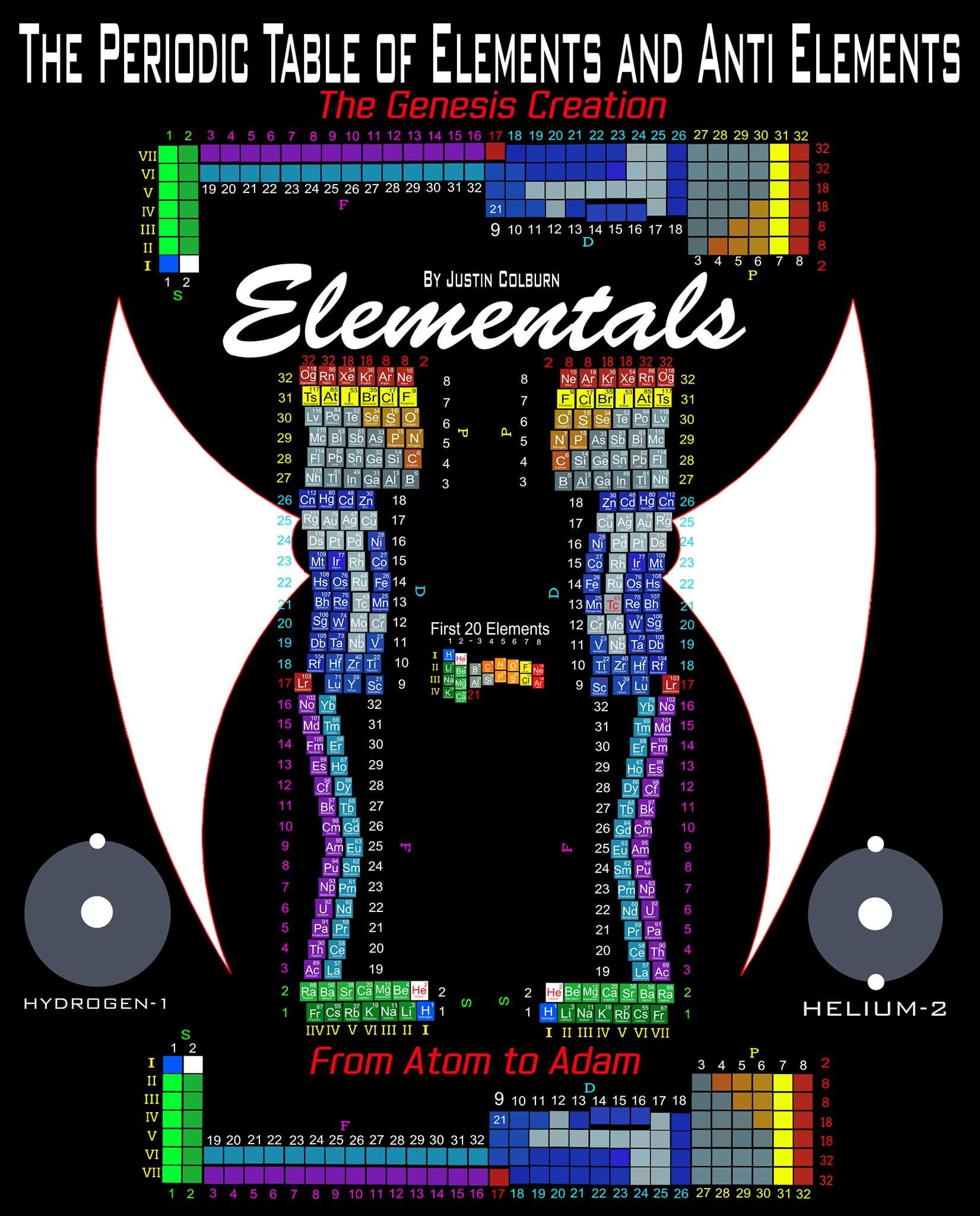
"The underlying order of the Atoms and asking the question of Intelligent Design has inspired my work. I have developed a new and improved Periodic Table of Elements that restores the Lanthanides and Actinides in their proper positions while also applying a complete and accurate numbering system. (1-8) numbering in s and p blocks correspond to Valence Electrons with (9-18) and (19-32) corresponding to core Electrons in the d and f block Elements.
"I have also inverted the Periodic Table of Elements which reflects the "missing" Anti Matter of our Universe. Unique to my Periodic Table of Elements is the ability to easily identify any Elements Electron Spin for Orbital Diagrams by shifting Elements up or down. Lastly I have highlighted some of the AUFBAU Exceptions or Electron Configuration anomalies in the transition metals. Interestingly, standing the Periodic Tables upright resembles the image of human beings, hence the title of my project, from Atom to Adam."
| Year: 2019 | PT id = 1125, Type = formulation |
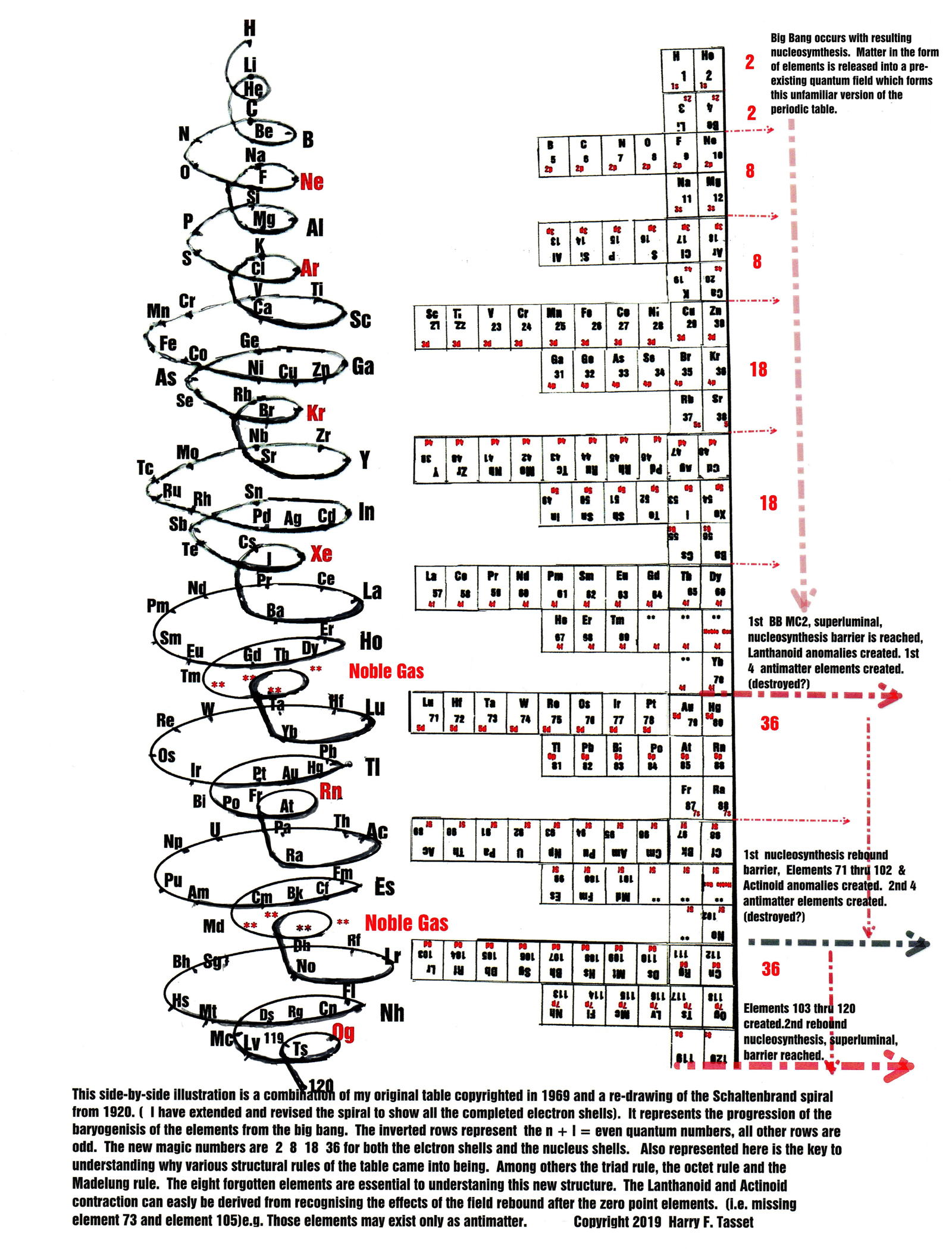
Tasset's Version of Schaltenbrand
Tasset's version of Schaltenbrand's Helical Periodic Table
Harry F. Tasset writes:
"I am very happy to be able to submit a periodic table that represents the culmination of many years of study. Much of my creative inspiration came from my father Everett and my two chemist brothers, Carl and Emmett. Also, I must mention Isaac Asimov.
"I have considered the gravity of this release and believe this is for the betterment of mankind. It is a moot point at this stage to regret the mistakes made in the 1930's when the Lanthanoids and Actinoids were separated, like the middle of a tree magically suspended to its side. Suffice it to say that the table was meant to be a complete whole."
Click the image to enlarge
| Year: 2019 | PT id = 1127, Type = data misc |
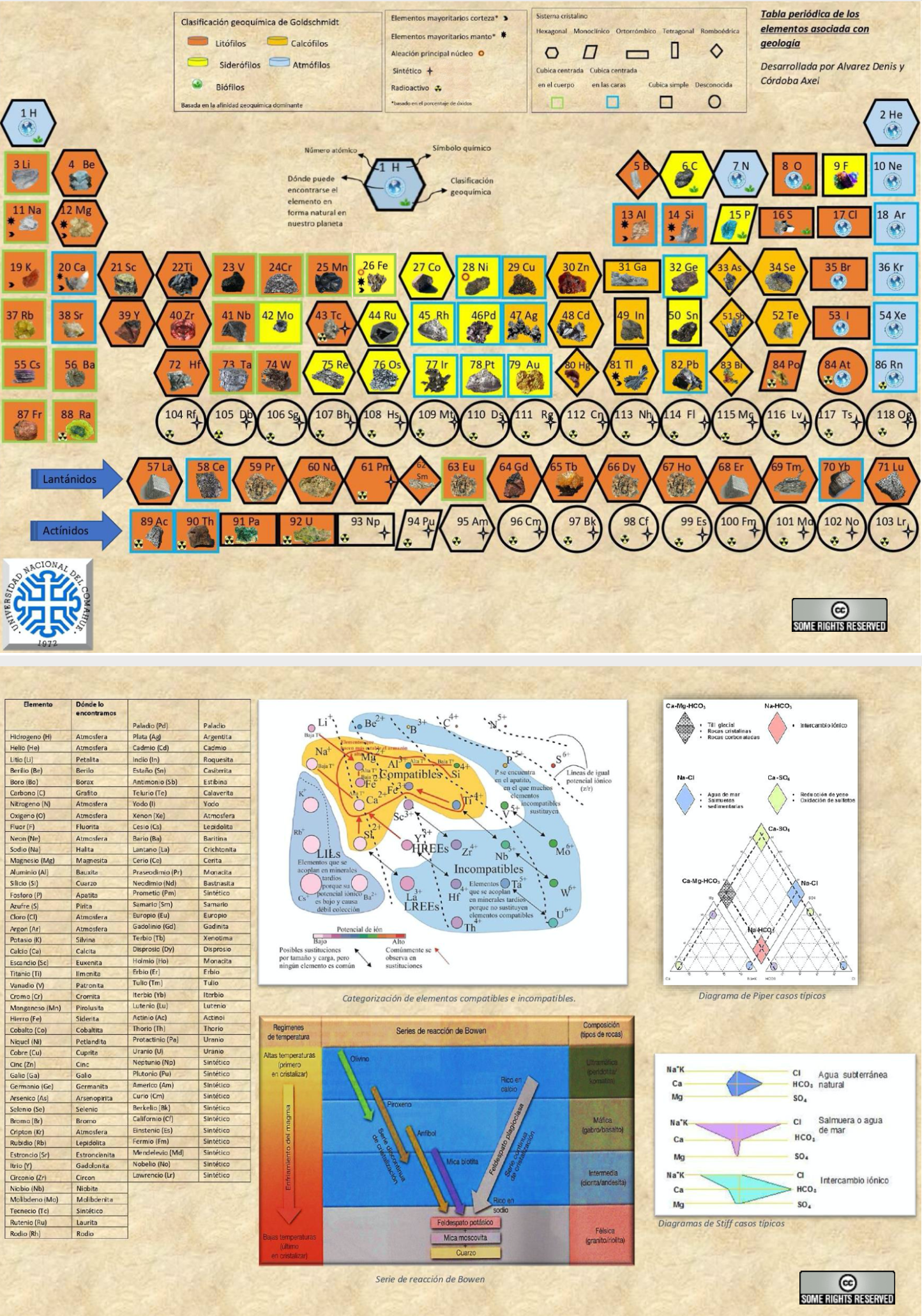
Geological Periodic Table
Alvarez & Cordoba's Periodic Table of the Elements Associated with Geology [from Spanish using Google Translate]
"It is a simple and innovative table where each element has the shape of its respective crystalline system. It also has several novelties linked to earth sciences such as: illustrative images that show where the element can be found naturally on our planet, geochemical classification and different types of relevant characterizations (radioactivity, synthetics, alloys, majority elements in bark and mantle). Likewise, various useful tools were included in the area such as the well-known Bowen series, categorizations of compatible and incompatible elements, typical cases of the Piper diagram and Stiff diagrams.
"To increase the interaction and understanding between the user and the table, it has elements external to it (letters) that incorporate augmented reality, which allows learning in a simpler, didactic and entertaining way about the atomic structure of chemical elements in 3D. Just scan the back of the letter with your cell phone to see its structure."
Click the image to see the PDF file
| Year: 2019 | PT id = 1128, Type = review |
Elemental Podcast
Radio New Zealand Podcast: Elemental, A journey through the periodic table of elements with chemistry professor Allan Blackman, from AUT, and Alison Ballance
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1133, Type = formulation data |
Vernon's Oxidation Number Periodic Table
René Vernon's periodic table showing oxidation number trends.
René writes:
- This table is based on two tables by Fernelius (1986), one of which is a portrait version of Bohr's 1922 table, and the second of which is a conventional table highlighting oxidation number trends in groups 4 to 10, and most of the p-block. Ref. Fernelius WC , 'Some reflections on the periodic table and its use', Journal of Chemical Education, vol. 63, no. 3, pp. 263–266 (1986).
- In my table, the transition metals are in groups 4 to 11. As per Mark's Leach's email: "...It's the 'incomplete d-subshell' that gives rise to properties such as: variable oxidation state, catalytic behaviour, d-orbital splitting and [thus] coloured ions/compounds."
- I've included oxidation number details for some elements. I've tried a different way of showing the composition of a bifurcated group 3, which is more in keeping with Bohr's table.
- The horizontal bar of the "T" is for Sc → Ti; the downward bar is for Y → Lu-Lr.
- Thus, Group 3 as Sc-Y-Lu-Lr could be called group 3T.
- La-Ac and Lu-Lr are duplicated in a greyed-out style to make it clearer where the lanthanides and actinides fit into the main body of the table.
- The inner transition metals are clearly delineated. Analogously to the transition metals they're all capable of forming ions with incomplete f sub-shells.
- The early actinides resemble the transition metals.

| Year: 2019 | PT id = 1143, Type = review |
Schmiermund's The Discovery of the Periodic Table of Chemical Elements
Torsten Schmiermund's book: The Discovery of the Periodic Table of Chemical Elements (Springer, in German)
Google Translate:
150 years ago, in 1869, D. I. Mendeleev and L. Meyer independently published their ideas on the arrangement of the chemical elements in a periodic system. The United Nations and UNESCO therefore declared 2019 the "International Year of the Periodic Table". The question arises what is so special about this "simple table". Embark on a short journey into the history of the periodic table with the author. Get to know its predecessors and see how the Periodic Table of the Elements has evolved over the years. Discover the periodic properties of the elements. Find out what makes the periodic table so interesting and timeless, and see what other ideas there are and have been.
The author, Torsten Schmiermund has worked as a chemical engineer in the chemical industry for many years.

| Year: 2019 | PT id = 1172, Type = data misc |
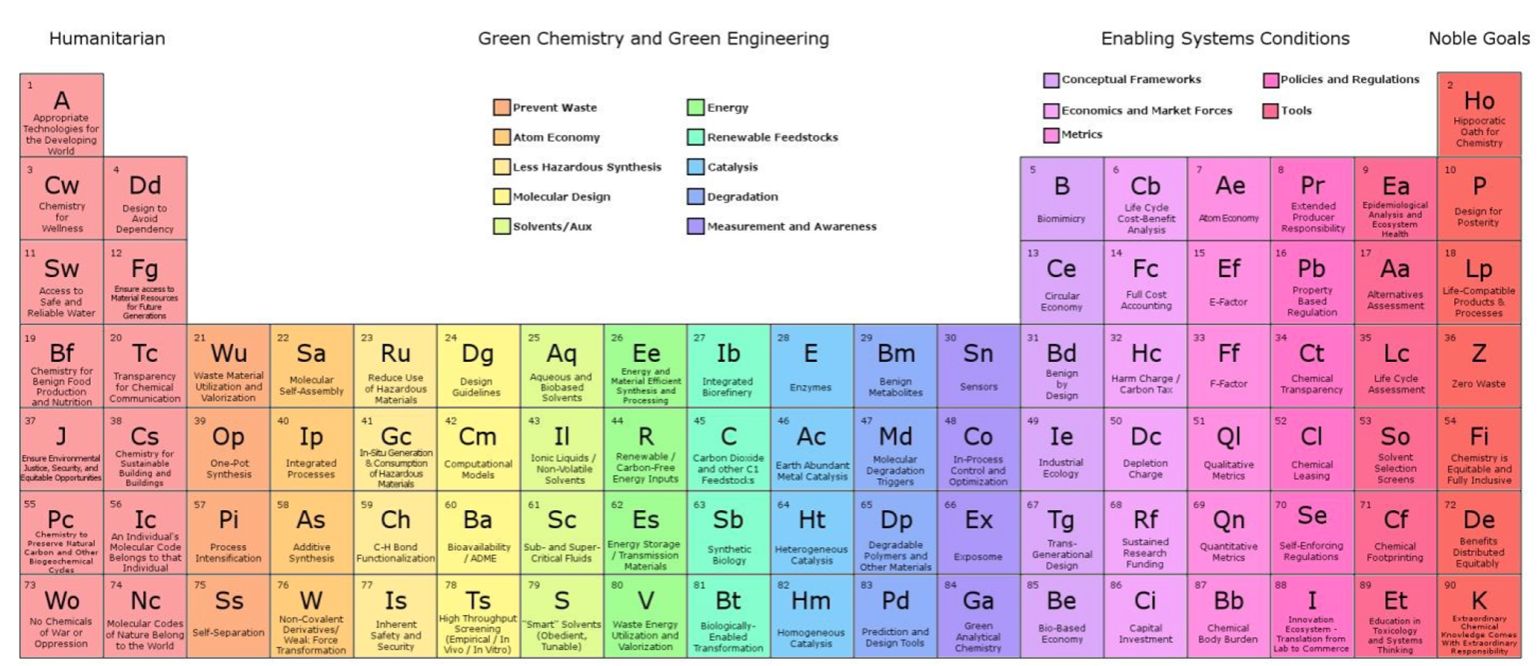
Green & Sustainable Chemistry, Periodic Table of
The periodic table of the elements of green and sustainable chemistry by Paul T. Anastas & Julie B. Zimmerman, Green Chem., 2019,21, 6545-6566, (DOI: 10.1039/c9gc01293a). Also, there is a review article in Chemistry World.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1178, Type = data |
Abundance by Atomic Number, Z
An article in De Gruyter Conversations: The Periodic Table & The Lanthanides by Simon Cotton has this interesting chart of elemental abundance with respect to 106 atoms of Si.
The image source is http://upload.wikimedia.org/wikipedia/commons/0/09/Elemental_abundances.svg
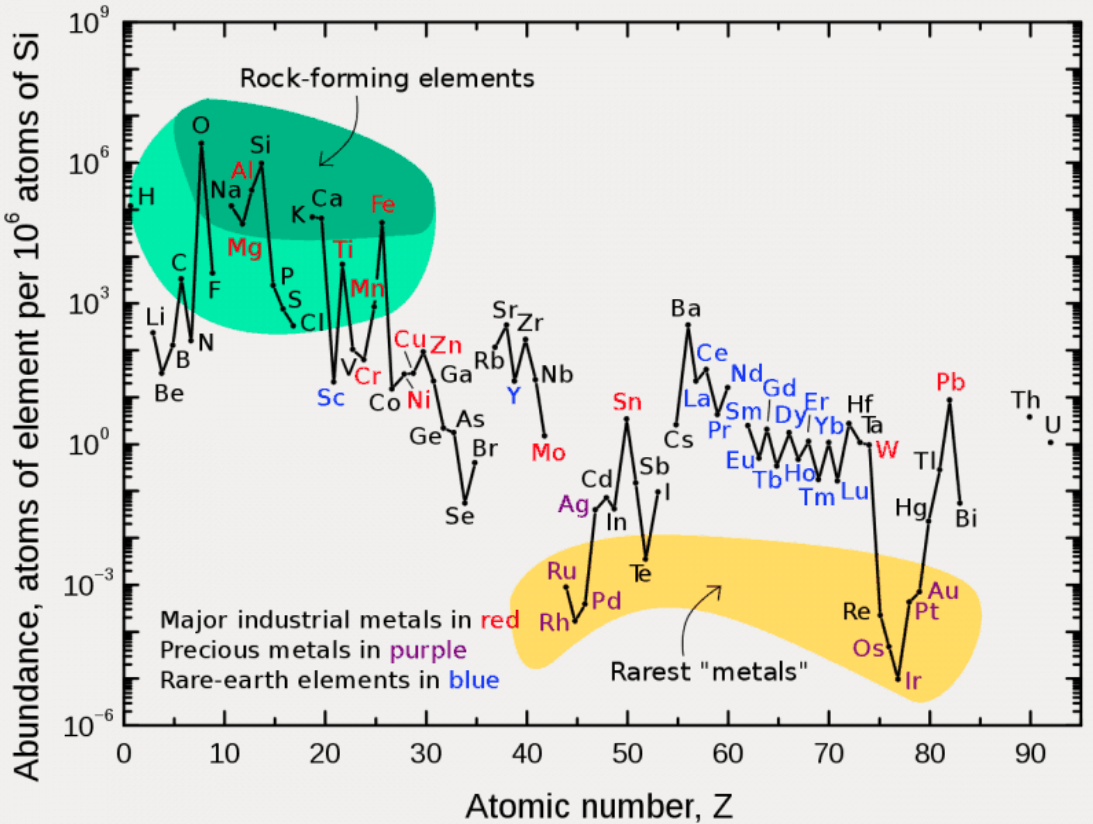
Thanks to René Vernon for the tip.
| Year: 2019 | PT id = 1194, Type = misc formulation |
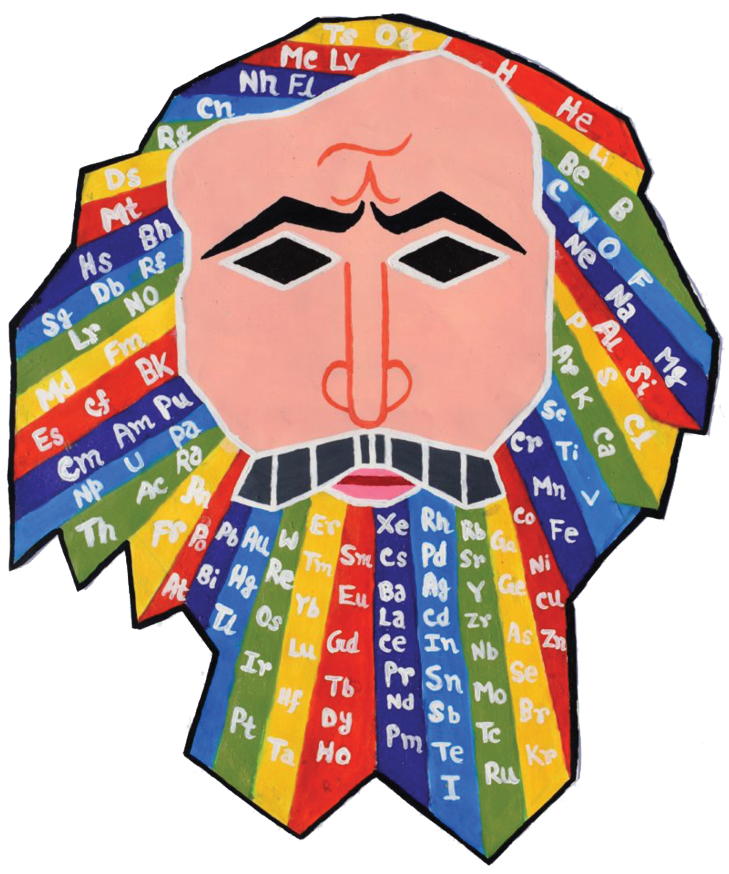
IUPAC Periodic Table Challenge: Nobelium Contest
IUPAC Periodic Table Challenge: Nobelium Contest. DOI: https://doi.org/10.1515/ci-2020-0204
After a first round based on the knowledge of the participant of the chemical elements, in this new phase of the Challenge, participants were invited to share their passion and creativity about chemistry. The entries were supposed to highlight the role of the Periodic Table in a creative manner. We received a variety of submissions including videos, poems, songs, and paintings. Entries were categorized into science, art, and outreach or community activities related to the science education and the Periodic Table. We were genuinely surprised and inspired by the dozens of pieces of art that we were receiving from all around the world. A jury as well as popular votes casted for each entry decided who were to receive the special limited edition IUPAC Periodic Tables signed by the Nobel laureates. That was one of the most difficult tasks of this activity, as the quality, originality, and diversity of the entries were truly amazing.
The artist is Swaprabha Chattopadhyay, a high school student from India.
- https://iupac.org/100/pt-challenge-entry/page/3/
- https://iupac.org/100/pt-challenge-entry/adorned-with-elements/
- https://iupac.org/100/summer-winners-of-the-nobelium-contest-announced/
| Year: 2019 | PT id = 1246, Type = misc element |
Periodic Table of the Elements Coloring Book
Periodic Table of the Elements Coloring Book
Project managing and chemistry overseen by Yann Brouillette (Faculty, Chemistry, Dawson College). Element representations and cover by Dawson College Illustration & Design students (2nd year)* overseen by Meinert Hansen (Faculty, Illustration & Design, Dawson College).
Thanks to René for the tip!
| Year: 2019 | PT id = 1247, Type = misc |
Spinor 150th Periodic Table Anniversary
Spinor Magazine's 150th Periodic Table Anniversary front cover:

| Year: 2019 | PT id = 1297, Type = formulation 3D |
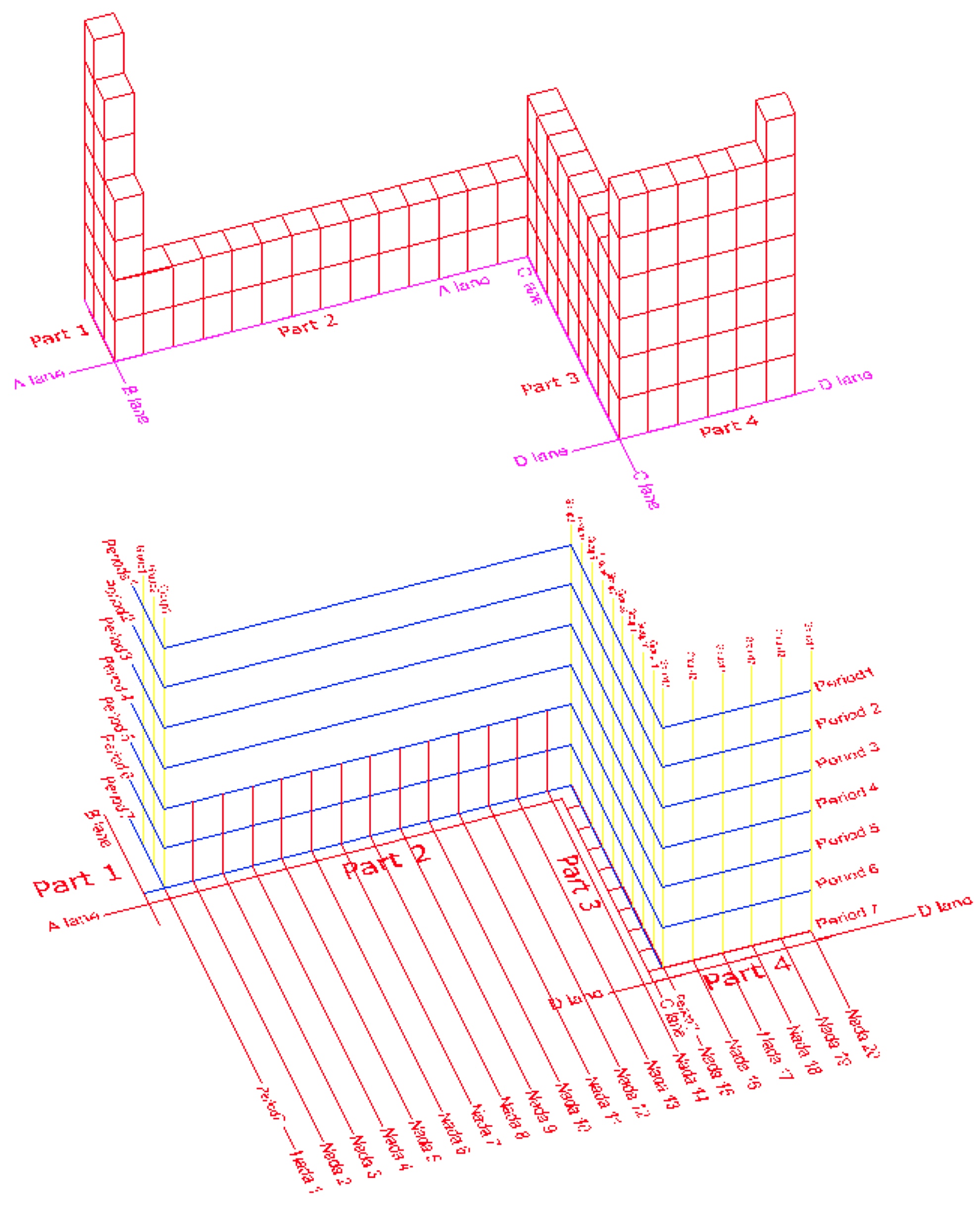
Ossmi LH & Chasib's Periodic Table
By Dr Laith Al- ossmi, Thi-qar University, Iraq: Al Ossmi LH & Chasib K F, A New Method of Elements Arrangement to Reattach the F–Block Elements of Lanthanides and Actinides in the IUPAC's Periodic Table of Elements, Med & Analy Chem Int J, 3, 4,2019, pp 1–18. DOI 10.23880/macij-16000148
The new 3-dimantional layout of the periodic table graphically shows the main body parts of the table and also these vertical and horizontal coordinators of Periods, Groups, and Nadas.
| Year: 2019 | PT id = 1320, Type = formulation misc |
International Year of the Periodic Table – Artwork Competition
From Chemistry A European Journal the results of a Periodic Table artwork competition, where the full stories can be read.
First Place: Víctor Duarte Alaniz from Mexico City with "Cycles in Space, In Time... and in Chemistry"

Second Place: Yuliia Oleksii from Vinnytsia, Ukraine "Noble Gases"

Third Place: Joanna Cwynar-Wojtonis from Poland

 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.