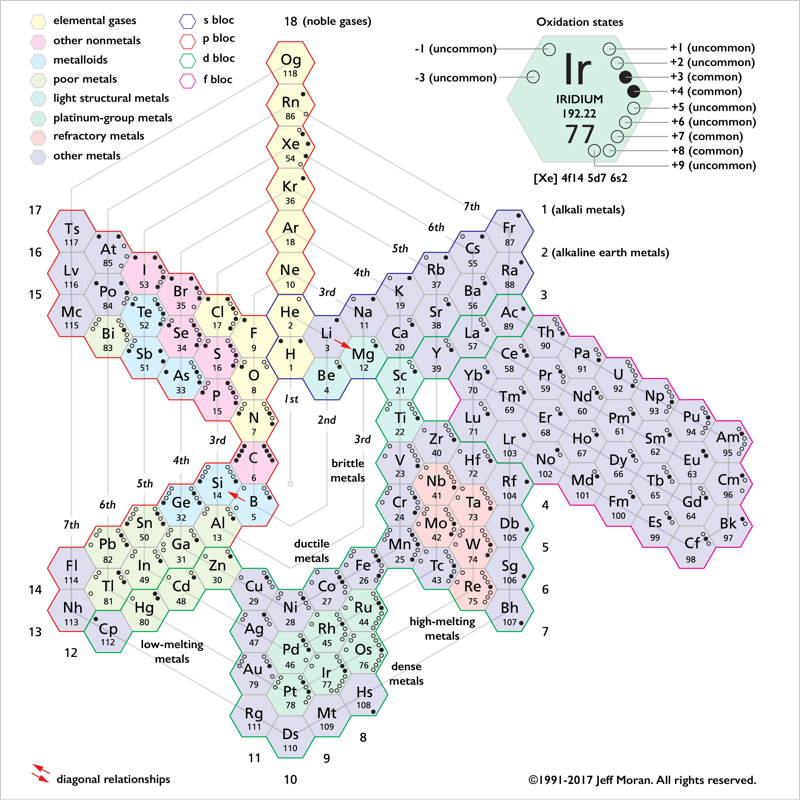
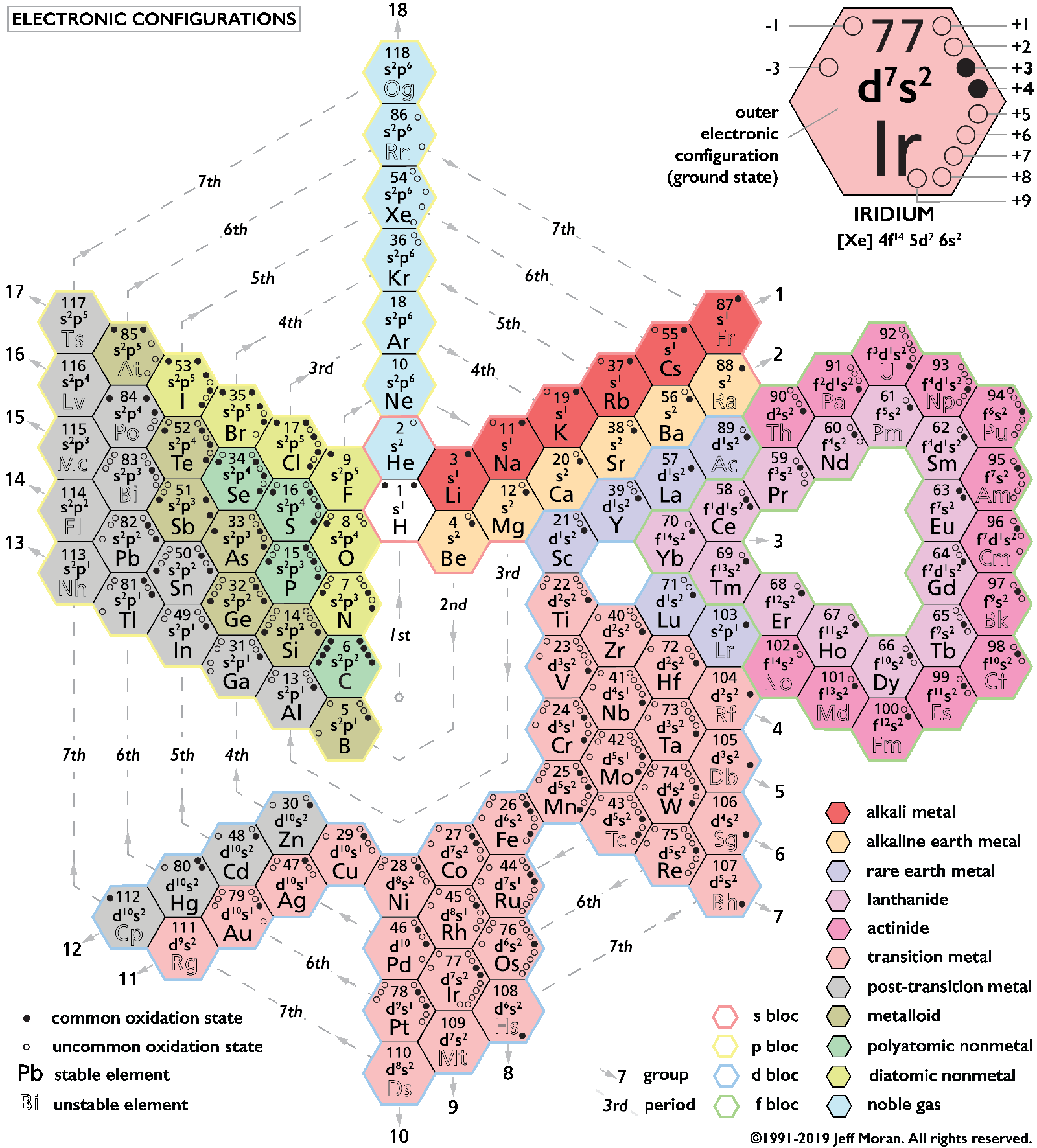
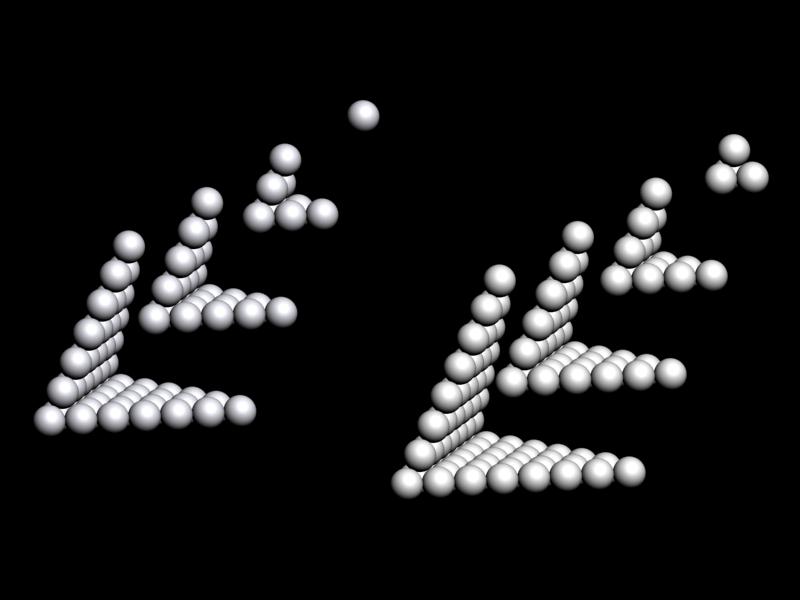Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables referencing the text string "spiral", listed by date:
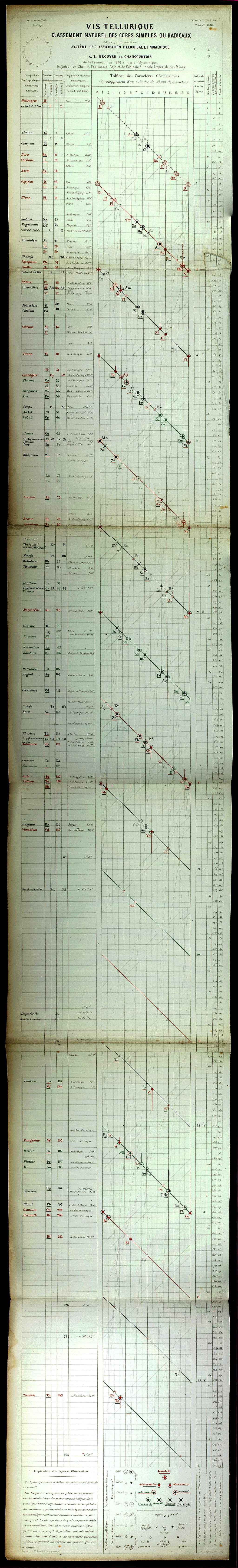
| Year: 1862 | PT id = 7, Type = formulation spiral 3D |
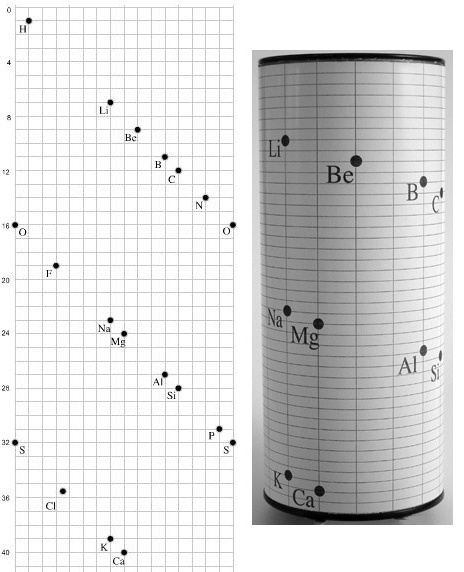
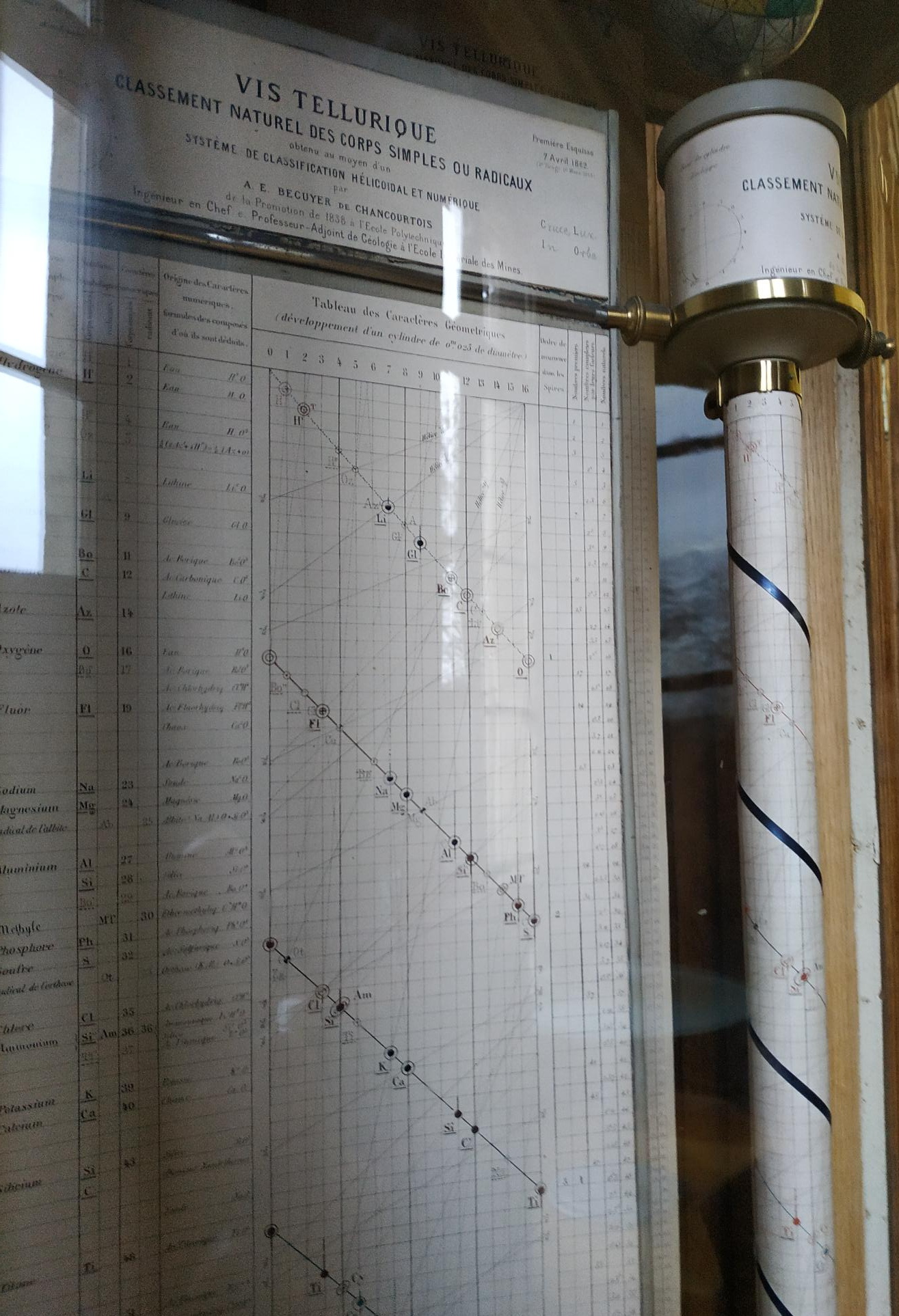
Béguyer de Chancourtois' Vis Tellurique
The French geologist , Alexandre-Émile Béguyer de Chancourtois was the first person to make use of atomic weights to produce a classification of periodicity. He drew the elements as a continuous spiral around a metal cylinder divided into 16 parts. The atomic weight of oxygen was taken as 16 and was used as the standard against which all the other elements were compared. Tellurium was situated at the centre, prompting vis tellurique, or telluric screw.
Many thanks to Peter Wothers – and courtesy of the Master and Fellows of St Catharine's College, Cambridge – comes a high quality image of the original 1862 formulation. Click here, or on the image to enlarge:
Watch Peter Wothers 'unravel' and show Prof. Martyn Poliakoff this first periodic table at 17min 50sec into the YouTube video below:
Some more information:
Chancourtois' original formulation includes elements in their correct places, selected compounds and some elements in more than one place. The helix was an important advance in that it introduced the concept of periodicity, but it was flawed.
It has been suggested that Chancourtois called his formulation a telluric helix because tellurium is found in the middle. However, most elements are found as there their 'earths' – tellus, telluris – or oxides, which for a mineralogist would have been highly significant.
The formulation was rediscovered in the 1889 (P. J. Hartog, "A First Foreshadowing of the Periodic Law" Nature 41, 186-8 (1889)), and since then it has appeared most often in a simplified form that emphasizes the virtues and eliminates its flaws. [Thanks to CG for this info.]
See also:
- Dutch Wikipedia
- ScienceWorld
- Science & Society Picture Library
- Roy Alexander's All Periodic Tables site
A three dimensional models of the telluric helix:
There are representations of the 1862 formulation at the School of Mines at ParisTech:

| Year: 1867 | PT id = 270, Type = formulation spiral |
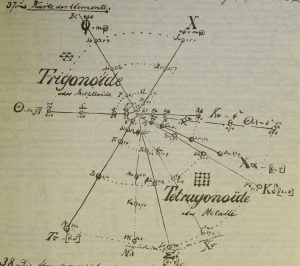
Hinrichs' Programme of Atomechanics
Gustavus Detlef Hinrichs' spiral "Programme of Atomechanics". Programm der Atomechanik oder die Chemie eine Mechanik de Pantome, Augustus Hageboek, Iowa City, IA (1867).
Hinrichs' system is based on the relationship of what he called: "pantogens, with its atoms called panatoms, which explains the numerical relations of atomic weights and gives a simple classification of the elements."
This classification system culminated in 1867 in his spiral periodic table, which better clarified the groupings of elements. Hinrichs' classification, while distinctly different from the other periodic tables of this period, "seems to capture many of the primary periodicity relationships seen in the modern periodic table... it is not cluttered by attempts to show secondary kinship relationships." (Scerri)
| Year: 1870 | PT id = 70, Type = formulation spiral |
Baumhauer's Spiral
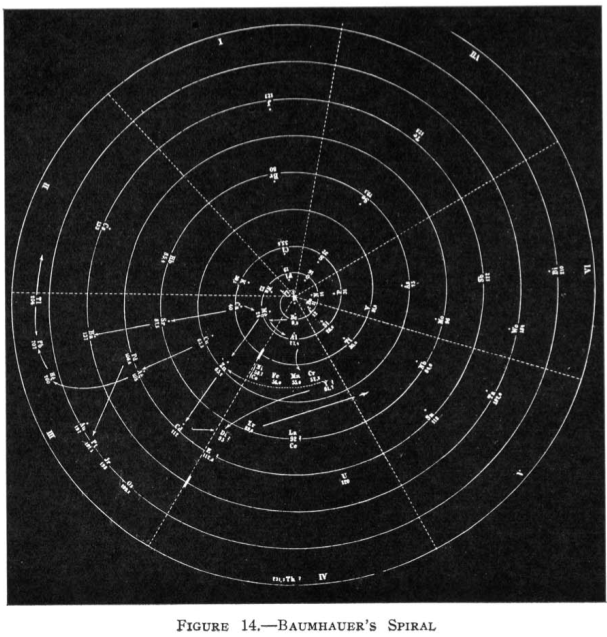
From Quam & Quam's 1934 review paper.pdf
| Year: 1872 | PT id = 284, Type = formulation spiral 3D |
Meyer's Spiral System
Meyer's Spiral System of 1872 (from van Spronsen):

| Year: 1886 | PT id = 1107, Type = formulation spiral 3D |
Shepard's Natural Classification
Shepard's Natural Classification of the Elements, a spiral formulation with instructions for turning it into a three-dimensional table. From: Elements of Inorganic Chemistry, Descriptive and Qualitative (pp221), by J. H. Shepard, (1886), Boston MA, pub. D. C. Heath
René Vernon writes:
Note the instructions along the side, to turn the table into a tube (spiral form) and the 19 spaces from La to eka-Ce. Here, Yb needs to be moved back one column into group II, so as to leave room for Lu under La. Then eka-Ce becomes Hf. This results in La + 15 lanthanoids.
The accompanying text says:
"Elements of most distinct basic character are found towards the left; non-metals predominate in the upper and middle parts of Groups V., VI., and VII. ; while the lower part of the table is marked by the more indifferent elements.
"A double spiral will be traced beyond Si (beginning with P and V respectively) and distinguished by heavy-face and light-face type.
"The harmony of nature here exhibited is most impressive. Is it possible that the so-called elements are really compounds? Did the various 'elements' of the earth and sun once exist as hydrogen, when our solar system was a nebula? And will modern chemists ever revive the famed problem of the alchemists, and seek to turn the base metals into gold? Far more precious than gold is the search for truth; and the more we learn of science, the broader becomes our conception of what we know in part, and the deeper should be our reverence for the infinite thought of the Creator."
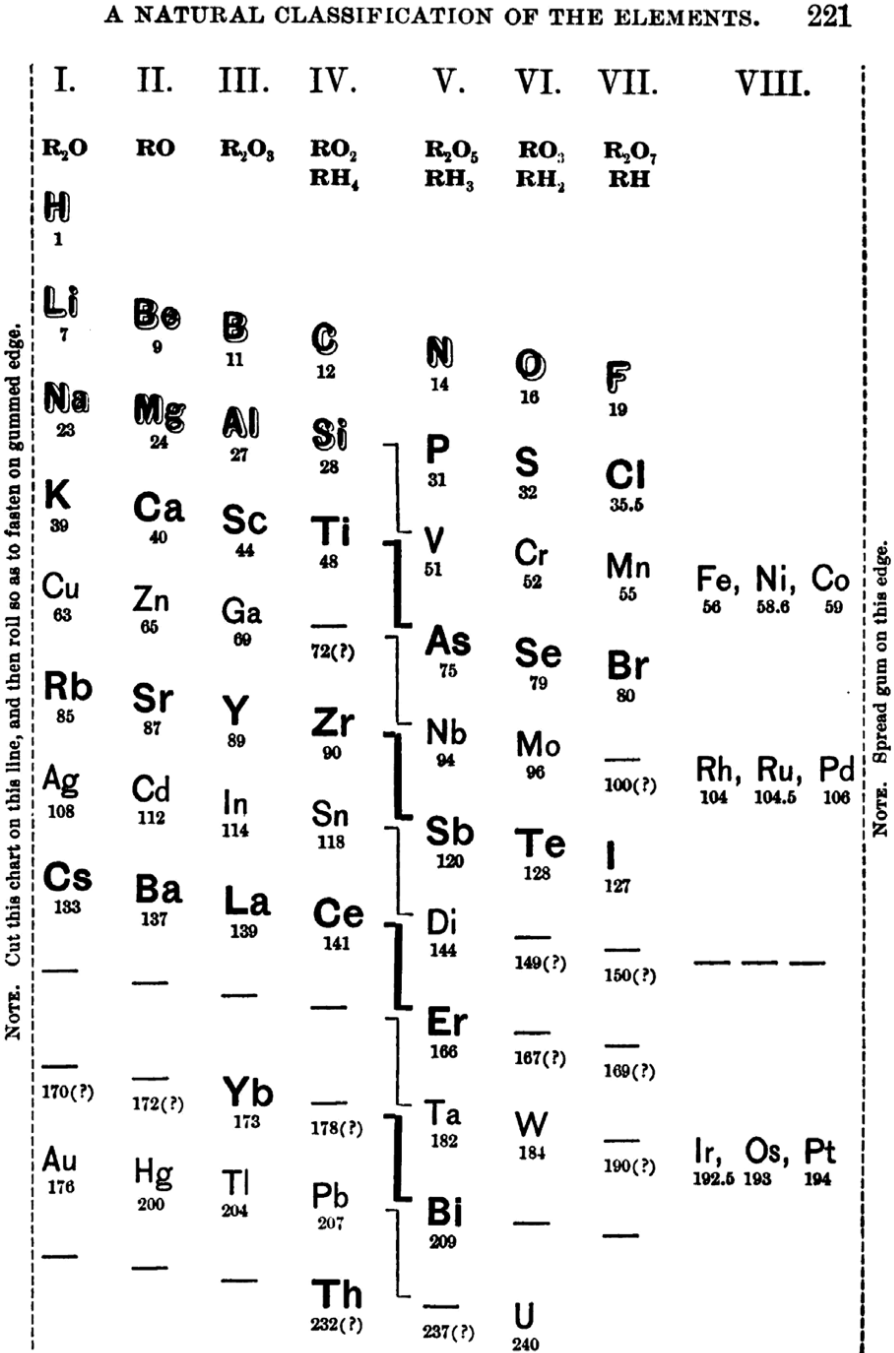
| Year: 1888 | PT id = 997, Type = formulation spiral |
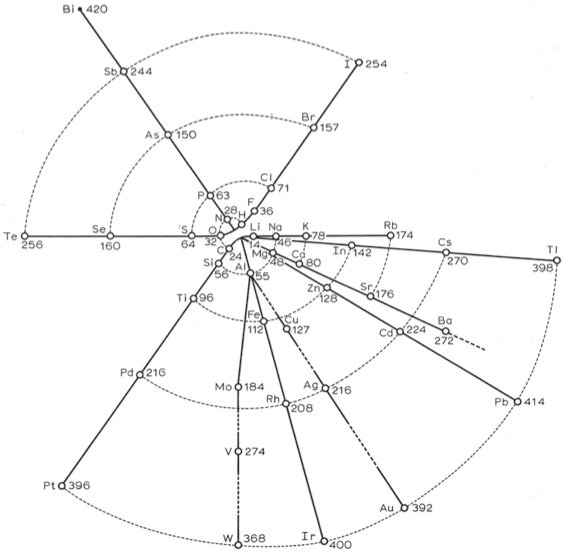
Stoney's Spiral
Johnstone Stoney's Spiral, taken from A. E. Garrett's The Periodic Law (page 167, 1909 pub. D. Appleton And Company). The reference is given – page 167 – is: Phil. Mag. [6], 4, pp 411 et seq.; Proc. Roy. Soc., 1888, p115.
Thanks to Roy Alexander for the tip!
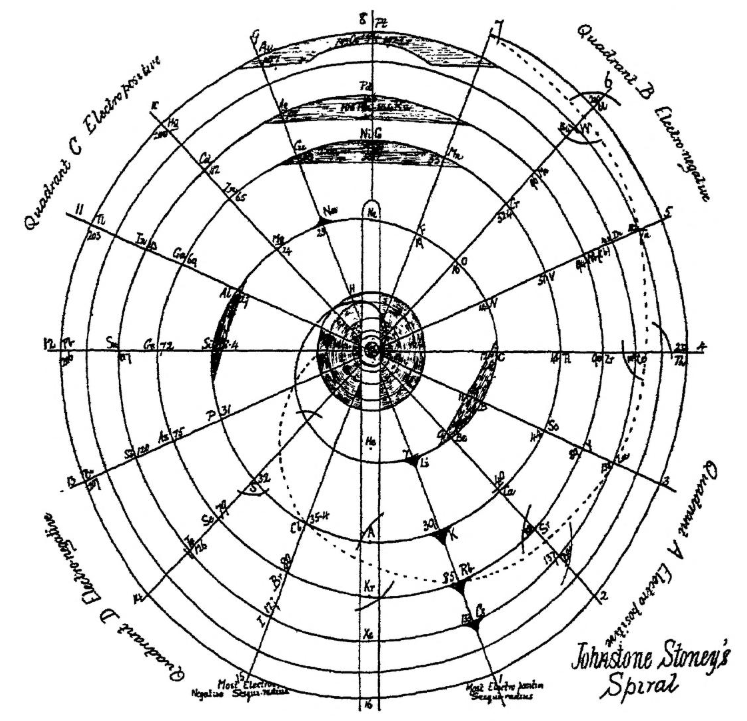
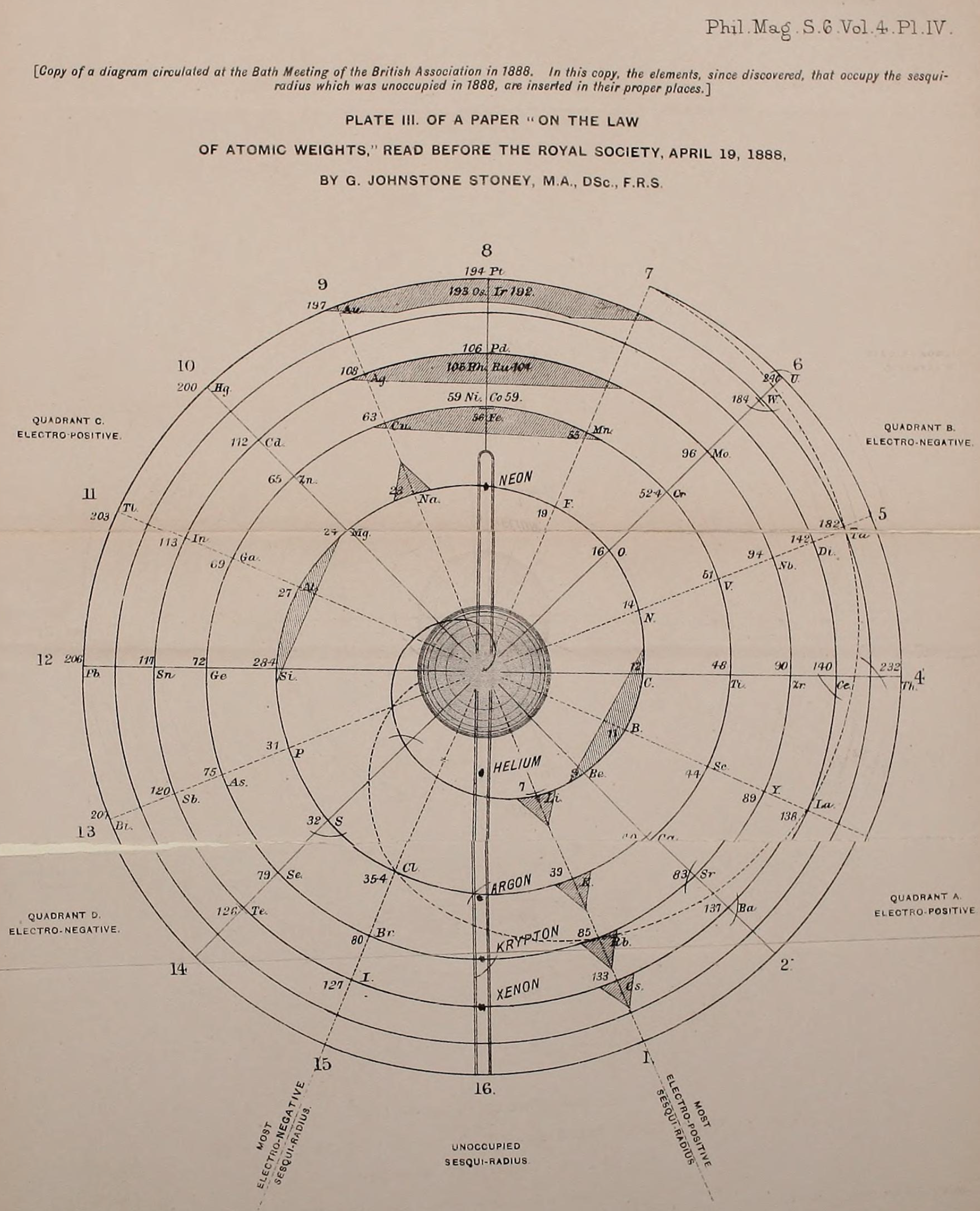
| Year: 1888 | PT id = 1267, Type = formulation spiral |
Stoney's Spiral Periodic Table
In the Proceedings of the Royal Society of London, Series A, Containing Papers of a Mathematical and Physical Character, Volume 85, Issue 580, Aug 1911, p. 472, there is an article On Dr. Johnstone Stoney's Logarithmic Law of Atomic Weights, by Lord Rayleigh (who co-discovered argon in 1894), who writes :
"In the year 1888, Dr. G. Johnstone Stoney communicated to the Society a memoir with title nearly as above, which, however, was not published in full. At the request of the author, who attaches great importance to the memoir, I have recently, by permission of the Council, consulted the original manuscript in the archives of the Society, and I propose to give some extracts, accompanied by a few remarks. The author commenced by plotting the atomic weights of the elements taken as ordinates against a series of natural numbers as abscissæ. But a curve traced through the points thus determined was found to be 'one which has not been studied by mathematicians.
"This sudden transition may have some connection with the fact that no elements have been found on sesqui-radius 16, although the investigation in § 3 shows that the values of m corresponding to the stations on sesqui-radius 16 cannot be dispensed with.
"The vacant places here pointed out are now occupied by the since discovered inert gases. The anticipation is certainly a remarkable one, and it goes far to justify the high claims made for the diagram, as representing in a telling form many of the leading facts of chemistry."
Comment from Mark Leach:
"Notice how the electronegative elements are positioned top right & bottom right and the electropositive elements top left & bottom right."
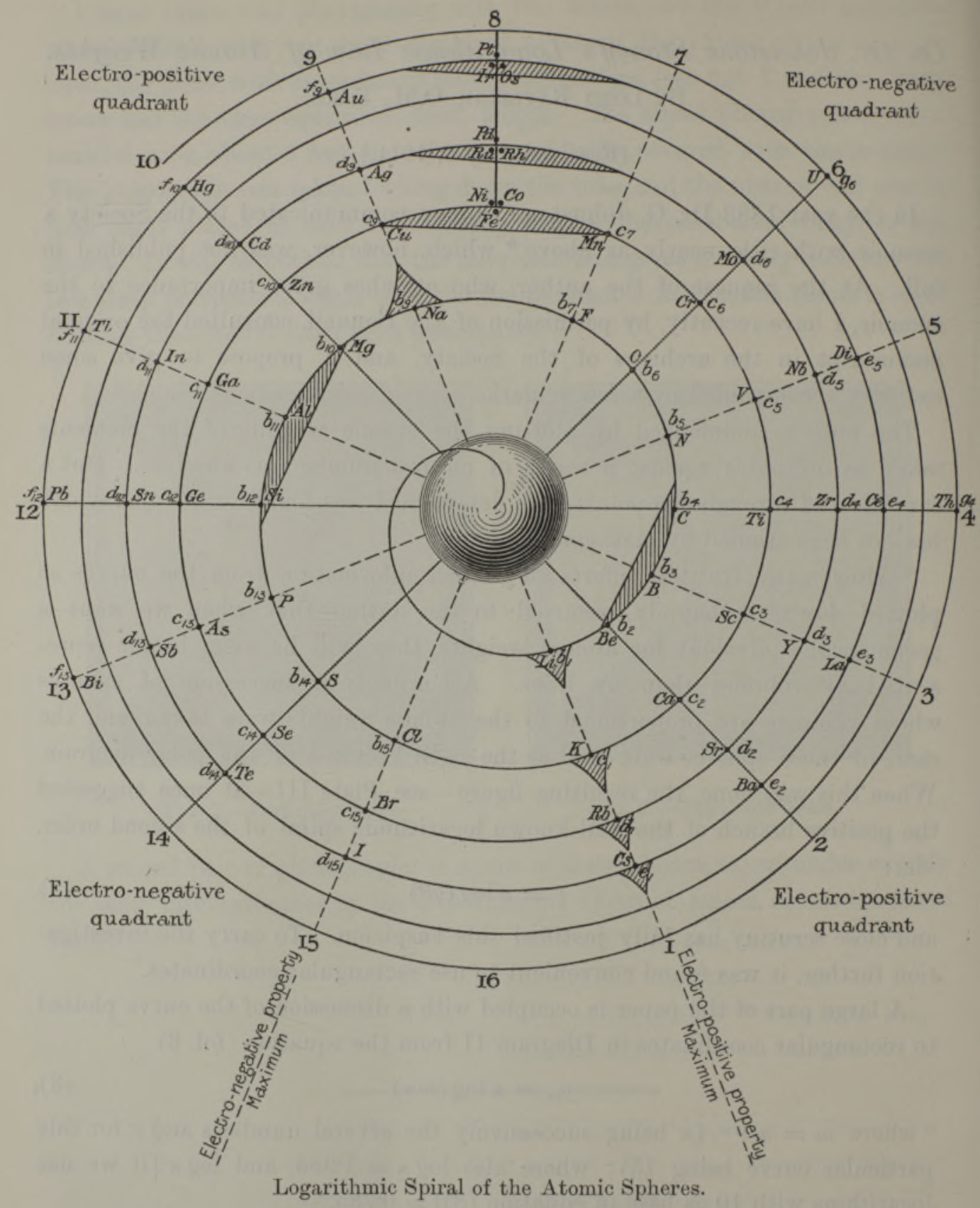
René Vernon writes:
"Stoney has another article in the September 1902 edition of the The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science, called Law of Atomic Weights, pp. 411–415. At the back of the journal is an updated fold-out version of Stoney’s table, image attached.
- Ar, Kr and Xe fit on the spiral, and on spoke 16.
- Neon fits on the spiral but is instead on spoke 8.
- Helium is on spoke 18 but is not on the spiral.
- The circle in the middle represents H (p. 414).
"On the page after the updated spiral, there looks to be some printed content, but it is hidden by what looks to be a folded over page."
Thanks to René for the tip!
| Year: 1902 | PT id = 71, Type = formulation spiral |
Erdmann's Spiral Table
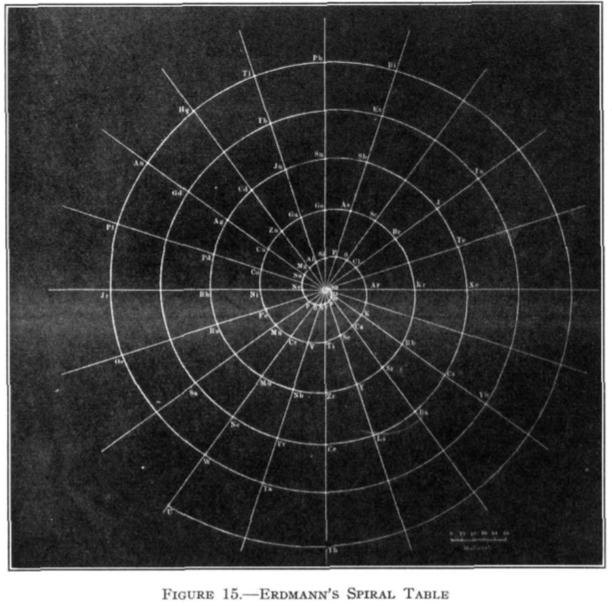
From Quam & Quam's 1934 review paper.pdf
| Year: 1905 | PT id = 773, Type = formulation |
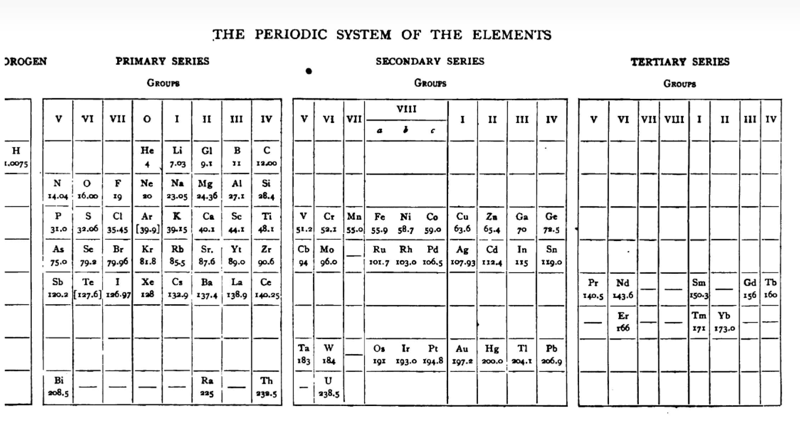
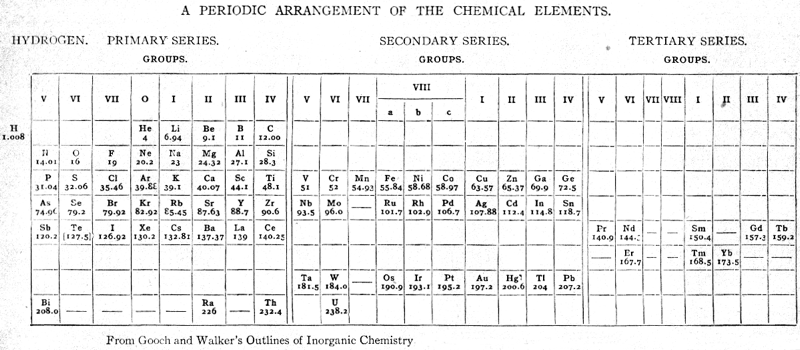
Gooch & Walker's Periodic System of The Elements
From a 1905 textbook by Gooch & Walker: Outlines of Inorganic Chemistry (see the Google Books scanned version pp273) comes an early 'right-step' periodic table. The formulation was reproduced in a 1917 textbook (lower image).
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1905 | PT id = 912, Type = formulation 3d spiral |
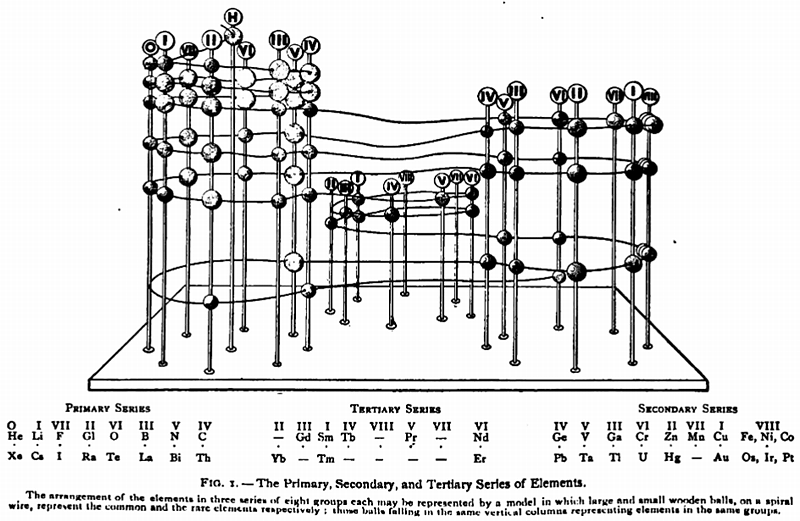
Gooch & Walker's Primary, Secondary, and Tertiary Series of Elements
This three dimensional formulation – clearly developed from the Crookes' vis generatrix model – is given a 1905 textbook by Gooch & Walker: Outlines of Inorganic Chemistry (see the Google Books scanned version pp273).
"The arrangement of the elements in three series of eight groups each may be represented by a model in which large and small wooden balls, on a spiral wire, represent the common and rare elements respectively; those balls falling in the same vertical column representing elements in the same groups":
| Year: 1913 | PT id = 973, Type = formulation |
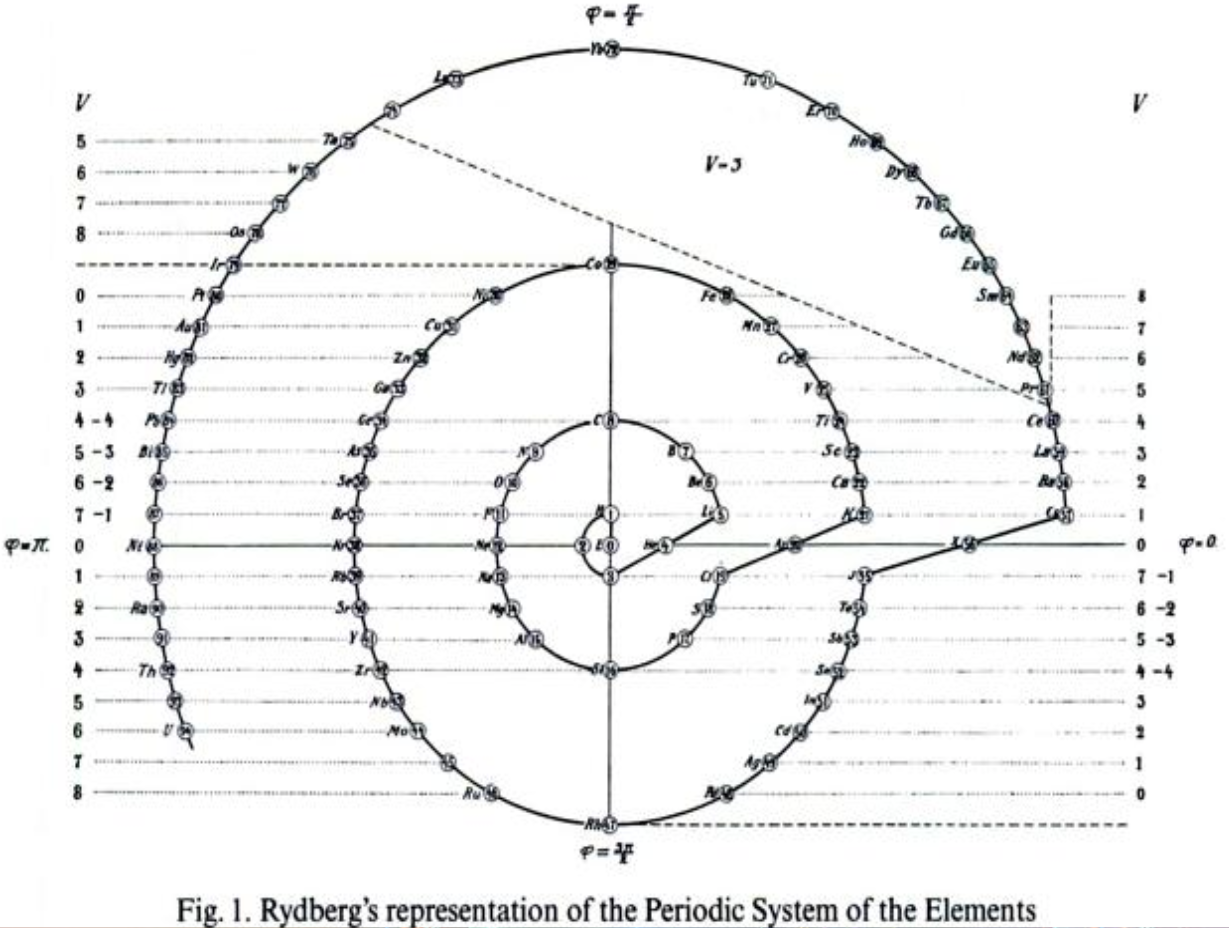
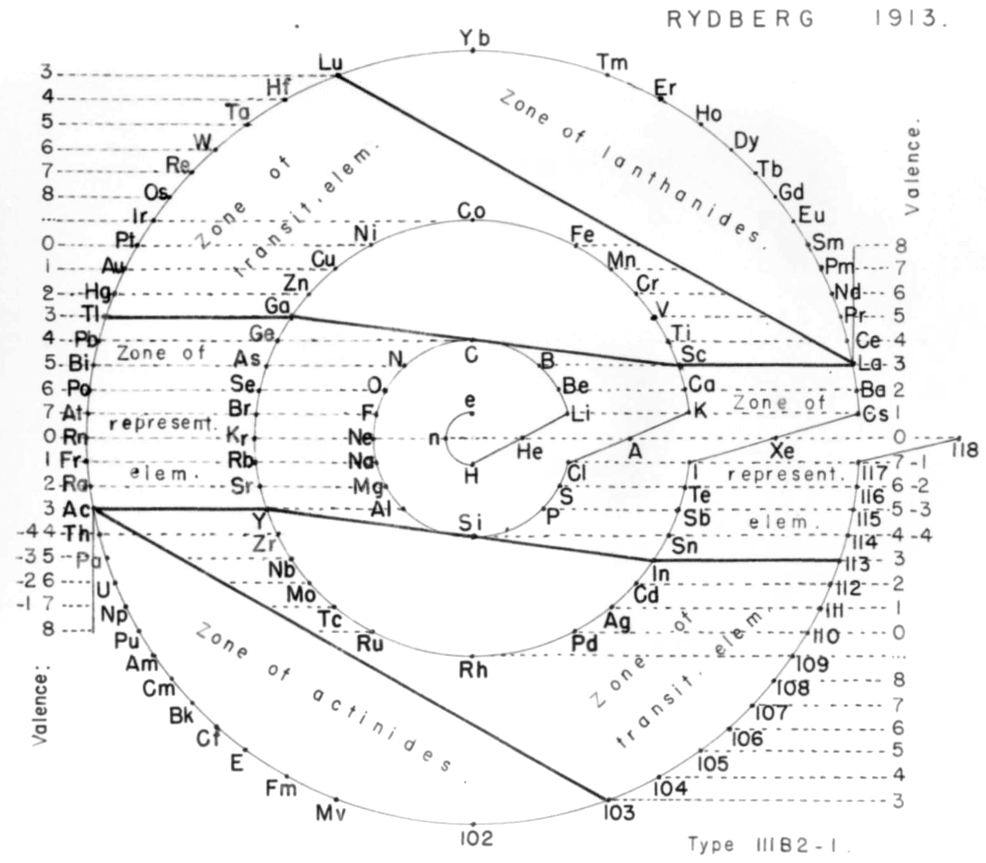
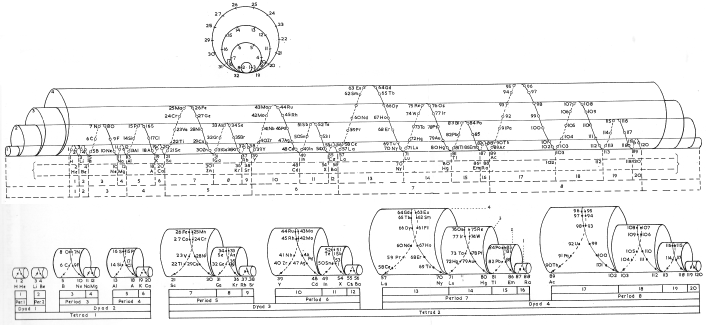
Rydberg's Periodic Table in style of Spiral with Four Revolutions
Periodic table in style of spiral with four revolutions circa 1913 (Original design) and 1957 (Date attributed to slide).
This table was originated by Swedish physicist Johannes Rydberg (1854-1919) in 1913 and classified by chemist Edward G. Mazurs as Type IIIB2-1 in his seminal work Types of Graphic Representation of the Periodic System of Chemical Elements (1957). The lower version of the table appears as Figure 63 on page 132 of Mazurs' 1957 publication.
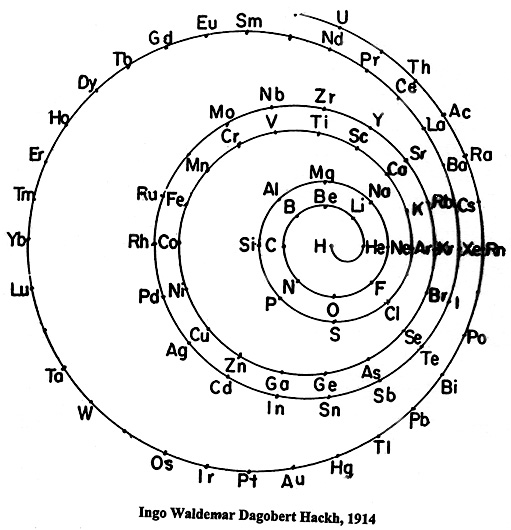
| Year: 1914 | PT id = 23, Type = formulation spiral |
Hackh's Spiral Periodic Table
Ingo Hackh's spiral periodic table of 1914, from Das Synthetisches System der Atome, Hamburg, Hephaestos.
Philip Stewart says:
"I believe that Hackh's 1914 spiral is of special interest it is the first spiral to take account of Mosley's atomic numbers, and the first to show successively larger pairs of coils. It is also interesting because H stands alone in the centre. I have only seen Mazurs' redrawn (as usual!) version, but Mazurs gives SciAm Supplement 1919 as one reference."
This is the Mazurs version:
| Year: 1919 | PT id = 548, Type = formulation spiral |
Hackh's Periodic Spiral
From a Scientific American in March 1919, an article by Ingo W. D. Hackh discussing the classification of the elements.
Included is a periodic spiral, developed from Hackh's 1914 version:
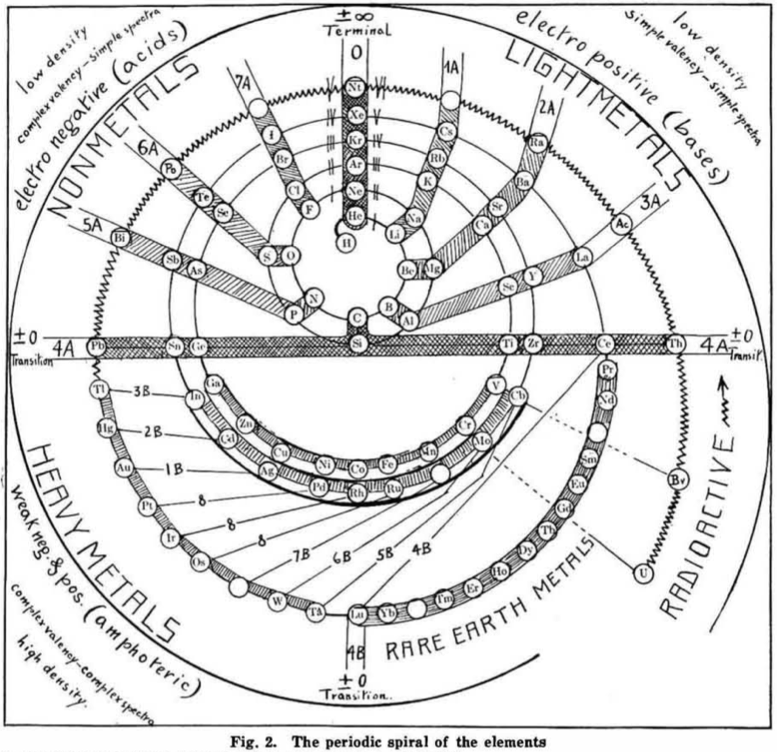
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1920 | PT id = 1075, Type = formulation |
Stewart's Arrangement of The Elements
From A.W. Stewart, Recent Advances in Physical and Inorganic Chemistry, 3rd ed., Longmans, Green and Co., London (1920)
René Vernon writes:
"Stewart discusses the 'forced symmetry' of Mendeleev's table, and the distinction between 'facetious symmetry' (as he calls it) and the actual correlation of facts (as he saw them at that time)."
Extracts:
237. Mendeleev... objected strongly to the employment of graphic methods of expressing the Periodic Law, on the ground that such methods did not indicate the existence of a limited and definite number of elements in each period.
239. The Periodic Table, as laid down by Mendeleeff in his writings, exhibits a symmetry which was one of its greatest assets. For some psychological reason, symmetry has an attraction for the human mind; and we are always apt to prefer a regular arrangement to one in which irregularities pre- dominate. Psychological peculiarities are, however, undesirable guides in the search for truth; and a careful examination of the Table in the light of our present knowledge will suffice to show that it can boast of no such symmetry as we are led to expect from the text-books of our student days.
For example, owing to the omission of some of the rare earth elements and by the insertion of blanks, the Table in its original form attained a very high degree of regularity; but since there are, as we know from the X-ray spectra results, only sixteen elements to fill the eighteen vacant spaces in the Table, it is evident that the symmetry of Mendeleeff s system is purely factitious.
Further, in order to produce the appearance of symmetry, Mendeleeff was forced to place copper, silver, and gold in the first group, although there is no known oxide Au2O and the stable chloride of gold is AuCl3.
These examples are well-known, and are mentioned here only for the purpose of enforcing the statement that the symmetry of Mendeleef's system cannot be sustained at the present day. Fascinating though its cut-and-dried regularity may be, we cannot afford to let symmetry dominate our minds when in actual fact there is no symmetry to be found.
240. The most superficial examination shows that, instead of being a symmetrical whole, the Table is really pieced together from a series of discrete sections.
250. The first attempt to arrange all the elements in a periodic grouping took the form of a three-dimensional model the Telluric Helix of de Chancourtois and it is not surprising that from time to time attempts have been made to utilize the third dimension as an aid to classification. It cannot be said that much light has been thrown on the matter by these essays; but some account of them must be given here for the sake of completeness.
251. The main drawback to the spiral representation appears to be that in it no new facts are brought to light, and there is no fresh collocation of the allied elements which might give it an advantage over the ordinary forms of classification. Also, in most cases it is more difficult to grasp as a whole.
253 ...if we have to choose between factitious symmetry and actual correlation of facts, we must decide in favour of the latter, discomforting though the choice may be.
255. The following new grouping seems worth considering. Although it has many good points, it is not to be regarded as a final solution, but is put forward mainly in the hope that an examination of it may suggest some more perfect system.
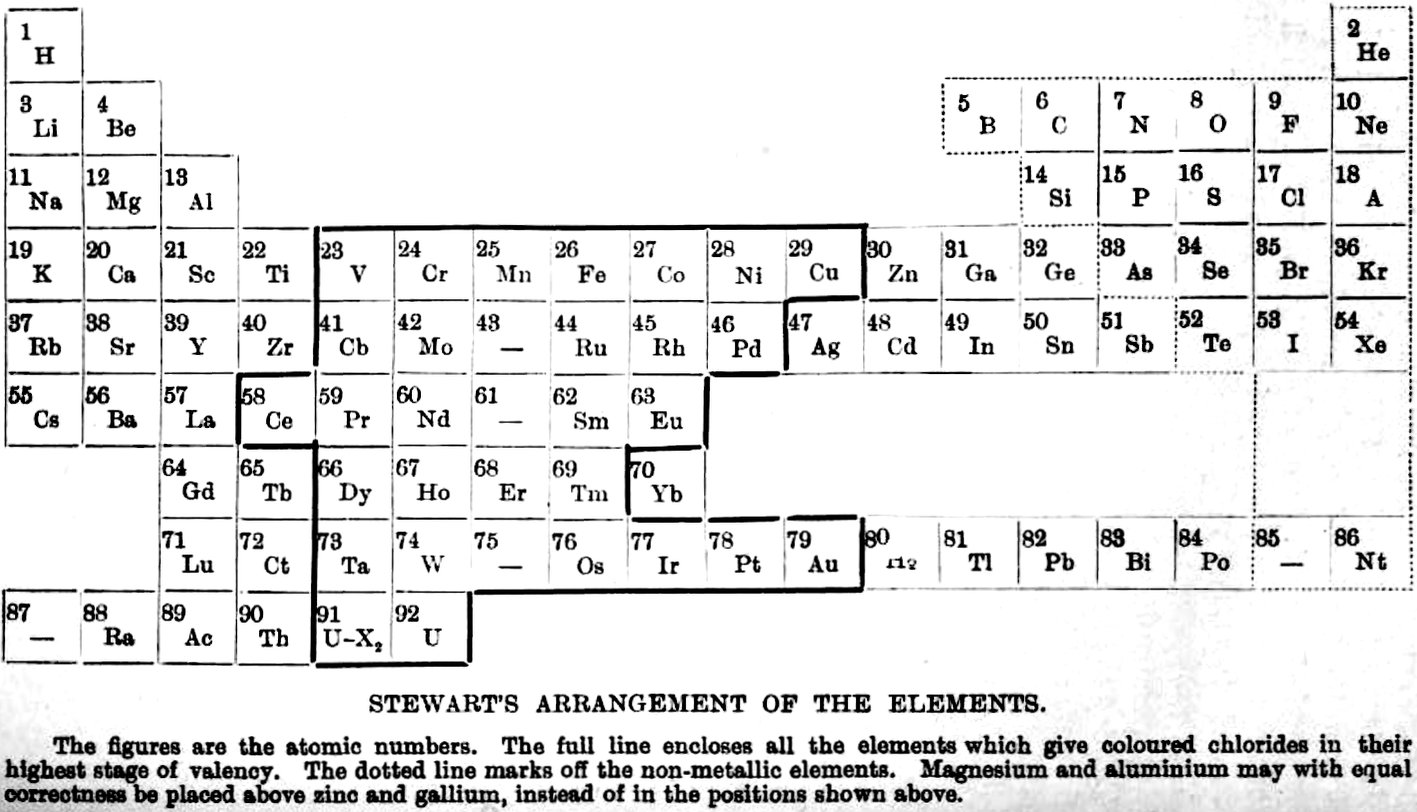
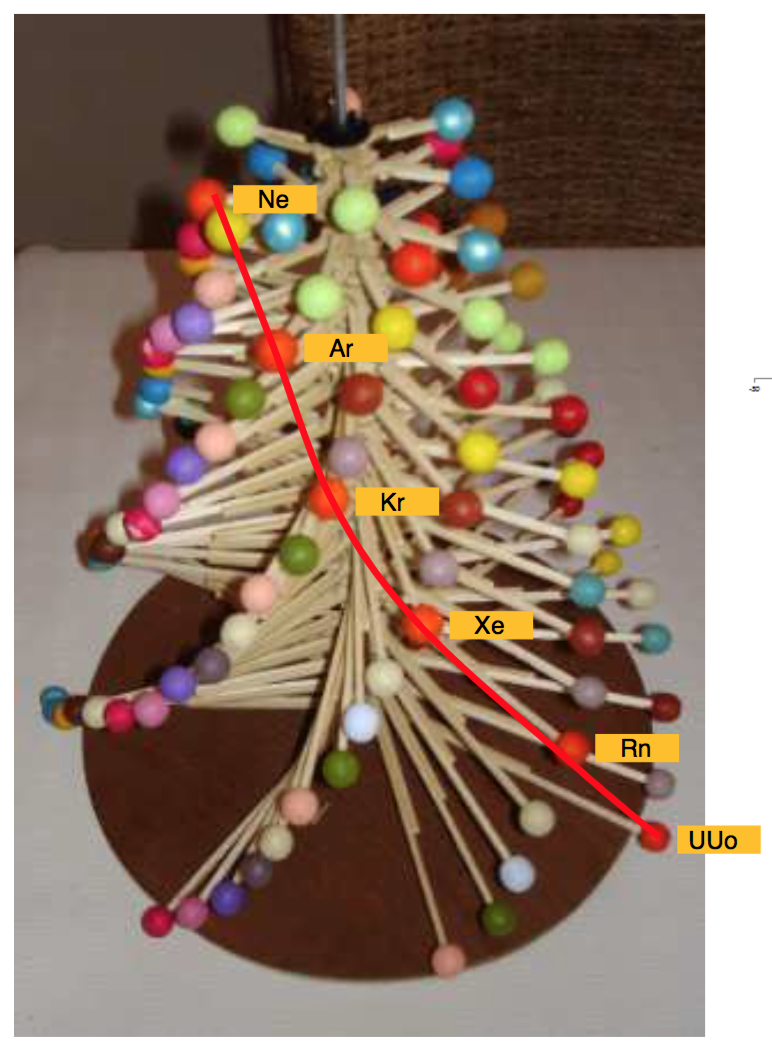
| Year: 1920 | PT id = 78, Type = formulation 3D spiral |
Schaltenbrand's Helical Periodic Table
G. Schaltenbrand, Darstellung des periodischen Systems der Elemente durch eine räumliche Spirale, Z. anorg. allgem. Chem., 112, 221-4 (Sept. 1920)
From Quam & Quam's 1934 review:
"The elements are arranged in order of atomic weights on an eccentric spiral. The four sets of curves include positions of similar elements. The first small turn carries H and He; the remainder of the inert elements and the halogens are on successive small turns in analogous positions.
"On the next larger turn are found the alkali, alkaline-earth, and aluminum family elements.
"The long periods require larger turns and the period containing the rare-earth elements requires the longest turn of all. Elements of the same group are found in the same plane passing through the axis of the spiral."
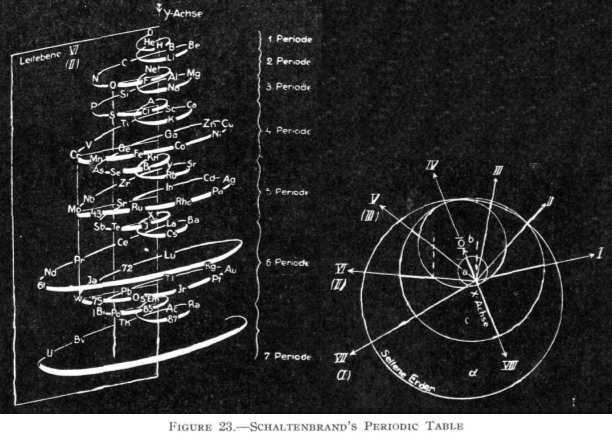
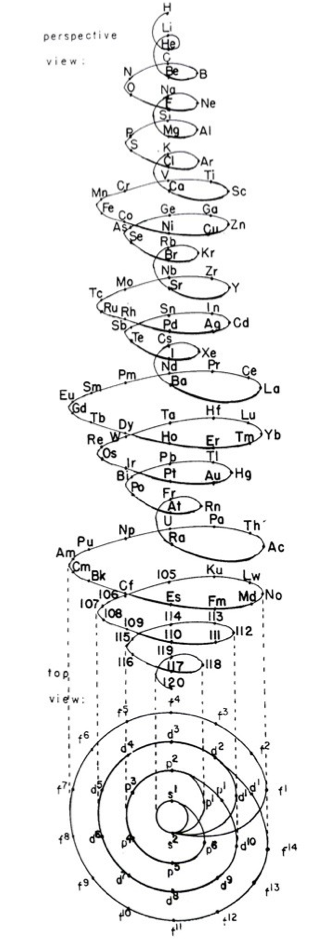
Commissioned in 2019 to match George Schaltenbrand's 1920 design for a helical gathering of the elements – albeit extended to all 118 current elements – and signed by Yuri Oganessian, it is almost certainly the most expensive periodic table in the world."


| Year: 1925 | PT id = 1035, Type = formulation 3D |
Model of the Periodic System of de Chancourtois
From the Science Museum in the UK collection, a model of the Periodic System of de Chancourtois from 1862:
"Model demonstrating the telluric screw periodic system of Alexander-Emile Beguyer de Chancourtois proposed in a paper published in 1862.
"This model, made by the Science Museum in 1925, provides a rare physical realisation of arguably the earliest periodic system of for the elements. It was devised by the French geologist, Alexander-Emile Beguyer de Chancourtois in 1862, 7 years prior to Dmitri Mendeleev's periodic table.
"De Chancourtois arranged the elements in the order of their atomic weights along a helix which was traced on the surface of a vertical cylinder, with an angle of 45 degrees to its axis. The base of the cylinder was divided into 16 equal parts (the atomic weight of oxygen), and the lengths of the spiral corresponding to the weights of the elements were found by taking the one-sixteenth part of a complete turn as a unit":

| Year: 1926 | PT id = 104, Type = formulation spiral |
Monroe & Turner's Spiral
Monroe and Turner's spiral, in which they correctly place the actinides. Information supplied by Philip Stewart.
Ref. is C J Monroe and W D Turner A new Periodic Table of the Elements, J Chem Ed, 3, 1058-65, 1926
| Year: 1928 | PT id = 289, Type = formulation spiral 3D |
Janet's Three-Dimensional Spiral-Tube System
Janet's Three-Dimensional Spiral-Tube System of 1928 (from van Spronsen):
Click here for large diagram.
| Year: 1928 | PT id = 305, Type = formulation spiral |
Janet's "Lemniscate" Formulation
From in The Helicoidal Classification of the Elements, Chemical News vol. 138, 21 June 1929, Fig. XI, p. 392:
Philip Stewart points out that this formulation is an 'end on' view of the Janet Cylinder or Three-Dimensional Spiral-Tube System formulation, and the term "lemniscate" comes from Mazurs.
| Year: 1928 | PT id = 74, Type = formulation spiral |
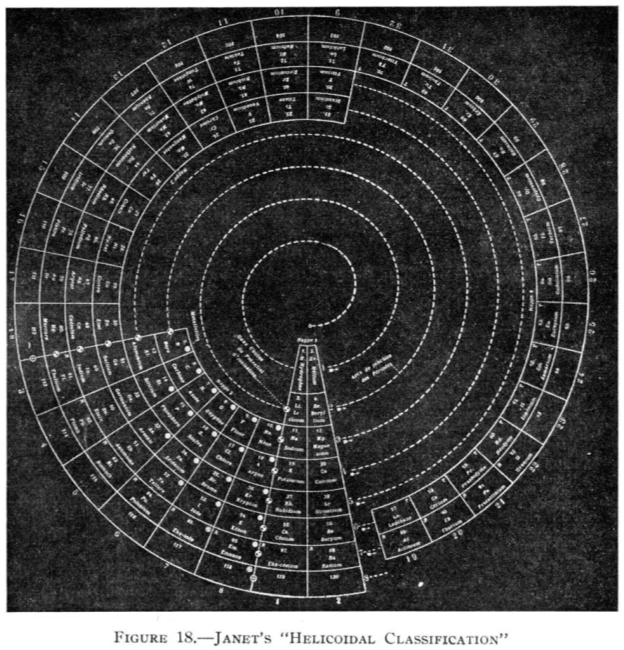
Janet's Helicoidal Classification
Janet's Helicoidal Classification, essentially his left-step formulation in its spiral version (ref. Charles Janet, La Classification Hélicoïdale des Éléments Chimiques. Beauvais: Imprimerie Départementale de l'Oise. 1928). Information supplied by Philip Stewart:
From Quam & Quam's 1934 review paper.pdf
| Year: 1934 | PT id = 105, Type = review formulation |
Quam & Quam's Graphical Representations of The Elements
Short Periodic Tables.pdf
Medium Periodic Tables.pdf
Spiral, Helical & Misc Periodic Tables.pdf
- Mendeléeff's Table (their spelling, 1872)
- Brauner's Table (1902)
- Rydberg Table (1913)
- Periodic Chart by Quam (1934)
- Rang's Periodic Table (1893)
- Werner's Periodic Table (1905)
- Courtines' Periodic Classification (1925)
- Bayley's Periodic System (1882)
- Adam's Periodic Chart (1911)
- Margary's Periodic Table (1921)
- Stareck's Natural Periodic System (1932)
- Baumhauer's Spiral (1870)
- Erdmann's Spiral Table (1902)
- Nodder's Periodic Table (1920)
- Partington's Periodic Arrangements of the Elements (1920)
- Janet's Helicodial Classification (1929)
- The Telluric Screw (1863)
- Crookes' Periodic Table model (1898)
- Emerson's Helix (1911)
- Periodic Table by Harkins and Hall (1916)
- Schaltenbrand's Periodic Table (1920)
- Rixon's Diagram of the Periodic Table (1933)
- Spring's Diagram (1881)
- Flavitzky's Arrangement (1887)
- Stephenson's Statistical Periodic Table (1929)
- Friend's Periodic System (1927)
- Many others, including: Vogel (1918), Stintzing (1916) and Caswell (1929) are discribed without the benefit diagrams.
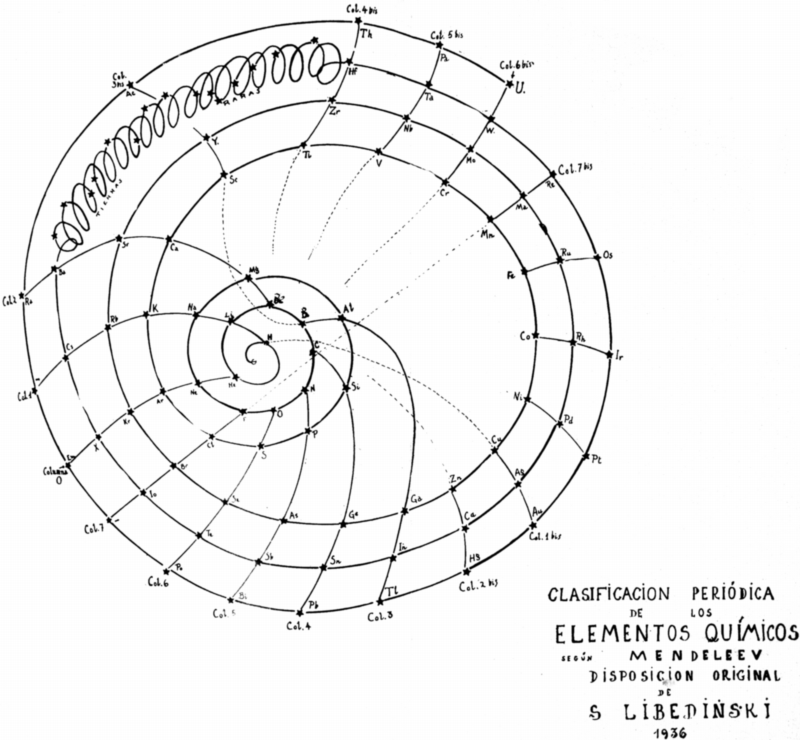
| Year: 1936 | PT id = 909, Type = formulation spiral |
Libedinski's Periodic Classification of The Elements
Simón Libedinski: PERIODIC CLASSIFICATION OF THE ELEMENTS, from his book: Dialectical Materialism, in Nature, in Society and in Medicine, Ediciones Ercilla, Santiago de Chile, 1938, pp 56-57:
"Mendeleev's Table, like that of Werner and others, are not, however, more than flat projections of the actual ordering of the elements. There is as much difference between Mendeleev's Table and the real group as there is between the planisphere and a rotating globe. A rational representation, starting from the simplest element – the negative electron –, would be a spiral line that, surrounding said central point, first gave a small turn, touching only two bodies: hydrogen and helium. From here it would jump to a much larger orbit, in which it would touch eight bodies and then another equal, also of eight. From here, another jump to a much larger orbit, comprising eighteen bodies, and then another equal; from this point one jumps to another orbit, again augmented, comprising thirty-two bodies (including rare earths); and when this round is over, the last one begins, to vanish a short distance.
"In the dialectical grouping of the elements, which I have the satisfaction of exposing, the classic arrangement of the same is respected. Only the arrangement changes, which instead of being rectilinear, is spiral. So I managed to suppress the anomaly of the double columns, and comfortably incorporate the important group of rare earths. I can not give my graphic the name of Tabla, because it is just the opposite: it aims to give the idea of ??space, and of movement in space. The double columns of the Classic Table can be found here as well, but only if you look through the whole, considered as a planetary system of conical shape, with the electron at the vertex. Effectively: column 1 coincides, through space, with column 1a; column 4 with column 4 bis, etc. The dialectical grouping also allows us to easily appreciate the remarkable dialectical character of the properties of matter: these properties are repeated periodically. These are the "returns" to qualities or previous properties, but not exactly equal to those, but only similar: and this resemblance, only to a certain extent. The difference is that that quality, those properties or some characteristic, are exalted to each dialectical return."
Contributed by Julio Antonio Gutiérrez Samanez, Cusco, Peru, March 2018 (using Google Translation)
| Year: 1937 | PT id = 25, Type = formulation spiral |
Pozzi Spiral Periodic Table
A spiral periodic table formulation constructed by E.C. Pozzi in 1937, from here.
Note the "Strong Positive, Strong Negative, Weak Positive and Weak Negative" corners:
| Year: 1939 | PT id = 627, Type = formulation spiral |
Periodic Chart in Spiral Form
Periodic Chart in Spiral Form from K. Gordon Irwin, Periodicity Patterns of The Elements J.Chem.Ed. 1939 16 (7), 335 DOI: 10.1021/ed016p335
Thanks to René Vernon for the reference:

| Year: 1942 | PT id = 1081, Type = formulation spiral |
Kipp (& Mazurs') Periodic Table in Style of Spiral and Plane Lemniscate
Kipp, Friedrich, and Edward G. Mazurs. "Periodic Table in Style of Spiral and Plane Lemniscate". Glass, circa 1942–1957. Edward G. Mazurs Collection of Periodic Systems Images, Box 1. Science History Institute, Philadelphia. https://digital.sciencehistory.org/works/nz806022g
Periodic table in style of spiral and plane lemniscate 1942 (Original design) circa 1957 (Date attributed to slide).
This table was originated by Friedrich Kipp in 1942 and classified by chemist Edward G. Mazurs as Type IIB2-2 in his seminal work Types of Graphic Representation of the Periodic System of Chemical Elements (1957).A version of this table appears as Figure 49 on page 122 of Mazurs' 1957 publication.
Thanks to Dhr. J.G. van Gils for the tip!
| Year: 1944 | PT id = 1305, Type = formulation spiral |
Emerson's Spiral Formulation
Emerson EI, 1944, A new spiral form of the periodic table, JChemEd., vol. 21, no. 3, pp. 111–115
René Vernon writes:
Emerson says that the elements in the A groups are called the representative elements because, as Eble states, they "include metals, nonmetals, inert elements, liquids, and gases." Eble RL, 1938, Atomic structure and the periodic table, JChemEd., vol. 15, p. 575
Note the inclusion in Emerson’s table of the neutron as element 0. Astonishingly, Emerson writes: "Element 0, possibly neutron [sic], is considered as a noble gas. Because of its probable chemical inertness and extreme density it might not be detected in a sizeable amount until some future scientist succeeds in sampling the center of the Earth." (p. 111)
(Mark Leach adds: The date is 1944 when the Manhattan Project was in full swing and nothing was being published about nuclear physics and/or neutron interactions. This idea may have come from some type of Popular Science story?)
Other features:
- The A and B groups are diametrically opposed in their positioning. "The electron structure of H either as H+ or H– finds a counterpart in the structure of element 0 or 2." (p. 113)
- The cell spaces for Be and Mg have been stretched on account of uncertainty as to whether they belong to group 2 or group 12. "The break between the periphery of the loops of the spiral along the spaces allotted to Mg and the transition metals of the fourth period serves to indicate that Be and Mg are not to be considered as a kind of prototype of these groups." (p. 111)
- "The C group is shown as a separate segment. If one were not concerned by plane representation the rare earths could be represented as a loop or bulge above the surface of the plane. One might imagine that this group of metals is a sort of hernia of nature that has been excised so as to maintain a flat surface." (p. 112–113)

| Year: 1946 | PT id = 776, Type = formulation |
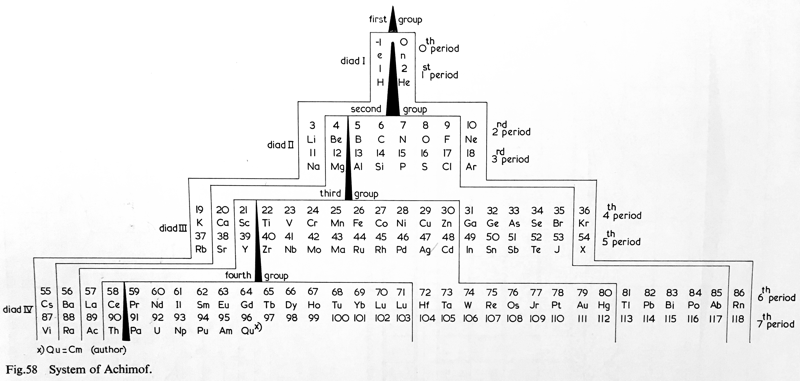
Achimof's System
Van Spronsen, on p. 157, says:
"Achimov's system took the form of a cross-section of a pyramid. He based his system on the principle that the lengths of the periods and the analogies in properties between the elements of these periods must be clearly demonstrated."
Achimov EI 1946 Zhur. Obshchei Khim., vol. 16, p. 961
Thanks to René for the tip!
| Year: 1949 | PT id = 1018, Type = formulation 3D |
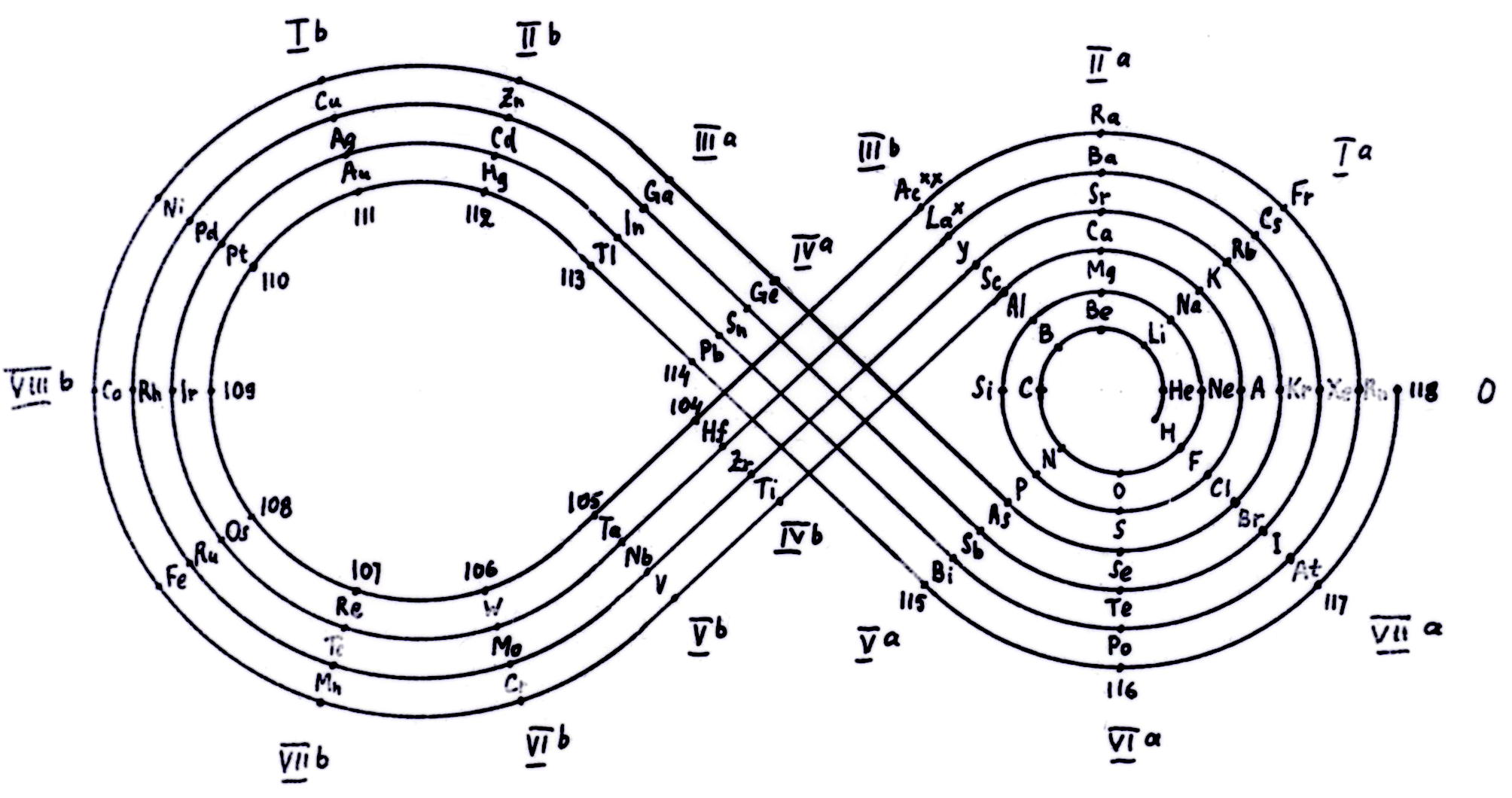
Scherer's Student Model of Spiral Periodic Chart
George A. Scherer, New Aids for Teaching the Periodic Law, School Science and Mathematics, vol. 49, no. 2 (1949).
René Vernon writes:
"This is a Left-Step periodic table with a split d-block, that can be rearranged into a cylinder. Students were expected to keep a copy of the two halves of the table in their note books, for reassembly as required. It was a clever way of introducing the 32-column form, and the transition from 2D to 3D (that faded into obscurity)":
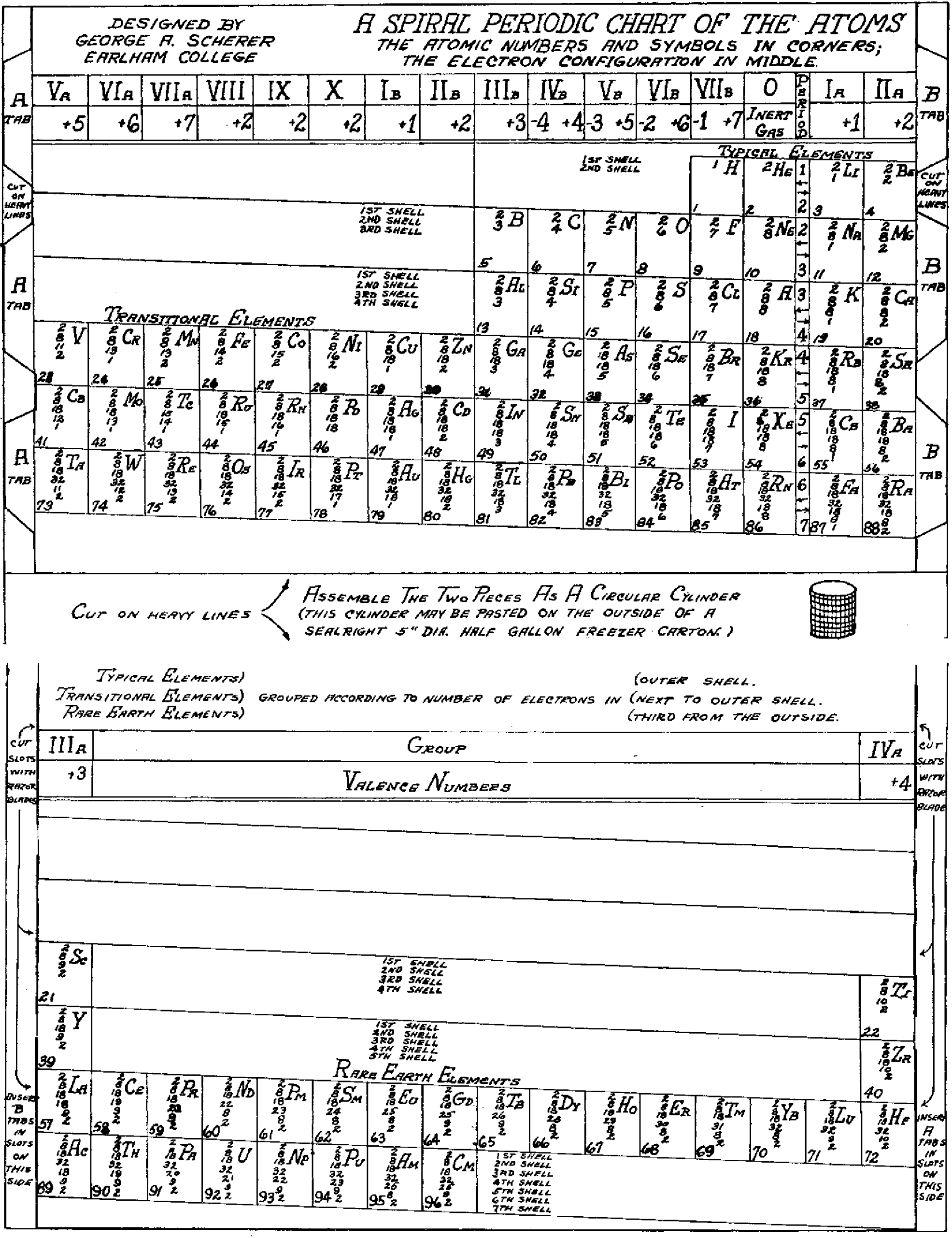
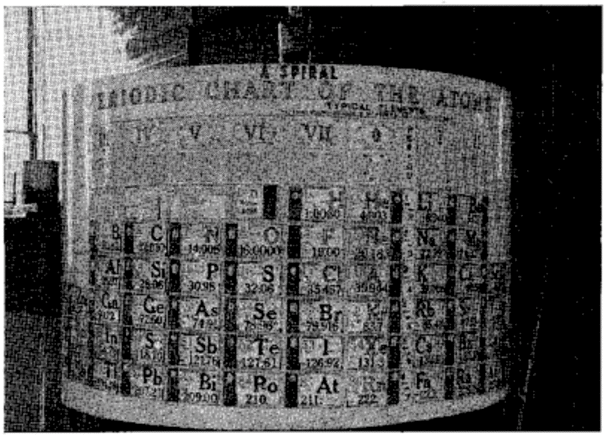
Thanks to René for the tip!
| Year: 1963 | PT id = 1033, Type = formulation 3D spiral |
Royal Military College of Science Three-dimensional Spiral
From a Science Museum blog, Rajay Shah writes:
"Supported by poles and twisting around itself in a snake-like manner, this object is one of many weird and interesting forms of the periodic table. It was built at the Royal Military College of Science in 1963. The Science Museum asked for this model to be made for them to display in their new chemistry gallery after the original model was seen at an exhibition held by the Physical Society.":


| Year: 1964 | PT id = 33, Type = formulation spiral |
Benfey's Spiral Periodic Table or Periodic Snail
Spiral Periodic Table by Otto Theodor Benfey:
From Wikipedia:
"One of the Benfey's publications in Chemistry was a model of an extended periodic table, sometimes referred to as the periodic snail. First published in 1964, it explicitly showed the location of lanthanides and actinides. The elements form a two-dimensional spiral, starting from hydrogen, and folding their way around two peninsulars, the transition metals, and lanthanides and actinides. A superactinide island is already slotted in."
Read more here in an article by OTB: Bull. Hist. Chem., VOLUME 34, Number 2 (2009)
| Year: 1964 | PT id = 732, Type = formulation spiral |
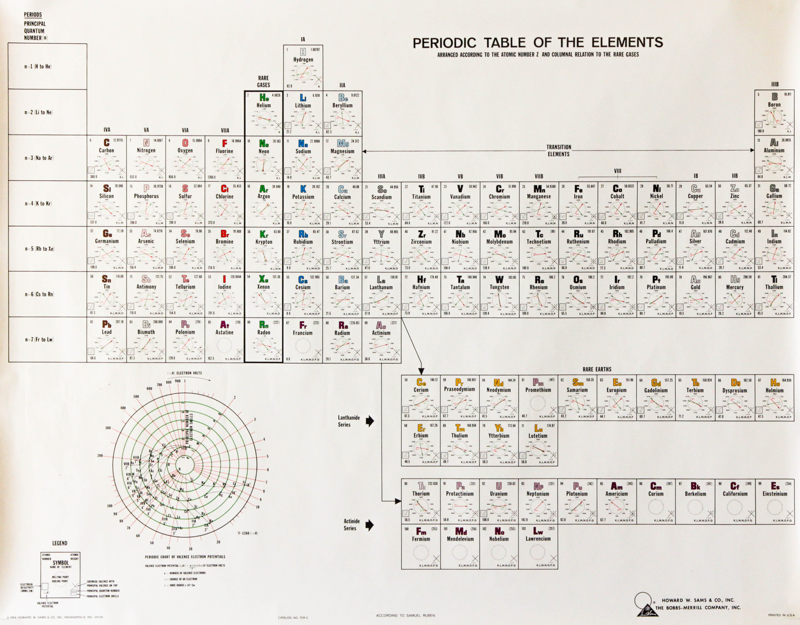
Samuel Ruben Periodic Table
An interesting periodic table from 1964, found at an estate sale. The text says that the elements are: "arranged according to the atomic number Z and column relation to the rare gases", and is by Samuel Ruben (wikipedia).
Click here to see the full size version.
Thanks to Rachel Helling for the tip!
| Year: 1965 | PT id = 525, Type = formulation spiral 3D |
Giguère's Periodic Table
Paul Giguère's Periodic Table formulation, "The 'new look' for the periodic system". Chemistry in Canada vol. 18 (12): 36–39 (see p. 37). More info here: https://github.com/groverlab/giguere-3D-periodic-table.
René Vernon writes:
"I have not considered Giguère’s table at any length, so the following pros and cons are off the top of my head:
Pros:
- Elegant and visually appealing overall design.
- Offers an impression of continuity through its (almost) spiral layout.
Cons:
- In practice, this formulation does not resolve the discontinuity of periods any better than the conventional table; one still needs to complete one turn of the spiral and then mentally leap to the next, much as one moves from one row to the next in the flat form.
- Includes the He-over-Be placement, which remains controversial.
- Quantitative comparisons (group or period properties) become less readable; there’s no immediate visual sense of columns.
- Despite its elegance, the f-block placement appears somewhat awkward.
- Communicates feel more than data.
- It seems to imply relationships between groups on opposite sides of the p-, d- and f-blocks (for instance, between Sc-Y-Lu-Lr and Zn-Cd-Hg-Cn), whereas the actual correspondences run the other way; that is, between the early and later transition groups, such as 3–7 and 8–12. As Imyanitov (2018) observed: "In a generalised form, the properties of the early d? (f?) elements and their compounds are similar to those of the late d? + 5 (f? + 7)."
It’s striking that there are only two pros but several cons, perhaps a reflection of the inherent difficulties faced by three-dimensional periodic tables in improving on the conventional form?"

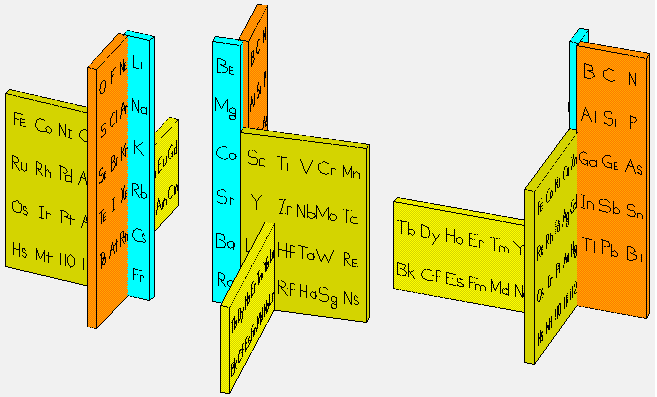
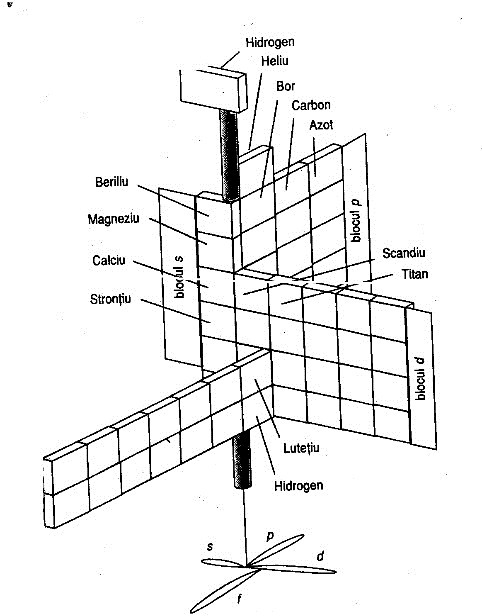
| Year: 1974 | PT id = 260, Type = formulation spiral |
Mazurs Version of Janet's "Lemniscate" Formulation
Janet's lemniscate formulation periodic table as modified by E.G. Mazur in his Graphic Representations of the Periodic System during One Hundred Years (1974), cited in Punyashloke Mishra's The Role of Abstraction in Scientific Illustration: Implications for Pedagogy (1999) republished in Carolyn Handa's Visual Rhetoric in a Digital World: A Critical Sourcebook", from the Island94 blog, here:
| Year: 1974 | PT id = 1058, Type = formulation spiral |
Mazurs' Redrawing of Stedman's Formulation
An spiral formulation by Mazurs, cited as being after Janet (1928). However, it is actually, it is after Stedman (1947).
In an article Bull. Hist. Chem., VOLUME 34, Number 2 (2009) O.T. Benfey writes:
"After we had developed our own [Periodic Snail] spiral design, we found that E. G. Mazurs had published a spiral with a separate protrusion for the lanthanides which, under the image, he misleadingly ascribed to Charles Janet in 1928, the same year that Janet had published a simple circular form also shown by Mazurs. The Mazurs diagram with the lanthanide protrusion was reprinted in [the journal] Chemistry. However, [Philip] Stewart informed me that the Mazurs figure bears no resemblance to the Janet diagram he indicated nor to any other of his designs. Detailed references given a few pages later by Mazurs suggested correctly that the spiral derives from Stedman and is so identified and depicted by van Spronsen. The Mazurs diagram is a mirror image of the Stedman spiral, updated to include elements discovered since 1947." [For references, see the article.]"
Mazurs (p. 77) writes:
"Subtype IIIA3–1a Helix on a modified cone. The transition and inner transition elements have special revolutions in the form of loops. This table, originated by Stedman in 1947 is not a successful one."
Thanks to René for the tip and information!
| Year: 1974 | PT id = 299, Type = formulation spiral 3D misc |
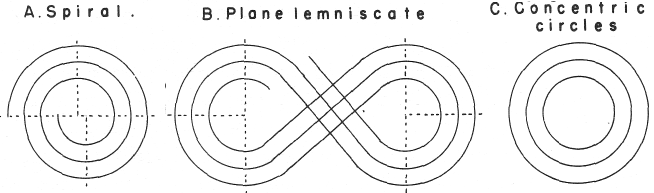
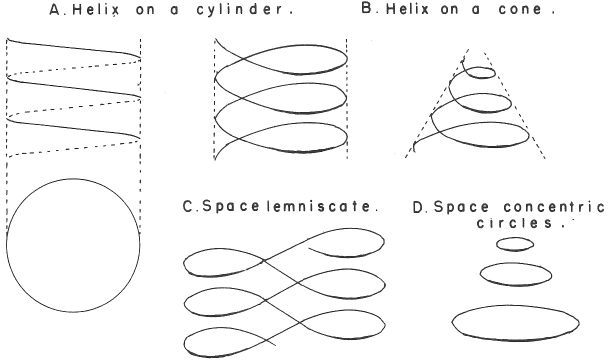
Mazurs' PT Formulation Analysis
In his 1974 book Edward G. Mazurs (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press gives a comprehensive analysis of periodic table formulations.
Mazurs identifies most PT formulations as being:
- Spiral
- Plane lemniscate
- Concentric circles
- Helix on a cylinder
- Helix on a cone
- Space lemniscate
- Space concentric circles
| Year: 1979 | PT id = 471, Type = non-chem formulation spiral |
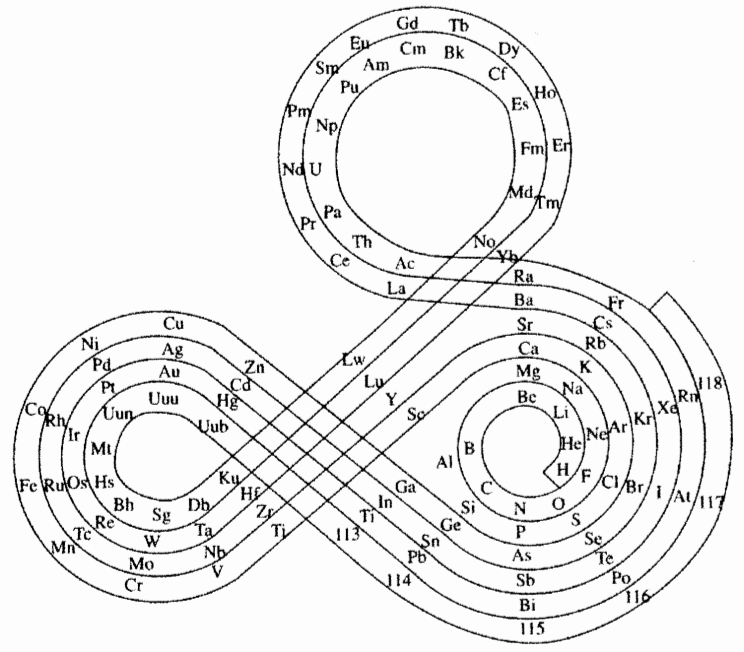
Mann's Spiral Periodic Table
From AT Mann:
"I designed a spiral periodic table which was published first in my book The Divine Plot: Astrology, Reincarnation, Cosmology and History (George Allen & Unwin, London, 1986) which attempts to correlate the PT with astrological understanding of the inherent properties of the signs and planets":
| Year: 1999 | PT id = 36, Type = formulation spiral |
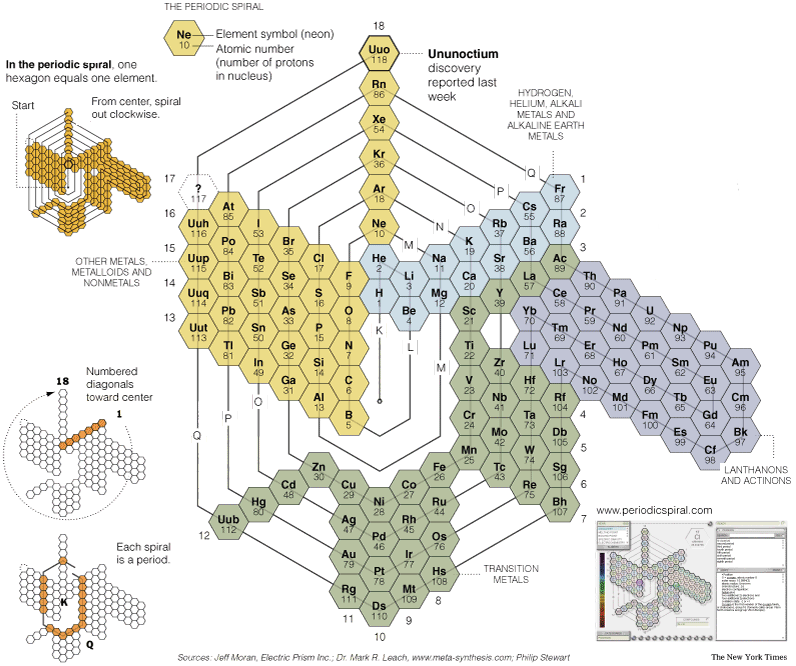
Moran's Spiral Periodic Table
Jeoff Moran's spiral periodic table can be found at periodicspiral.com.
See an article in the New York Times:
| Year: 2003 | PT id = 573, Type = formulation spiral |
Bird of Prey Periodic Table
From Edmond (Ned) Maurice Peyroux:
"I am a self-taught, underground cartoonist - around the end of 2005 I began studying ether physics, & mid 2006 orgone biophysics. End of 2008 I was going through old note & sketch books while compiling pieces for a poetry book, & came across a sketch I did in 2003 of the first 20 elements of the periodic table in a spiral. I had just begun studying the ether vortex model of the atom & thought a vortex model of the periodicity might be a fun experiment so I played with it more. I didn't remember what inspired the original concept sketch 5 years later, but my guess was I had stayed up too late watching public television again. It probably had to do with some 4-Dimension ring concepts I was playing with, but by 2008 I was thoroughly involved in 3-D biophysics & wasn't thinking back to earlier thought experiments I had done."
"The compositions are largely artistic, naturalistic, & most are like steps on a story board, showing transformation of the table, distorting from from rectangular to spiral, then splitting between metals & noble gases like the wings of a bird, flapping, then joining again to make the spiral (then the spiral inflates to make a flower, wilts into a spider's web) - there are many transitions I have in mind, but I my work is not limited to the periodic table.":
| Year: 2003 | PT id = 600, Type = formulation spiral |
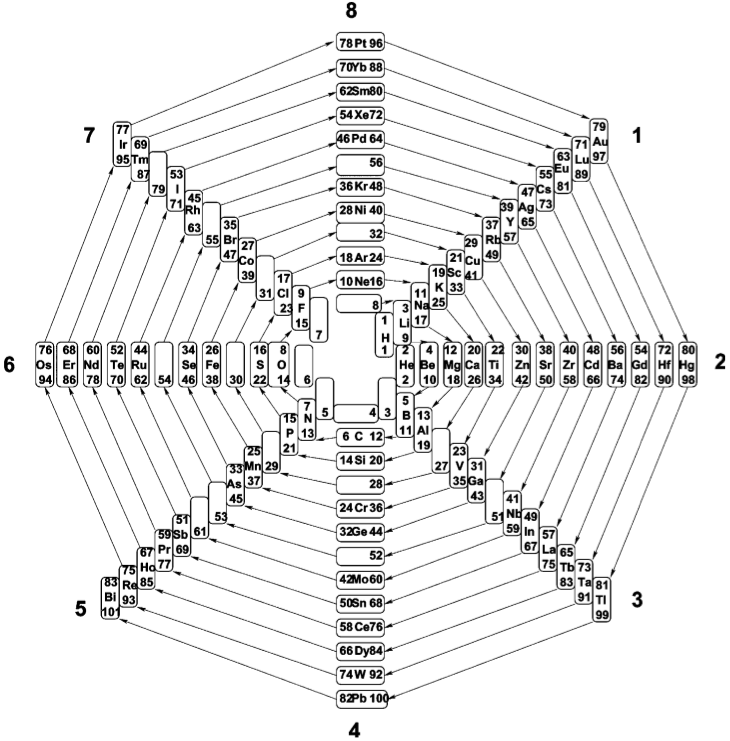
Eight-Group Periodic Table
From Number Patterns in Nature by Jan C.A. Boeyens, Crystal Engineering 6 (2003) 167–185.
The Eight-Group Periodic Table of the 81 stable elements, in spiral form. Available sites on the prime-number cross, starting from zero, number 102.,
| Year: 2004 | PT id = 143, Type = data misc |
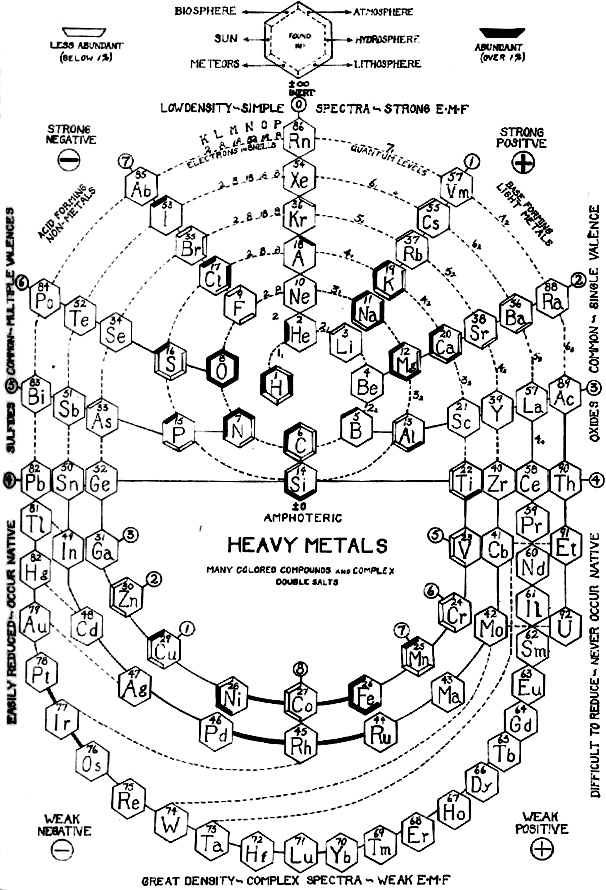
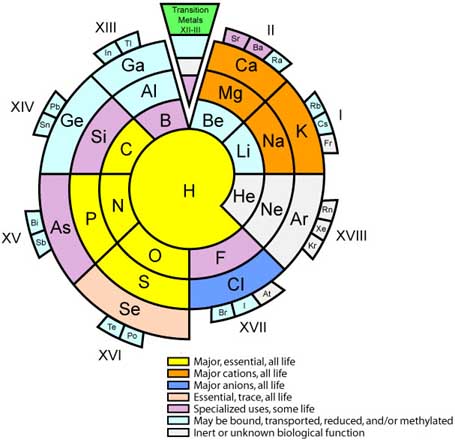
Biologist's Periodic Tables
A periodic table showing where biologically essential (green), essential trace (purple), toxic (red), radioactive (yellow) and of low – but not zero– biological impact (gray) elements are found. Only highly toxic elements are shown in red. Li (as Li+) is biologically active and is used as an antidepressant.
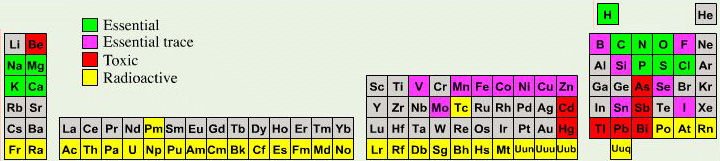
By Mark Leach
or here:
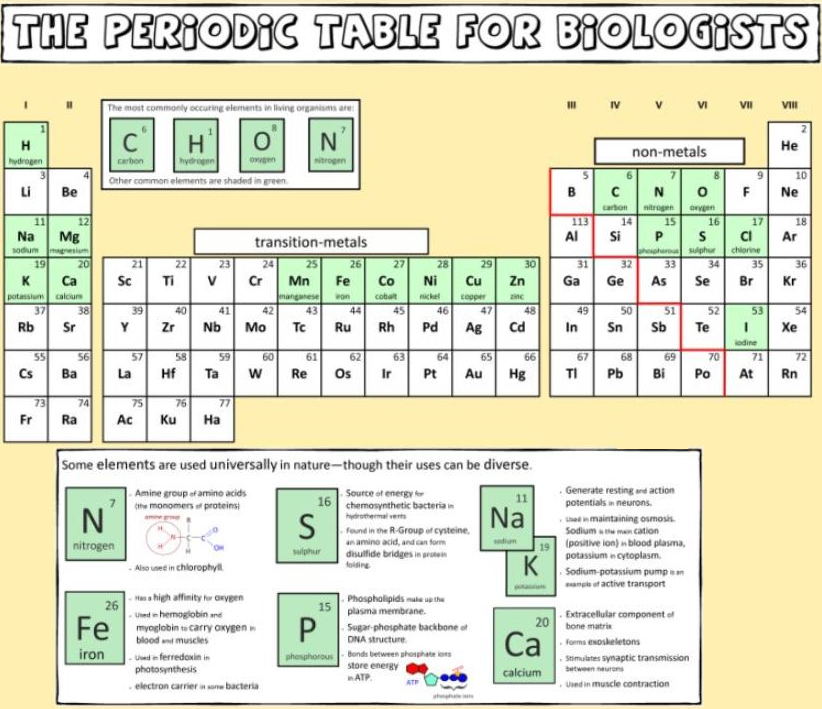
And a periodic table for biologists from Science Videos:
| Year: 2005 | PT id = 37, Type = formulation spiral |
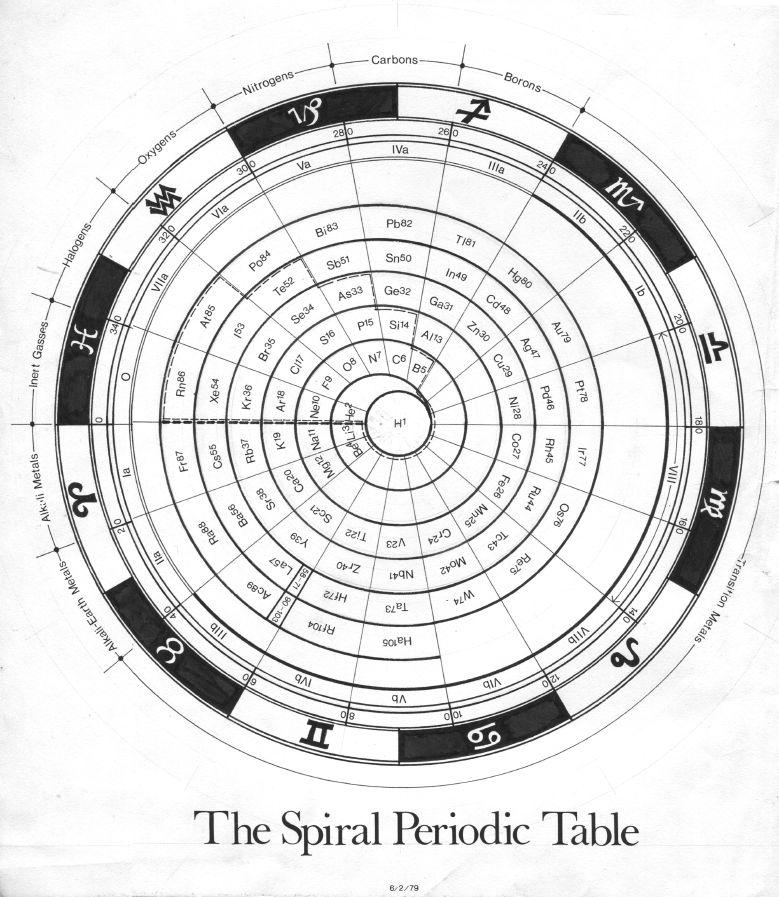
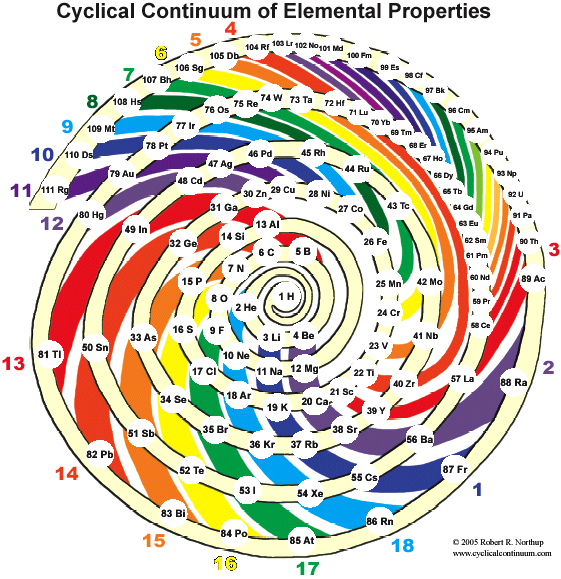
Cyclical Continuum of Elemental Properties by Robert R. Northup
The Cyclical Continuum of Elemental Properties Periodic Table by Robert R. Northup
"The Cyclical Continuum of Elemental Properties is a user-friendly teaching tool that is intended to accompany the Periodic Table of Elements. Hydrogen is shown at the center, atomic numbers and symbols form an unbroken spiral, and element groups 1 through 18 (noble gases, alkali metals, halogens, etc.) are displayed by colored arcs. Beginning chemistry students can visually see the continuity of atomic numbers in the Cyclical Continuum as a way to introduce and orient them to the Periodic Table. Advanced chemistry students can test their understanding of the Periodic Table's organization by applying that knowledge to interpretation of the Cyclical Continuum."
Read more and buy the poster at the Cyclical Continuum web site.
| Year: 2006 | PT id = 563, Type = formulation spiral |
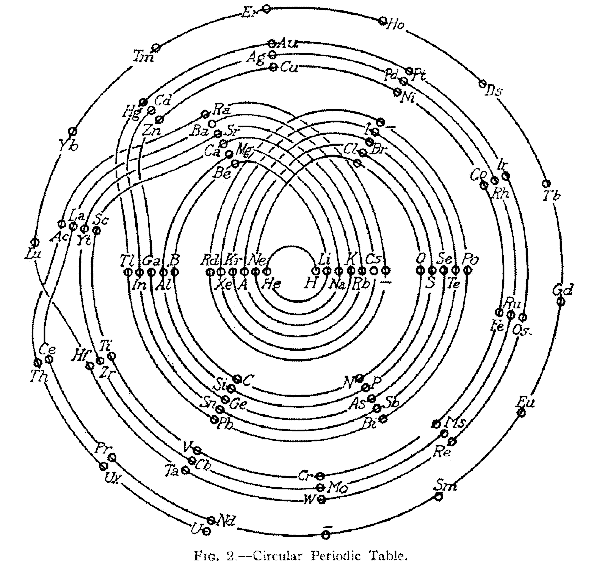
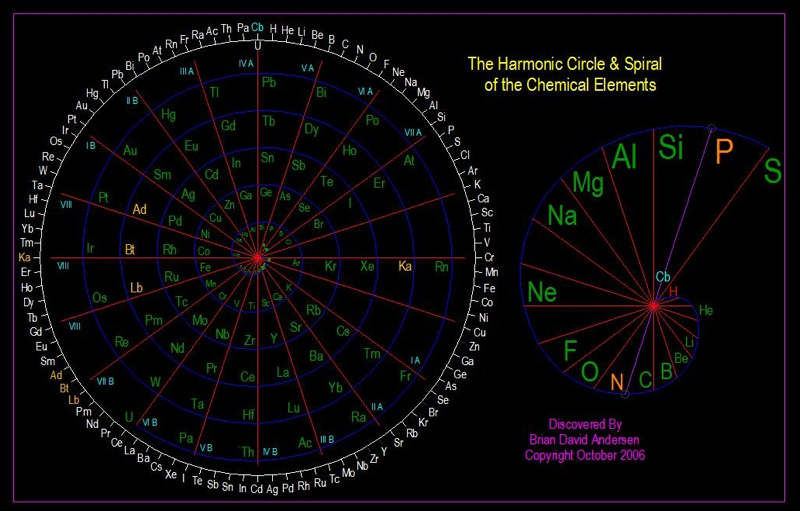
Harmonic Circle & Spiral of the Chemical Elements
Brian David Andersen of Tri-Vortex Technology (Researcher/Inventor/Scientist), Subtle Energy Products trivortex.com:
| Year: 2008 | PT id = 167, Type = formulation spiral |
Jan Scholten's Periodic table (Spiral Format)
A spiral format periodic table by Jan Scholten:
| Year: 2008 | PT id = 175, Type = formulation spiral misc |
Spiral Periodic Table
A spiral periodic table available as a poster, binder, cup, T-shirt, etc. by Vectoria:
| Year: 2010 | PT id = 357, Type = formulation spiral |
Harrison Spiral Periodic Table
This spiral, inspired by Stewart's Chemical Galaxy, is based on the modern periodic table with the elements strictly arranged in the increasing order of their atomic number and in accordance with their electron configurations.
The spiral separates the elements into the eight dominant 'A' groups of normal elements, and the eight corresponding 'B' subgroups of transitional and inner transitional elements, which have been incorporated as the inner spiral. The organisation of the elements closely follows H.G. Deming's 1923 Periodic Table where A B numeration was first utilized to correspond the characteristic oxides of the 'B' groups to those of the 'A' groups. The result of this design places Group VIII, the triads Fe, Co, Ni, etc. as a subgroup of Group 0 (or 18 Helium Group) which conflicts with some modern periodic tables, though broadly agrees with Deming's original proposal (VIIIA and VIIIB).
Hydrogen, which generally cannot be considered as part of any group, has been placed with the Fluorine group VII which appears its natural place in the spiral. Common names have been used where practicable to make the table more educational and reader-friendly. Element symbols have been included in the expanded poster of this table.
Look at a larger PDF.
| Year: 2010 | PT id = 358, Type = formulation spiral |
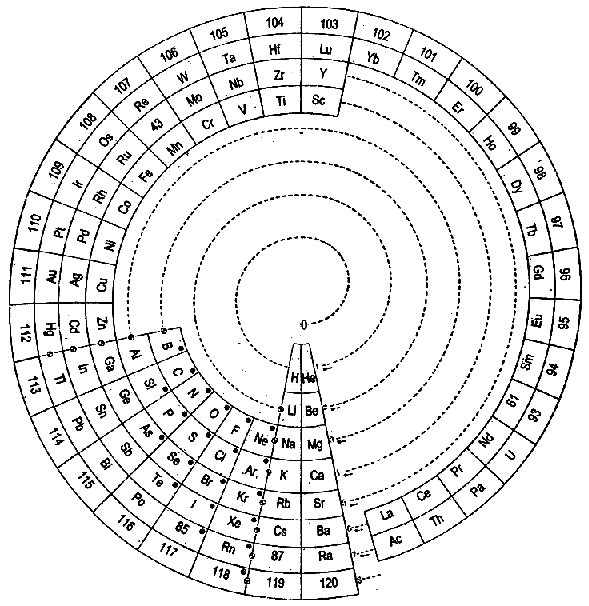
Spiral of Atoms and Their Periodic Table
Page 8 of my website (in Russian) shows The Spiral of Atoms and Their Periodic Table, which depicts a spiral disk of atoms with a periodic table of their relative masses.
This information clarifies the options published in the editions of my book The Axiomatics of Nature (2007-2009). Mark Adelman Samuilovich (Mark S. Eidelman)
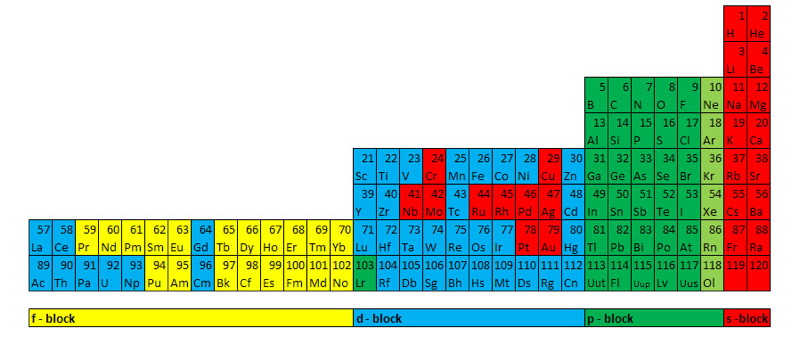
| Year: 2011 | PT id = 775, Type = formulation 3D |
Weise's Tetrahedron
Dmitry Weise shows how it is possible to go from the Janet [left-step] periodic table formulation, to a tetrahedral formulation.
Dmitry writes:
"Three-dimensional table of the periodic law can be constructed in the form of a tetrahedron having an inner order. A comparison of the tetrahedron shells and the table of elements shows, that one tetrahedron shell corresponds to 4 periods of the 2D table."
Jess Tauber adds:
"The spheres here also aren't labeled, but I explain how they get labeled in the text accompanying the pic. Each such period (except for s-only, which are obviously simpler) we have a 'switchback' configuration. Like a road going up a mountain back and forth to minimize verticality, or a parachute folded into a pack. There are 8 different ways to do this (4 basic types in 2 chirally opposite mappings). And the original Weise-style non-continuous tetrahedron is just another way to organize half tetrahedra."
| Year: 2011 | PT id = 410, Type = formulation spiral 3D |
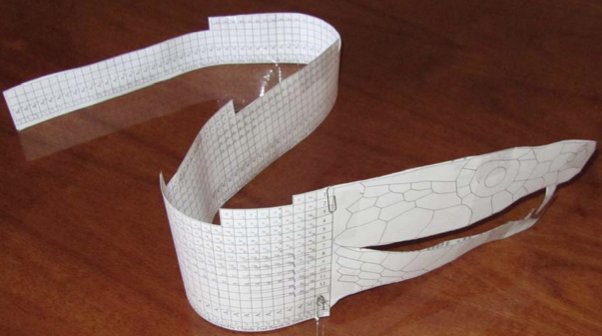
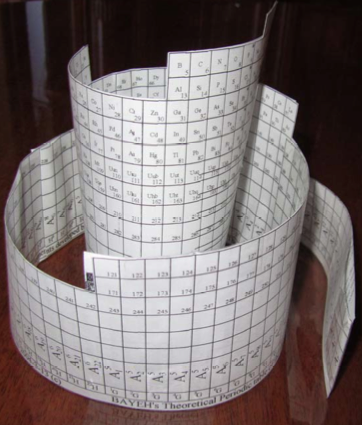
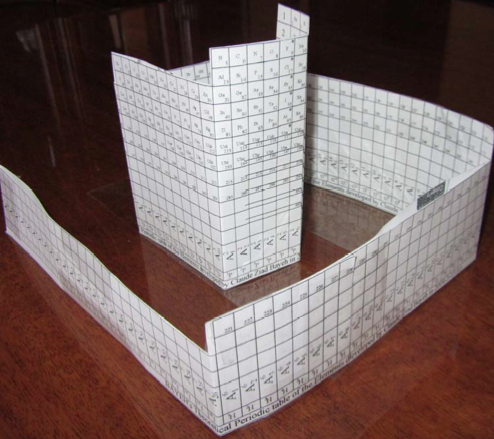
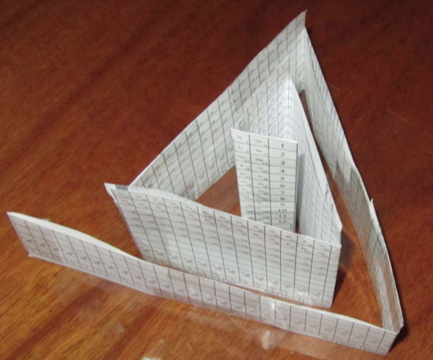
Bayeh's Theoretical 3D Periodic Tables
From Bayeh Claude: "I have designed these periodic tables as developments of Bayeh's Theoretical Periodic Table, but I have introduced new shapes and 3D versions":
- Crocodile Periodic Table
- Ship Periodic Table
- Snake Periodic Table
- Spiral Periodic Table
- Spiral rectangular Periodic Table
- Spiral triangular Periodic Table
| Year: 2012 | PT id = 551, Type = formulation |
Piazzalunga's Pyramidal Periodic Table Formulations
Three Pyramidal Periodic Table Formulations, and a Spiral, from Marco Piazzalunga:
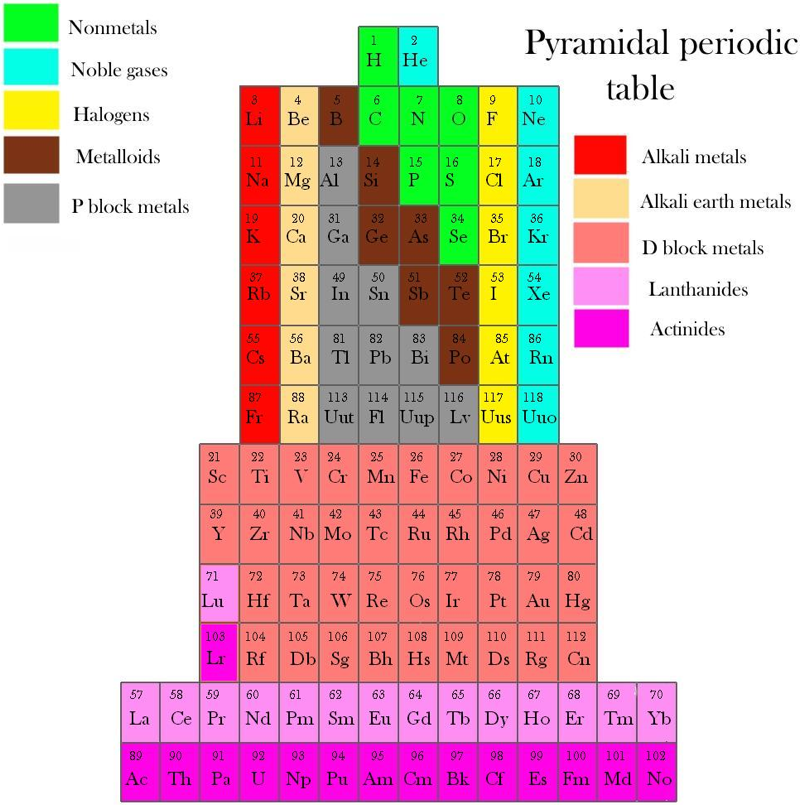
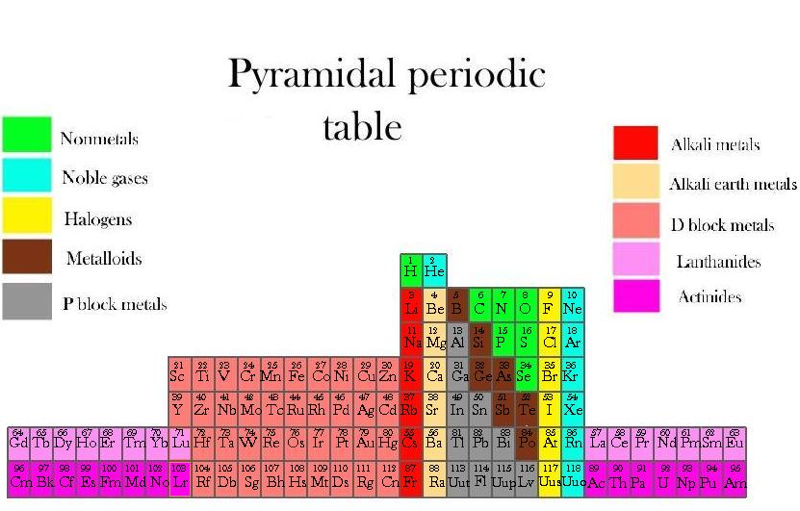
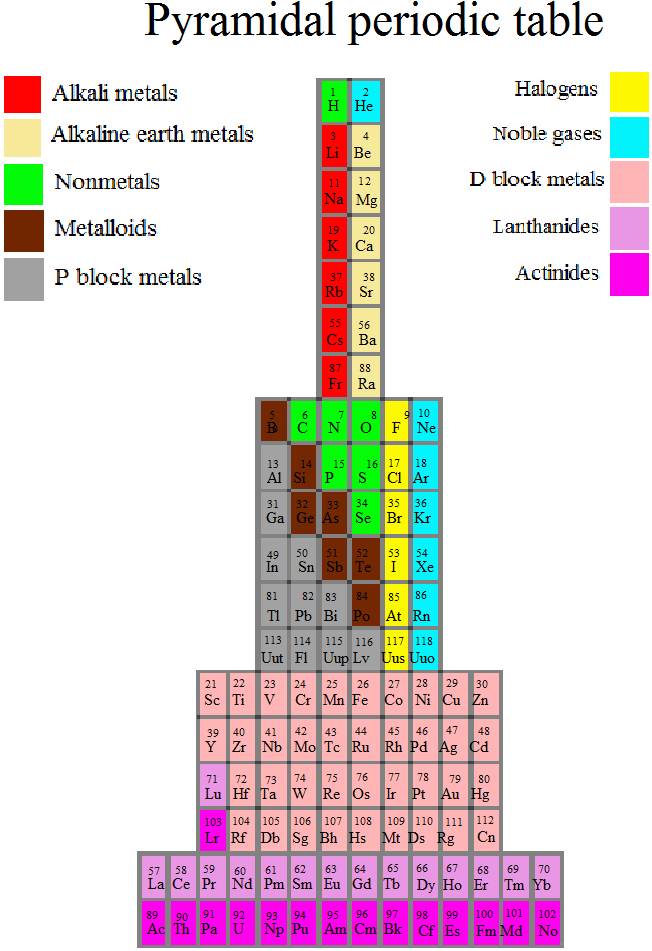
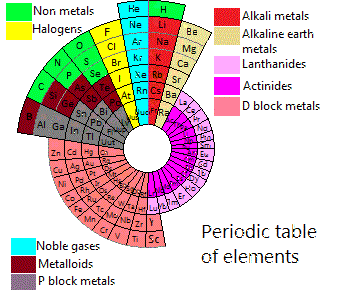
| Year: 2013 | PT id = 560, Type = formulation |
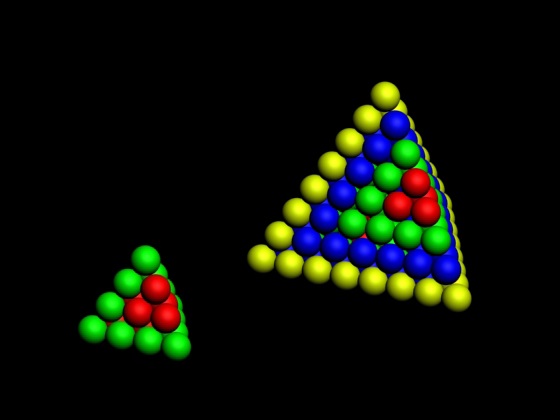
Twin Spiral Pi Trinomial - Based Periodic Table
A Twin Spiral Pi Trinomial - Based Periodic Table by Bill Harrington, Founder/CTO of Rainforest Reactor Research and Temporal Dynamics Laboratory. For full size, click the image:
| Year: 2013 | PT id = 598, Type = formulation 3D spiral |
Bernard Periodic Spiral
The Bernard Periodic Spiral of the Elements (BPSE), depicts a novel rendition of the Periodic Table that replaces the flat rectangular format with a continuous unidirectional spiral that maintains all the properties of Group and Period formation.
Comparisons may be made with similar models spanning the last three decades of the 20th century (Alexander, 1971; Mazurs, 1974; & Kaufman, 1999).
In the chart form, this new rendition is referred to as the Elliptical Periodic Chart of the Elements. In the three-dimensional form, the model resembles a Christmas tree in shape with the 7 Periods represented as circular platforms situated at various levels with the elements placed appropriately at the outer edges of each of these platforms as a Period builds up. The elements may be represented as spherical objects or flat discs with radii proportionate to atomic radii (or reasonable approximations). Color schemes accentuate the four different Blocks of elements: the s-Block (green), the p-Block (blue, with the exception that the last Group is red signifying the end of a Period), d-Block (orange), and the f-Block (yellow). The grey section, called the Group-Period Interchange, is where the end of a particular Period connects to the beginning of the next Period, and, at the same time, transitions from Group 18 to Group 1.
Watch the video here:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2013 | PT id = 610, Type = review |
Top 10 Periodic Tables
There are more than 1000 periodic tables hosted by the Chemogenesis Webbook Periodic Table database, so it can be a little difficult to find the exceptional ones.
Here we present – in our humble opinion – The ten most significant periodic tables in the database.
We present the best:
- Three best data rich periodic tables
- Five formulations which show the development of the modern PT
- One, of many, interesting alternative formulations
- One example of the periodic table being used as an infographic template
Three Excellent, Data Rich Periodic Tables
The first three of our top 10 periodic tables are classic element data repositories.
They all work in the same way: click on the element symbol to get data/information about the selected element. The three are Mark Winter's WebElements, Theo Gray's Photographic Periodic Table & Michael Dayah's Ptable.
- Since 1993 – and with its rather bland interface – WebElements has given access to vast quantities of in depth chemical data & information. This is the professional chemist's periodic table:
- Theo Gray's Photographic Periodic Table is undoubtedly the most attractive PT available in web space, but there is more. Clicking around the website gives access to a host of information, pictures & anecdotes from Theo's extraordinary and extensive collection of chemical elements:
- Ptable has a super-slick, and very fast interface. It is data/information rich and is available in 50 languages:
Five Formulations Showing The History & Development
The next five examples deal with history and development Periodic Table. The first is Dalton's 1808 list of elements, next is Mendeleev's 1869 Tabelle I, then Werner's remarkably modern looking 1905 formulation. This is followed by Janet's Left Step formulation and then a discussion of how and why the commonly used medium form PT formulation, is constructed.
- There are several early listings of chemical substances, including Valentinus' Alchemy Table and Lavoisier's Table of Simple Substances (1789). In 1803 Dalton proposed that matter consists of discrete atoms that combine in fixed ratios, stoichiometry, to form chemical elements. Thus, Dalton's list of chemical elements, plus mass data, must be included in any top ten listing:

- If you examine the periodic tables from Antiquity to 1899, you will see that from about 1830 onwards, proto-periodic tables were coming thick and fast. Significant developments include: Daubeny's Teaching Display Board of Atomic Weights (1831), Chancourtois Telluric Helix (1862) and Newlands octaves (1864).
But, it was Mendeleev's Tabelle I that was first near complete periodic table formulation of the then known elements (no Group 18 rare gasses, note). Crucially, Mendeleev identified gaps and was able to make predictions about the chemical properties of the missing substances. Plus, Mendeleev promoted his ideas with great energy:

- Werner's 1905 Periodic Table is remarkably modern looking. The formulation is a long form that separates transition metals and rare earths, but he guessed wrong on how many existed:

- Janet's Left Step formulation of 1928 is one for the purists as it clearly shows the chemical elements arranged into s, p, d & f-blocks of the recently developed quantum mechanical description of atomic structure:
- The modern (and commonly employed) periodic table is obtained by transforming Janet's Left Step into the modern long form periodic table by rearranging the blocks around. This transformational mapping is discussed in some detail here.
The long form and medium form PTs have electronegativity trending from top-right (electronegative) to bottom left (electropositive), and many aspects of periodicity corollate with electronegativity: atomic radius, first ionisation energy, etc.
Thus, the long form and medium form periodic tables are commonly used in the classroom:
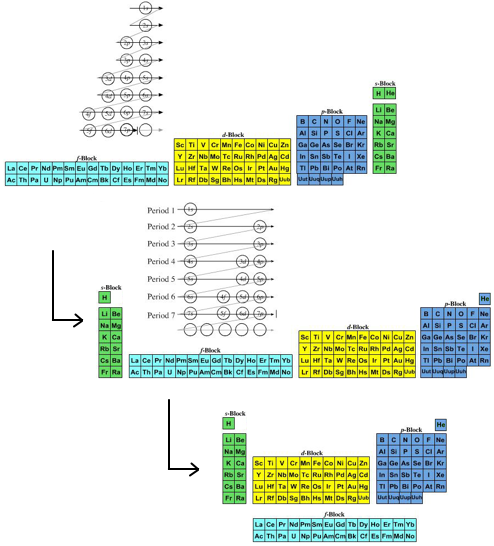
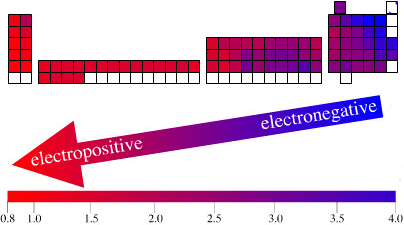
An Alternative Formulation
The internet database contains many, many alternative formulations, and these are often spiral and/or three dimensional. These exemplified by the 1965 Alexander DeskTopper Arrangement. To see the variety of formulations available, check out the Spiral & Helical and 3-Dimensional formulations in the database:

Non-Chemistry PTs
The periodic table as a motif is a useful and commonly used infographic template for arranging many types of object with, from 50 to 150 members.
There are numerous examples in the Non-Chemistry section where dozens of completely random representations can be found:
- Adobe Illustrator Shortcuts
- Adult Positions
- Airline Customer Reviews
- Beer Styles
- And, chosen more or less at random, European Nations:
| Year: 2014 | PT id = 705, Type = formulation 3d helix |
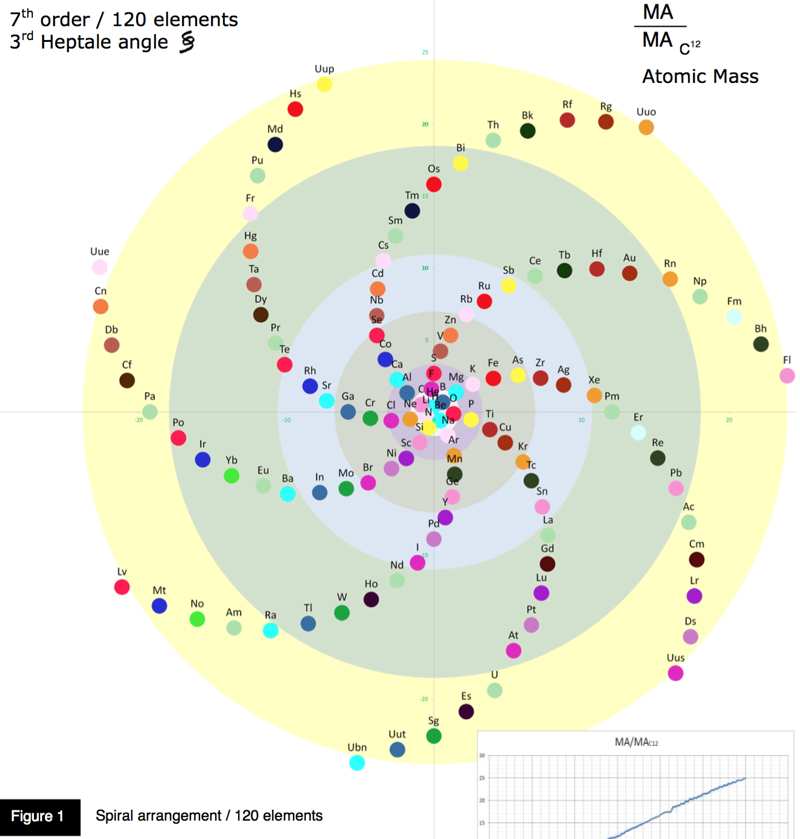
Arrangement of Elements 7th Order & Element Sequences
An exploration of some mathematics underlying the periodic table, read the PDF here, by Olivier Joseph.
Oliver says:
"May I propose you the following pattern, as the result of a personal study concerning the arrangement of the Elements, including sequences. Based on some hypothesis and as depicted in the enclosed illustrations, the elements are positioned according to a spiral function of atomic number and atomic mass, representation in 2D in a spiral form pattern, or in 3D conical helix model.
"The elements are numbered and placed consecutively along this spiral according to a specific angle, appropriately established between each element, forming a seven arm spiral pattern. With such an angle, specifically defined, a link is established between the various elements of a same group (corresponding to chemical elements with similar properties) and different layers. These latter becoming distributed among each arm of the spiral in a notable arranged way."
| Year: 2015 | PT id = 674, Type = formulation 3D |
UVS Periodic Table Model of a Klein Bottle Topology
This configuration can topologically suggest the g-block cycle in the 8th period for extended periodic table.
In the Klein bottle topology as illustrated, it is plausible that after the s-block cycle in the 8th periodical cycle, the topological path continues to spiral around the outer f-block cycle to harmonically form 14 elements.
And then subjected to the spiral Möbius strip topological twist, it could resonate to form 4 more elements in the anti-cyclonic path around 17th, 18th, 1st, and 2nd angular phases of the anti-cyclonic core; this would render the 18 elemental positions for the hypothetical g-block cycle in the entire half-integral anti-cyclonic cycle of the Klein bottle topology.
Hypothetically, the topological path then moves into the cyclonic cycle, and harmonically forms its d-block and p-block cycles with 16 elemental positions to complete the 8th periodical cycle with a total of 36 elements.
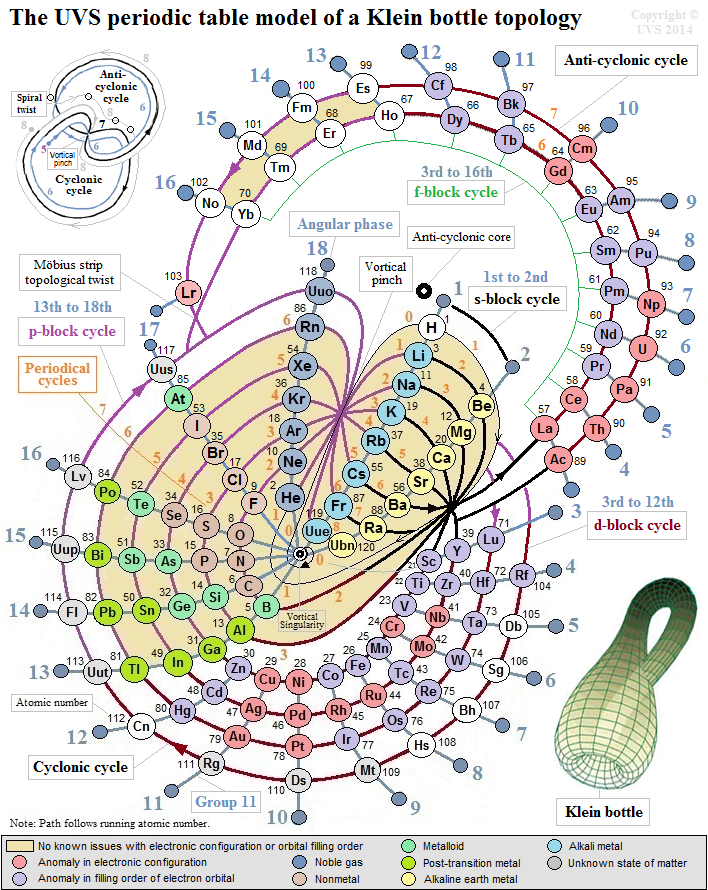
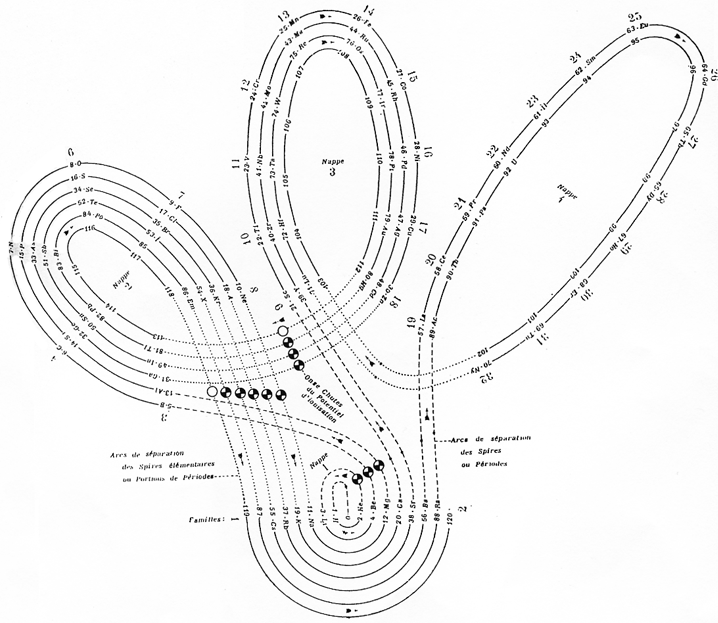
| Year: 2016 | PT id = 725, Type = formulation sprial |
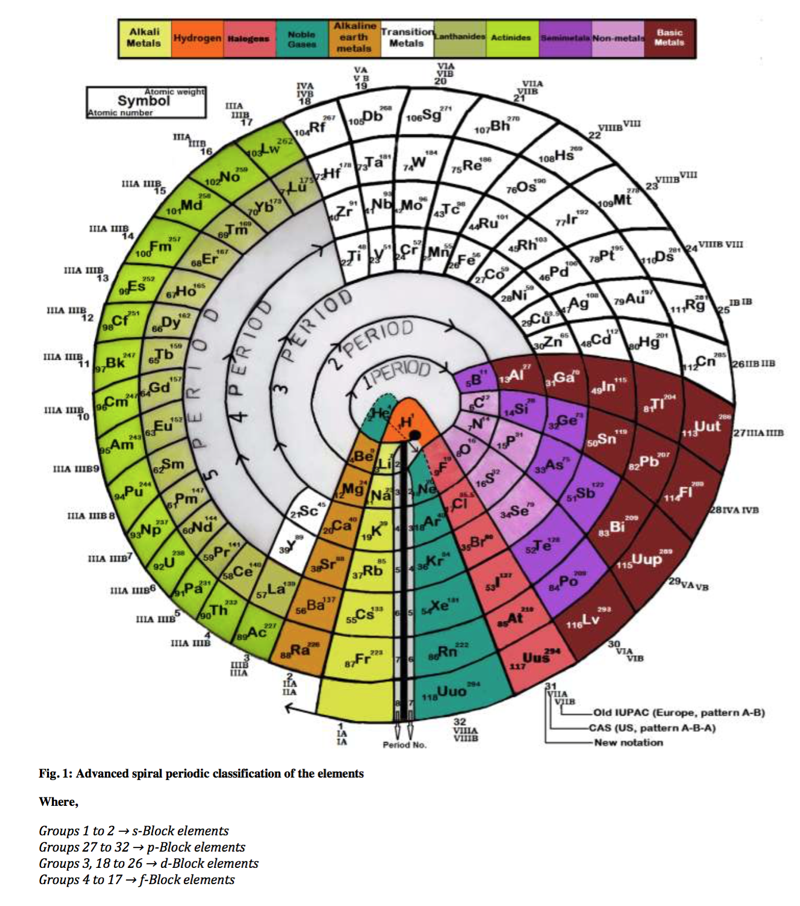
Advanced Spiral Periodic Classification of the Elements
By Imran Ali, Mohd. Suhail and Al Arsh Basheer an Advanced spiral periodic classification of the elements. Read the paper here.
| Year: 2016 | PT id = 742, Type = formulation 3D spiral |
Instructables 3D Periodic Table
From Makendo on the Instructables website:
The first periodic table was developed in 1862 by a French geologist called Alexandre-Émile Béguyer de Chancourtois. He plotted the elements on a cylinder with a circumference of 16 units, and noted the resulting helix placed elements with similar properties in line with each other. But his idea - which he called the "Telluric Spiral" (see here), because the element tellurium was near the middle - never caught on, perhaps because it was published in a geology journal unread by chemists, and because de Chancourtois failed to include the diagram and described the helix as a square circle triangle.
Mendeleev got all the glory, and it is his 1869 version (dramatically updated, but still recognizable) that nearly everyone uses today.
This instructable [project] documents my efforts to reimagine a 3D periodic table of the elements, using modern making methods. It's based on the structure of a chiral nanotube, and is made from a 3D printed lattice, laser cut acrylic, a lazy susan bearing, 118 sample vials and a cylindrical lamp.
| Year: 2017 | PT id = 917, Type = formulation 3d spiral |
Kurushkin's Spiral Periodic Table
Mikhail Kurushkin has a way of constructing the standard long form periodic table from the Janet Left-Step formulation.
Mikhail writes in his J.Chem.Educ paper DOI: 10.1021/acs.jchemed.7b00242; J. Chem. Educ. 2017, 94, 976?979
"Addition of another s-block to the left of the left-step periodic table [enables it] to be rolled into a spiral so that the left and right s-blocks are merged together and the number of elements is exactly 118. The resulting periodic table is called the "spiral" periodic table, which is the fundamental representation of periodicity":
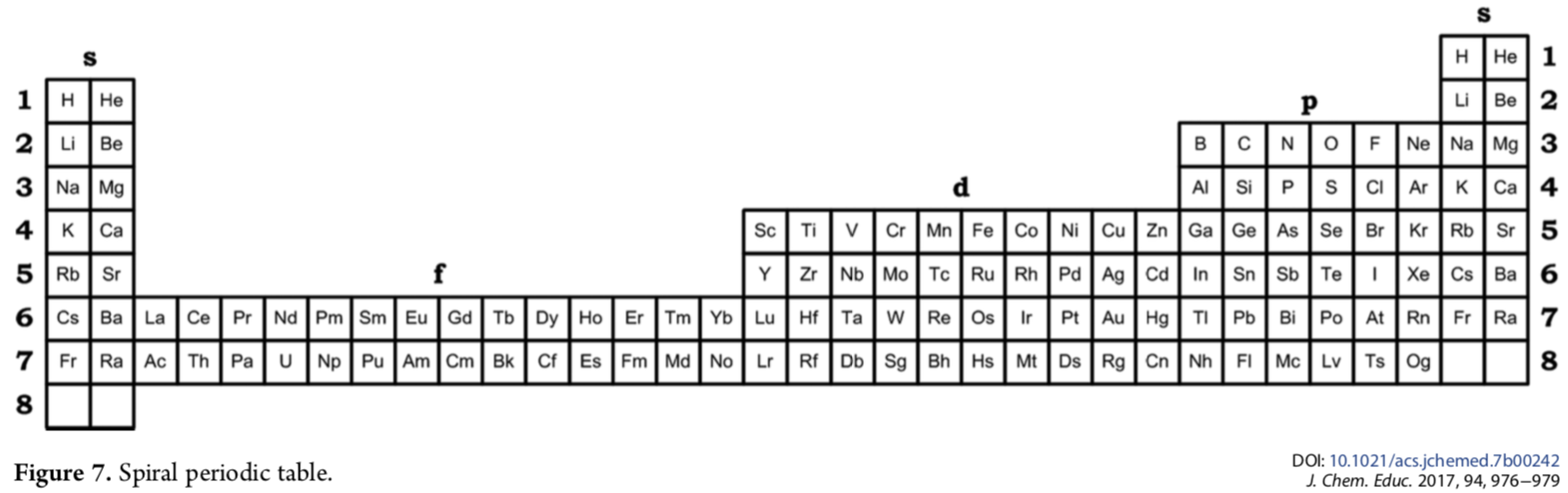
| Year: 2017 | PT id = 764, Type = formulation spiral |
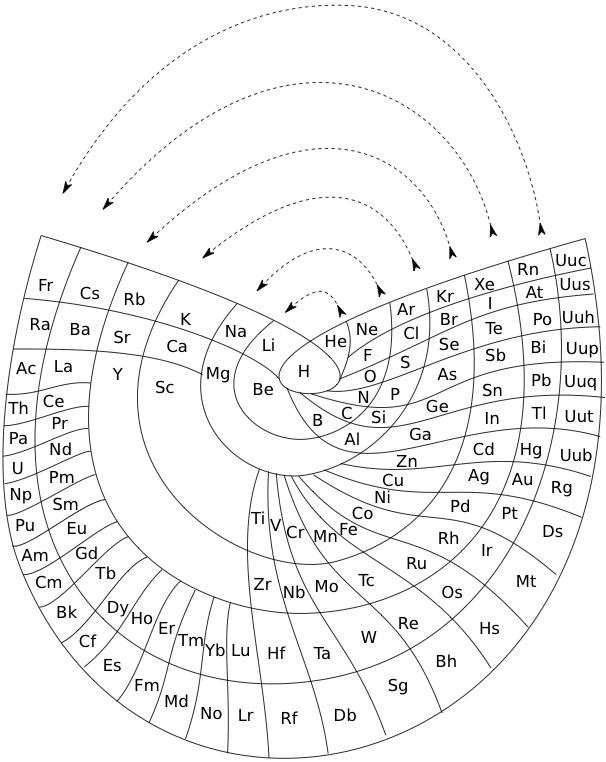
Moran's Periodic Spiral (Updated)
Jeff Moran has updated his 1999 Periodic Spiral.
Click here for a larger version.
Jeff says: I offer the attached spiral formulation as a way of expressing the relationships of the f and d blocs to group 3:
- La and Ac are assigned to the Ln and An series, respectively
- The f block series is within, though apart from, the d block
- The group 3-ish relationship of Ln and An to Sc (and, by extension, to Y) is implied
- The group 3 status of Lu and Lr is explicit
| Year: 2018 | PT id = 913, Type = formulation spiral |
Nawa–Scerri Octagonal Periodic System
A spiral periodic table formulation by Nawa, called the Nawa–Scerri Octagonal Periodic System.
Click here for a larger version:
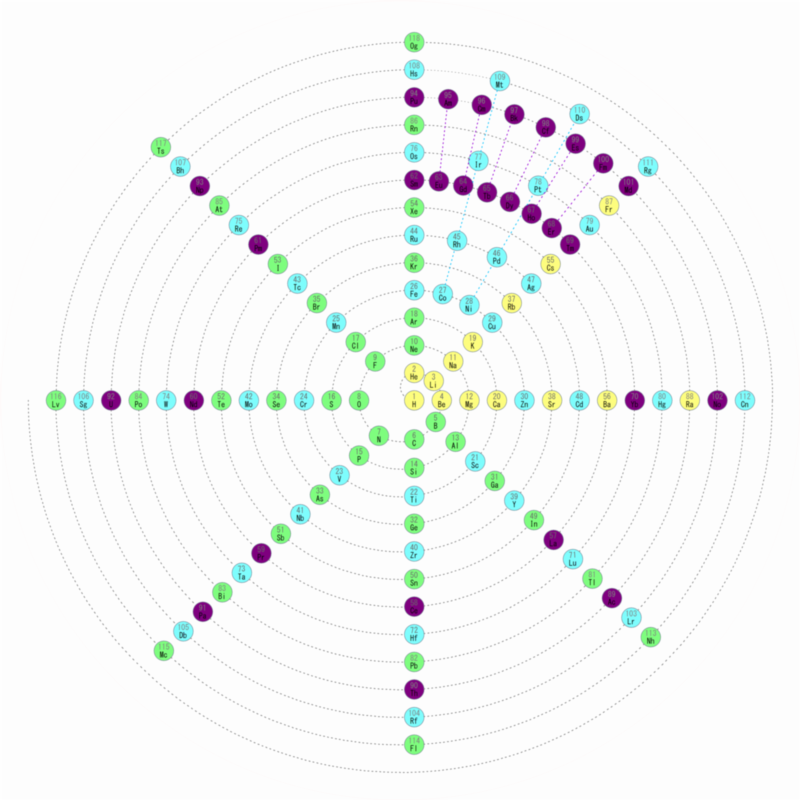
| Year: 2018 | PT id = 920, Type = formulation 3d |
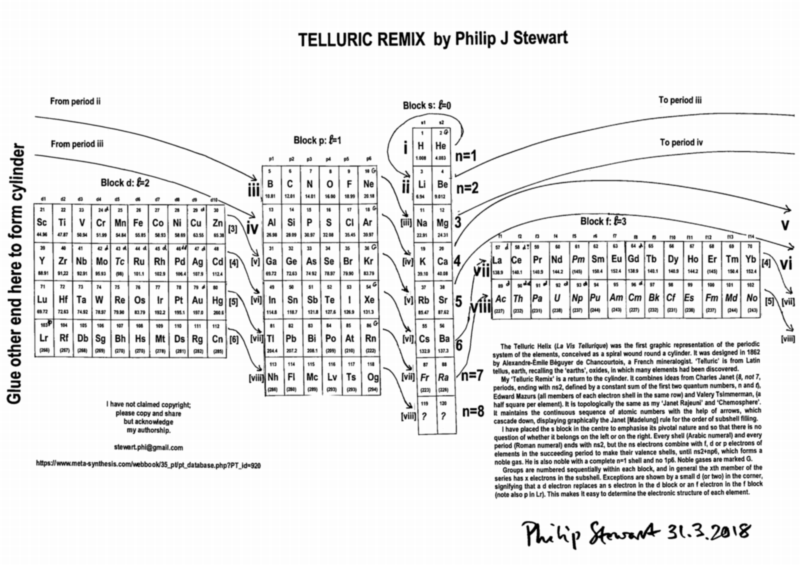
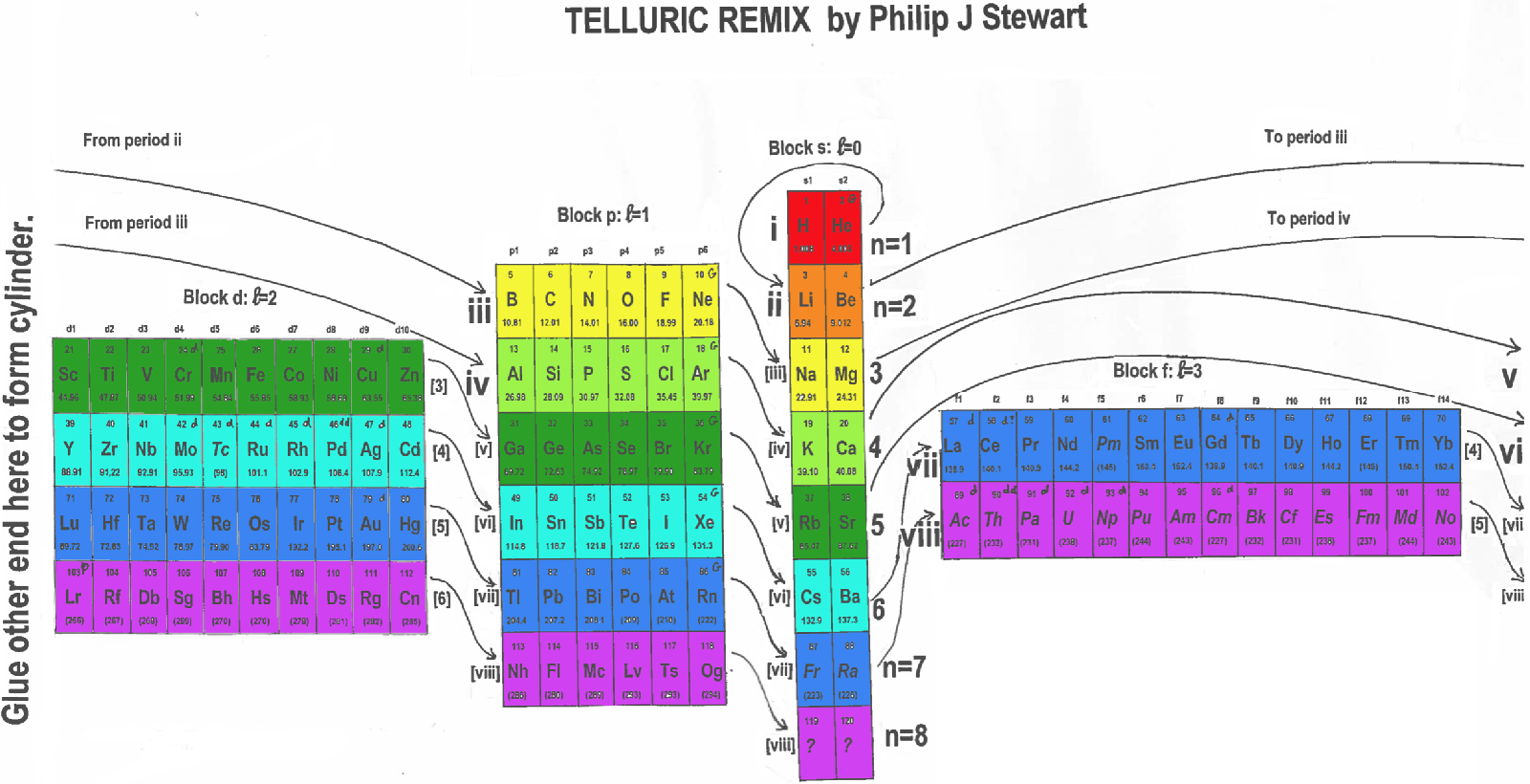
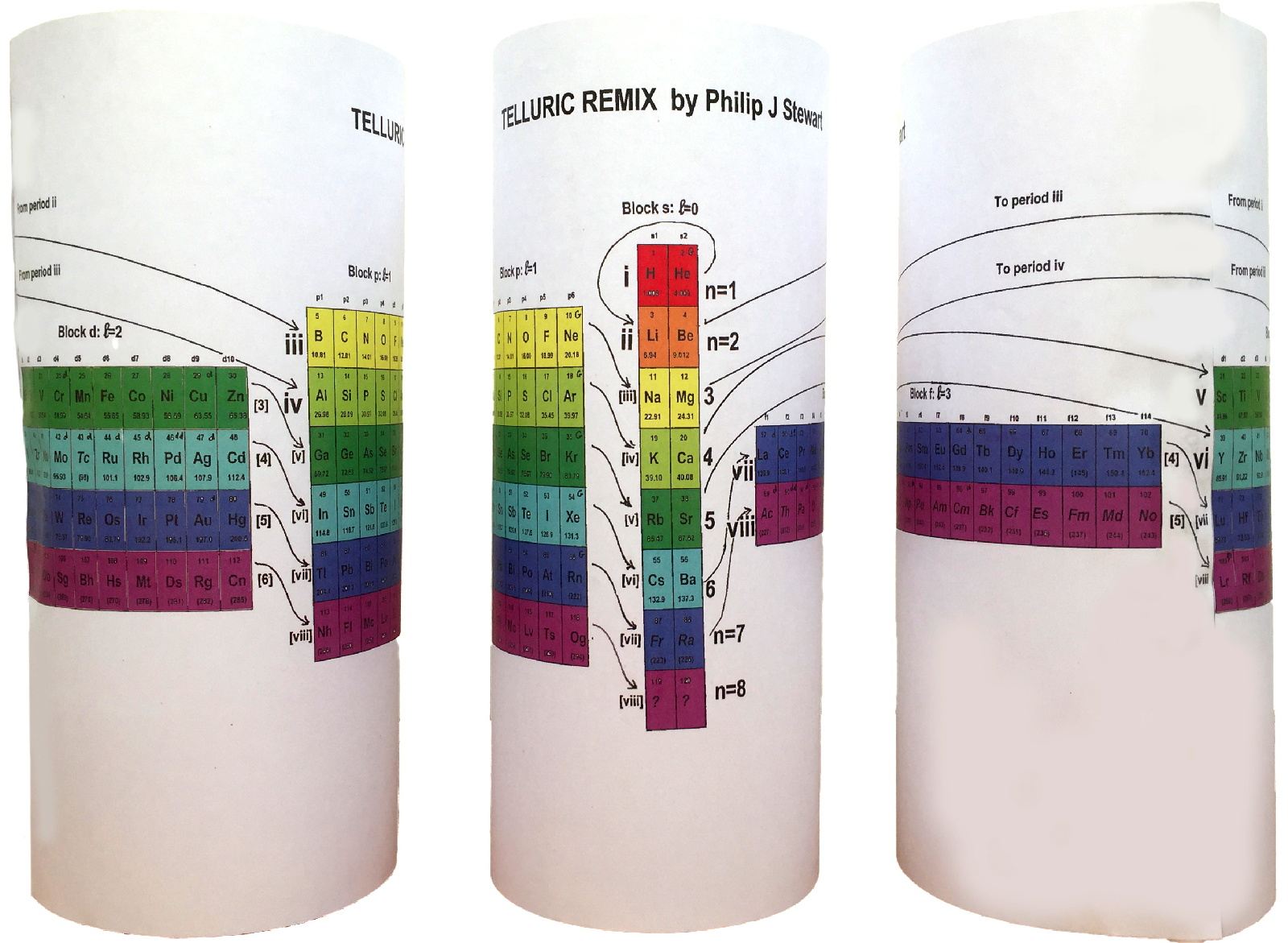
Telluric Remix
Philip Stewart writes:
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version.
The printable version is available (click here for the full size version) to make your own:
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
| Year: 2018 | PT id = 946, Type = formulation 3D |
Periodical System (Binodic Form): a new mathematical paradigm
By Julio Antonio Gutiérrez Samanez, who writes:
"System devised and prepared by the Peruvian chemical engineer, Julio Antonio Gutiérrez Samanez, deals with a new conception of Mendeleev's Law as a mathematical function and a new description of the process of forming the series of chemical elements according to mathematical laws and dialectical processes of changes quantitative and qualitative under a dynamic spiral architecture in 3D, which is postulated as a new scientific paradigm."
| Year: 2018 | PT id = 1202, Type = formulation |
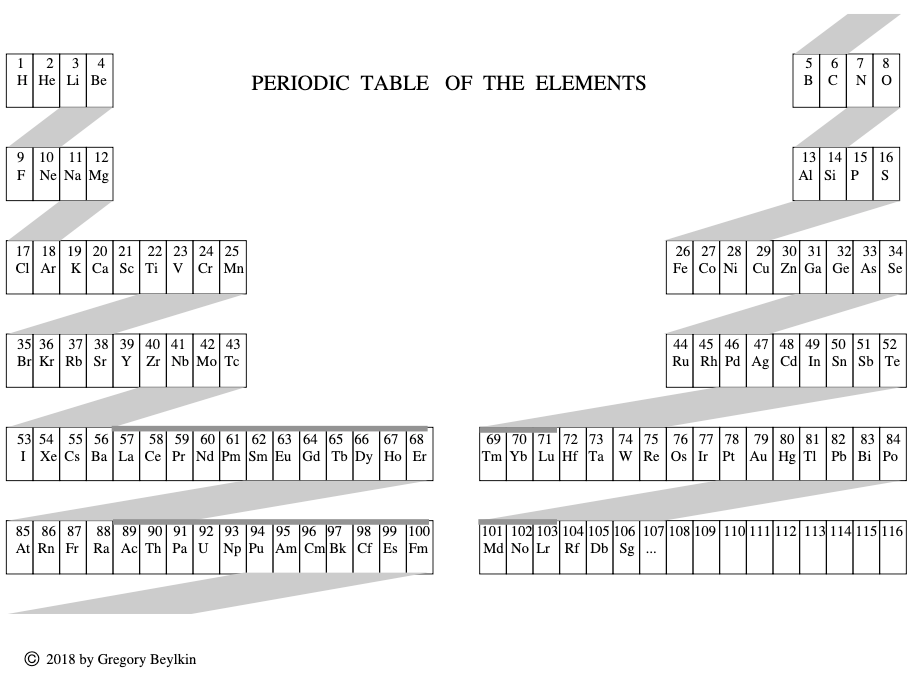
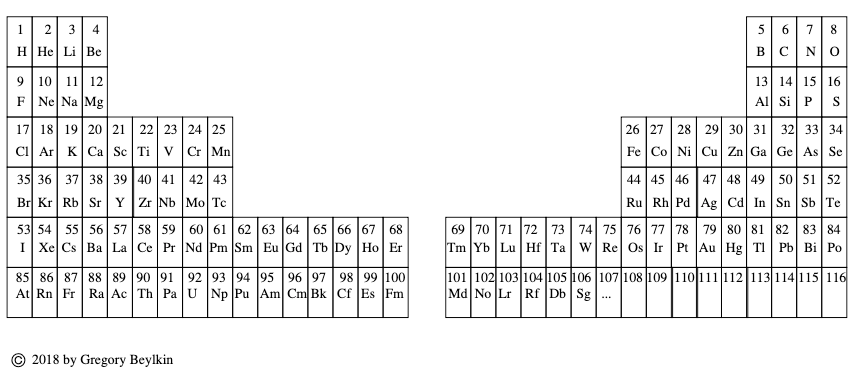
Beylkin's Periodic Table of The Elements
René Vernon writes: Beylkin's Periodic Table of The Elements has 4n2 periods, where n = 2,3..., and shows symmetry, regularity, and elegance, more so than Janet's left step table.
Beylkin (an applied mathematician) writes:
"Let us take a continuous strip of paper and, on one side of the strip, write all the elements in the order of their atomic numbers. We then form a spiral with the strip such that the two most chemically distinct groups, the group of halogens (in which we include hydrogen) and the group of noble gases, are properly aligned. By flattening the strip on a plane and folding it in the middle, we obtain the new periodic table..."
Other features:
- H is over F, which is a smoother fit in terms of physicochemical trends down the group
- He is over Ne, which is a smoother fit etc
- group 3 has lanthanum in it
- the modern relationships Ti-Zr-Hf, V-Nb-Ta, Cr-Mo-W, and Mn-Tc-Re can still be traced
- the lanthanides and actinides are integrated into the main body of the table
- 15 lanthanides and 15 actinides(!)
- the old school arrangement of B-Al-Sc-Y-La can still be traced, as can the less smooth alternative B-Al-Sc-Y-Lu
- the 1s "block" starts at H; the s block proper at Li; p at B; d at Sc; f at Ce
There are four new(ish) groups: Ti-Zr-Ce-Th, V-Nb-Pr-Pa, Cr-Mo-Nd-U and Mn-Tc-Pm-Np. For the actinide elements of these groups, the resemblance of the earlier actinides to their lighter transition metal congeners is well known. For the lanthanide elements, Johansson et al. (2014) wrote a nice article about Ce and its cross-road position. For Pr, Nd, and Pm, all of these are known in multiple oxidations states (+2, +3, +4 excl. Pm, and +5 for Pr only), just as the transitions metals are so known.
- Beylkin G 2018, The periodic table of the elements with 4n2 n = 2,3... periods, https://arxiv.org/pdf/1901.02337.pdf
- Eric 2006, https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=20
- Johansson, B., Luo, W., Li, S. et al. 2014, Cerium; crystal structure and position in the periodic table. Sci Rep 4, 6398. https://doi.org/10.1038/srep06398
- Gregory Beylkin: https://en.wikipedia.org/wiki/Gregory_Beylkin
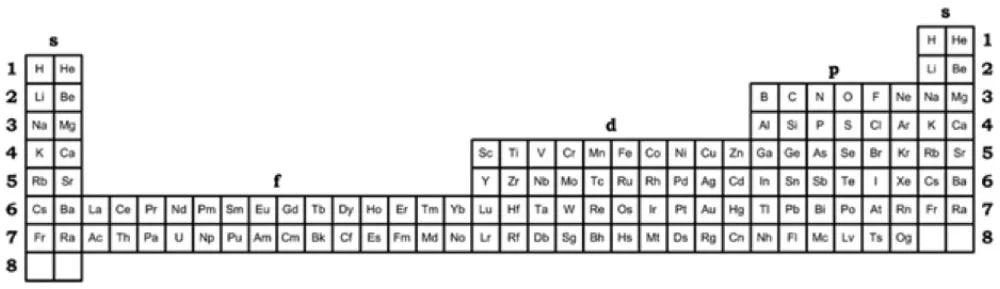
| Year: 2018 | PT id = 1261, Type = formulation |
Kurushkin's 32-Column Periodic Table & Left-step Periodic Table United
Dr Mikhail V. Kurushkin, 32-column Periodic Table & Left-step Periodic Table United: https://bernalinstitute.com/events/bernal-seminar-by-dr-mikhail-v-kurushkin-itmo-universityrussia/
ABSTRACT
The pursuit of optimal representation of the Periodic Table has been a central topic of interest for chemists, physicists, philosophers and historians of science for decades (Leigh, 2009; Scerri, 2009). Should the Periodic Table of Chemical Elements first and foremost serve the needs of chemists as implied by its name? Or should it start from considerations of before quantum mechanics and thus be more appealing to physicists (Scerri, 2010, 2012b)? Is there a representation which overcomes this problem? The Periodic Table is from a fundamental point of view a graphic representation of periodicity as a phenomenon of nature. While the 32-column Periodic Table, popularized by Glenn T. Seaborg, is considered by chemists the most scientifically correct representation (Scerri, 2012a), physicists apparently prefer the Left-step Periodic Table above all (Scerri, 2005; Stewart, 2010). Alternatively, it is suggested that a rigorously fundamental representation of periodicity could only take the form of a spiral as, evidently, the abrupt periods of 2-D Periodic Tables contradict the gradual increase of atomic number, and the spiral representation reconciles this debate (Imyanitov, 2016). An optimal representation is eagerly sought after both for the needs of philosophy of chemistry and chemical education as their never-ending dialogue secures a thorough methodology of chemistry. The aim of the present work is to show that the 32-column Periodic Table and the Left-step Periodic Table can co-exist in mutual tolerance in a form of what Philip Stewart has already called Kurushkin’s Periodic Table (Kurushkin, 2017), Figure 1 below.
René Vernon writes:
"Kurushkin reminds us that the Janet left step table (with Sc-Y-Lu-Lr, and He over Be), and the version of the table with the s-elements on the right (also with Sc-Y-Lu-Lr, and He over Be) are interchangeable.
"For an earlier paywall version which includes a short video see:
Kurushkin M 2018, Building the periodic table based on the atomic structure, Journal of Chemical Education, vol. 94, no. 7, pp. 976–979, https://pubs.acs.org/doi/10.1021/acs.jchemed.7b00242
"Kurushkin’s interchangeable approach extends to tables with group 3 as either Sc-Y-La-Ac or Sc-Y-Lu-Lr. See Vernon's Yin Yang of The Periodic Table https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=1252"
| Year: 2019 | PT id = 1067, Type = formulation 3D spiral misc |
Scott Van Note Periodic Table Sculpture
On the Saatchi Art website, a 3D periodic table Sculpture by Scott Van Note.
Sculpture: Metal (Bronze). Ten made for the local ASM international chapter.
Loops and changes of direction show electron shell filling. S,P,D,F with S just a change of direction. Continuous spiral from top to bottom. New loops introduce as the electron shell would. Does not show the out-of-order shell filling.
Keywords: periodic, science, sculpture, functional, nerd
Thanks to Roy Alexander for the tip!
| Year: 2019 | PT id = 984, Type = formulation 3D spiral |
Telluric Remix in Colour
Philip Stewart writes (this is the same text that accompanies the 2018 B/W version):
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version (pdf).
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
| Year: 2019 | PT id = 1005, Type = formulation misc 3D spiral |
Schaltenbrand's Helical Gathering of the Elements
From the RSC Website:
"A glistering, shining spiral made of silver, gold, platinum, palladium and a diamond forms the show-stopping apex of the tribute from the University of Cambridge's St Catharine's college to the International Year of the Periodic Table.
"Commissioned to match George Schaltenbrand's 1920 design for a helical gathering of the elements – albeit extended to all 118 current elements – and signed by Yuri Oganessian, it is almost certainly the most expensive periodic table in the world."


Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1013, Type = formulation spiral |
Moran's Periodic Spiral (2019)
Jeff Moran has been working on his Periodic Spiral for more than twenty years. Here is the latest iteration, click to enlarge:
| Year: 2019 | PT id = 1014, Type = formulation spiral |
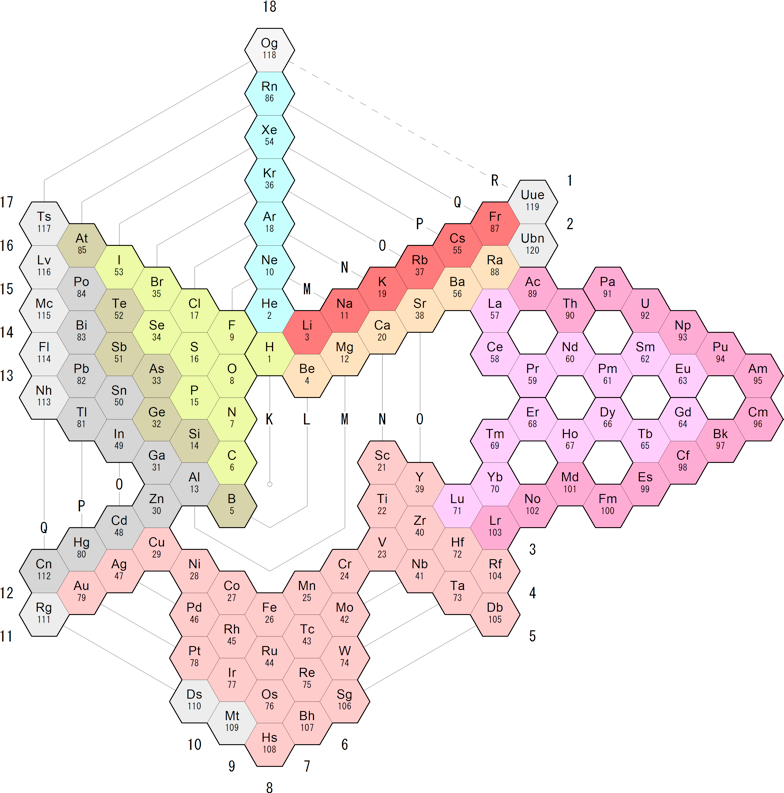
NAWA's Version of Moran's Periodic Spiral
Periodic table designer Nagayasu Nawa has put his spin on Moran's Periodic Spiral:
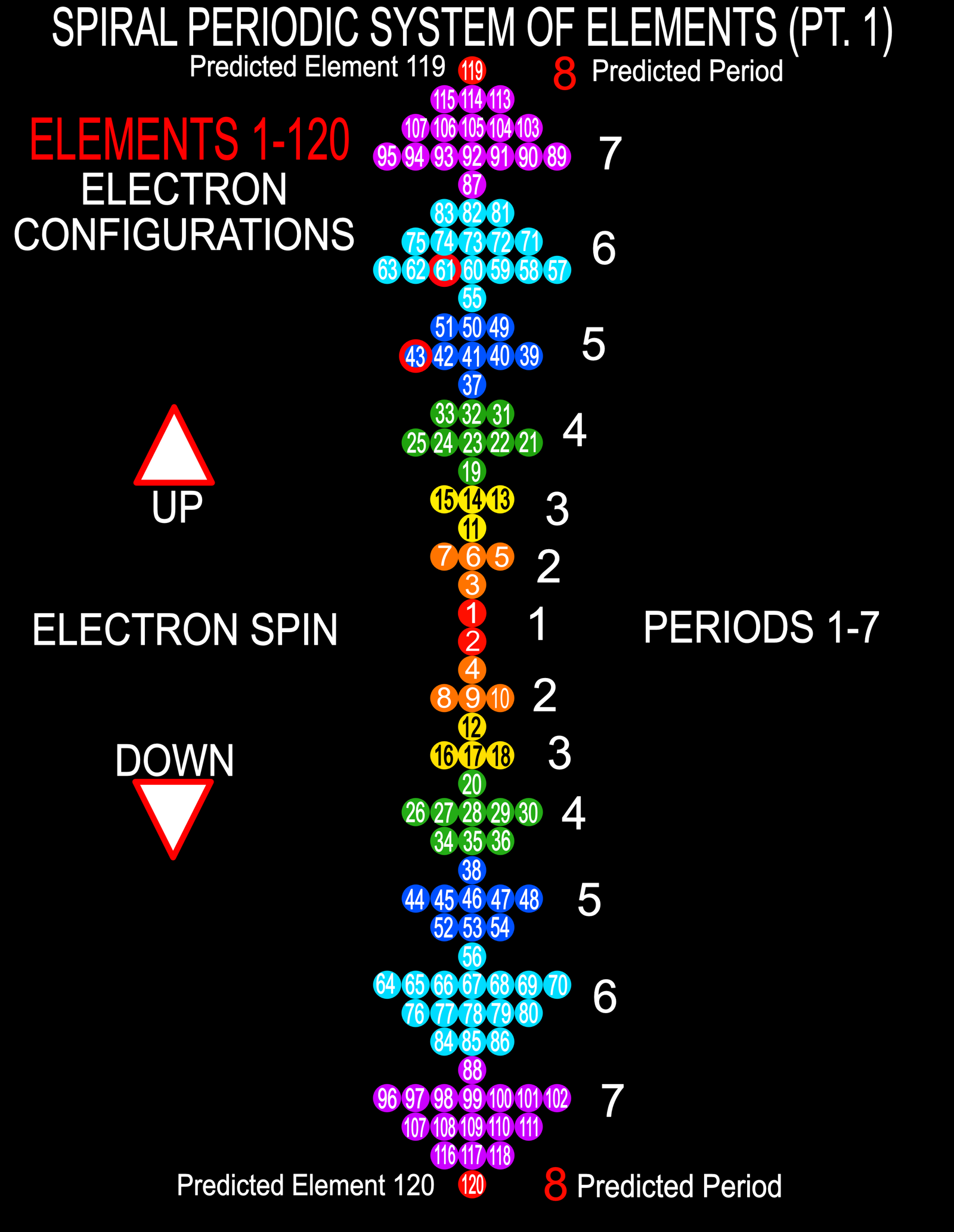
| Year: 2020 | PT id = 1154, Type = formulation spiral |
Spiral Electron Spin Periodic Table
The Spiral Electron Spin Periodic Table, By Justine Colburn, who also developed the Genesis formulation.
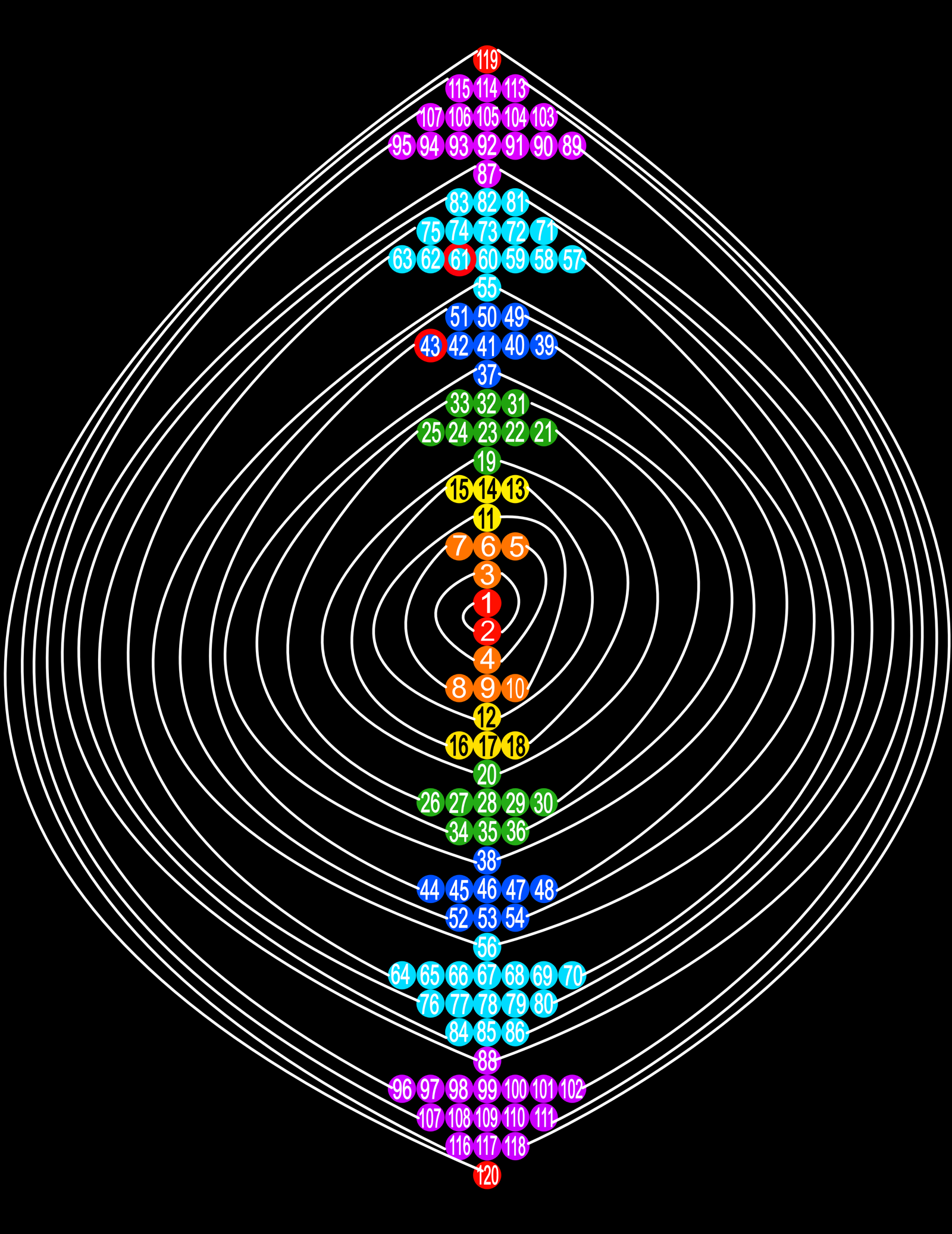
| Year: 2021 | PT id = 1186, Type = formulation |
Helix vs. Screw
Julio Antonio Gutierrez Samanez writes:
Until today, when they write about the work of Chancourtois and his telluric helix wound in a cylinder, still no one alludes to this other telluric helix wound in a cone or screw, the idea is the same: a rope that winds a geometric solid.
The first was devised in 1862, the other in 2008 (146 years later). But, there is a big epistemological difference. In the first, the elementary series presented was: 8, 8, 8, 8, 8 ..., etc., in the second it is: 2, 2, 8, 8, 18, 18, 32, 32. Furthermore, the division of conical radii is regulated by the function 2 (n ^ 2) = 2, 8, 18, 32...
Each binode has two spirals or two periods with the same number of elements, which correspond to the function 4 (n ^ 2). I don't think it is a discovery, it is just the conclusion of the contributions of Rydberg, Janet, and, of course, Chancourtois.
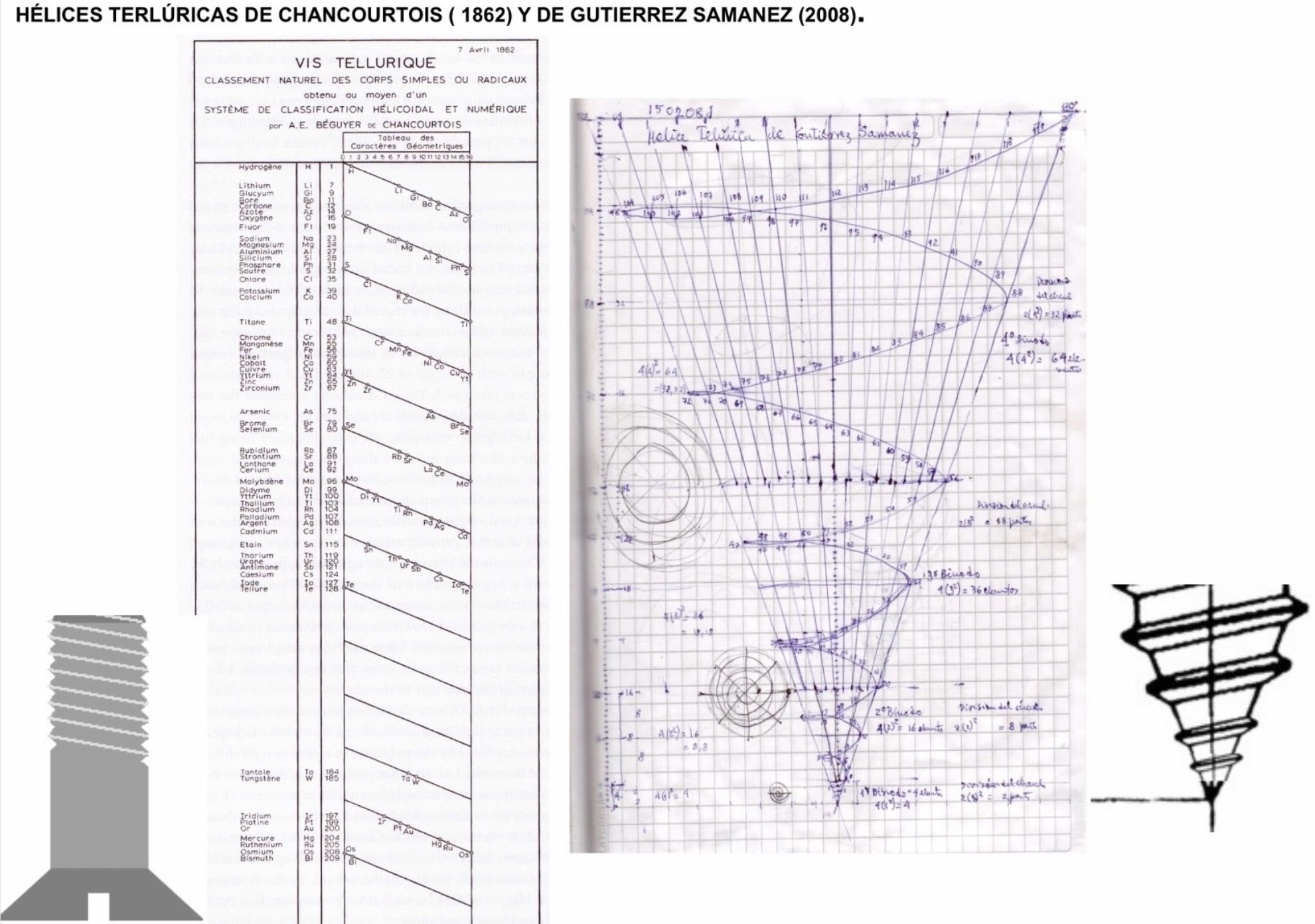
| Year: 2021 | PT id = 1187, Type = formulation |
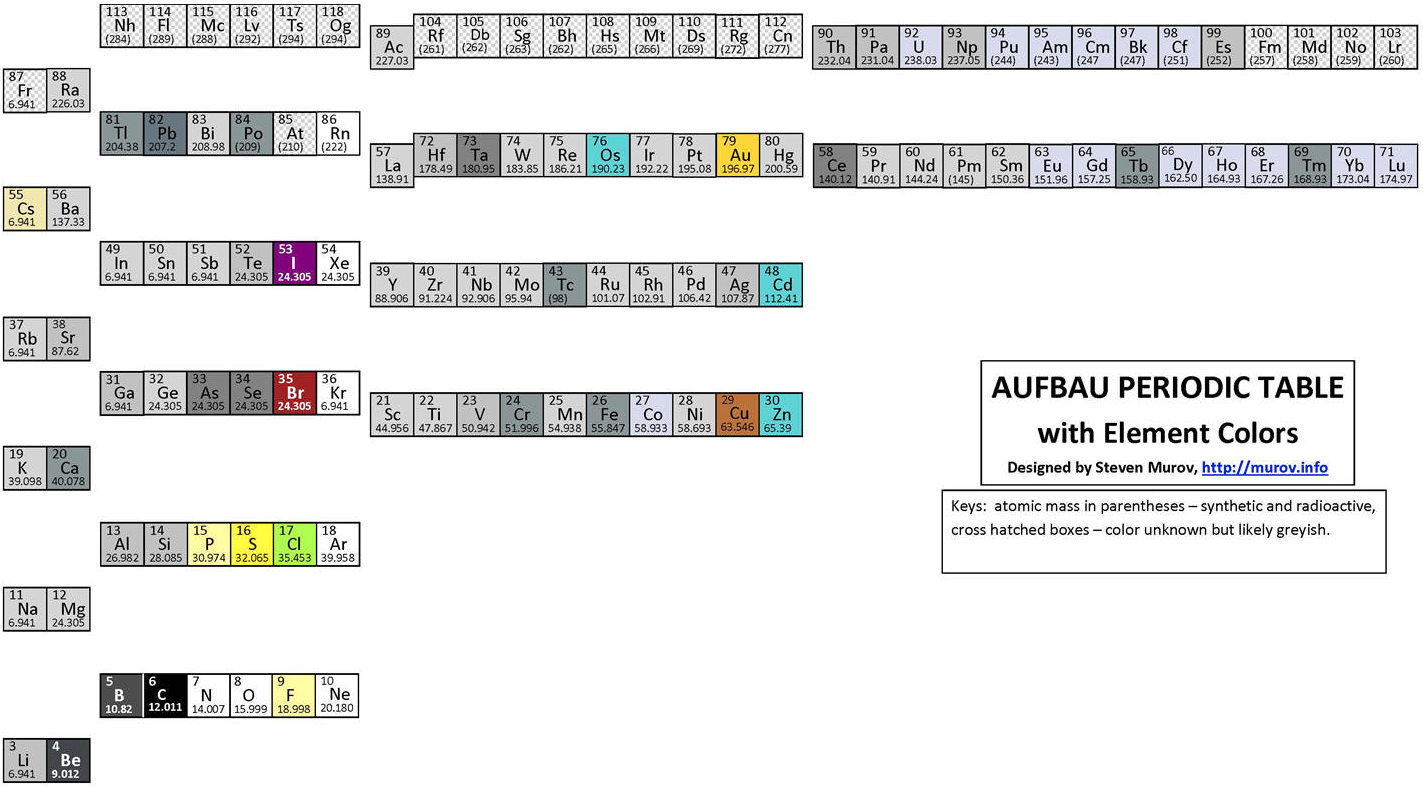
Aufbau Periodic Table
An Aufbau Periodic table designed by Steven Muov at http://murov.info/aufbaupt.htm
Steven writes:
"Science has aptly been described as a search for order in the Universe. It follows that chemistry is a search for order in matter. While the search will always be a work in progress, great strides towards the finding of order in matter resulted in 1869 when Dimitri Mendeleev stood on the shoulders of many others and published his periodic table. The table has since been modified and improved but still has a remarkable resemblance to the original Mendeleev table. Excellent compilations of many alternate periodic tables have been published that use novel and intriguing approaches (e.g., circles, spirals and 3d, but the contemporary versions of the Mendeleev table are the charts found on the walls of thousands of lecture rooms around the world. The periodic table deserves recognition as one of the milestones of science along with contributions from other sciences including but not limited to: physics by Newton and Einstein, biology by Darwin, Rosalind Franklin, Watson and Crick, astronomy by Copernicus and Galileo and geology by Wegener..."
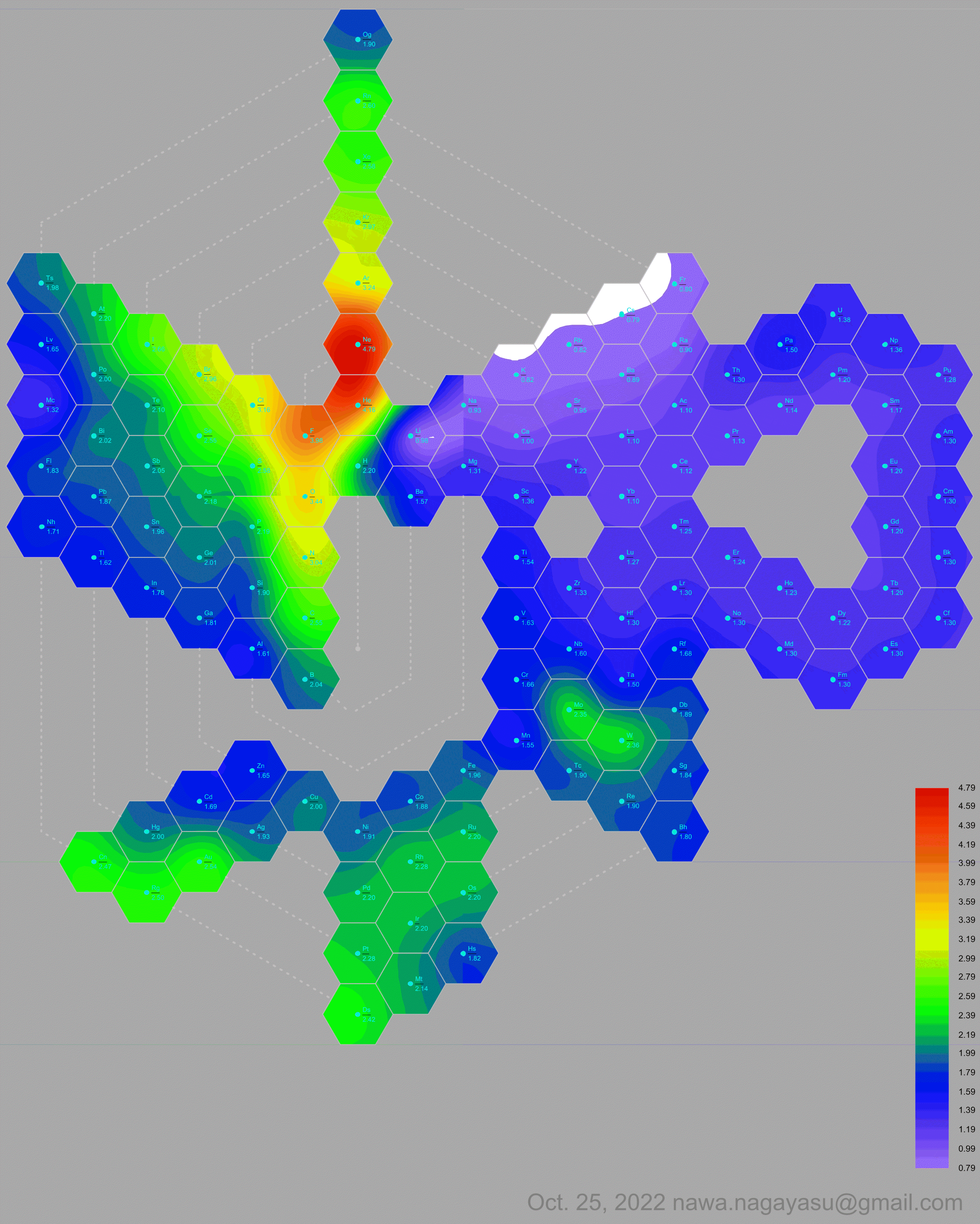
| Year: 2022 | PT id = 1241, Type = data |
Electronegativity Seamlessly Mapped Onto Various Formulations of The Periodic Table
A discussion on the Google Groups Periodic Table Discussion List, involving a René Vernon, Nawa Nagayasu & Julio Samanez (all contributors this database) lead to the development of the representations below, showing electronegativity seamlessly mapped onto a modified Left-Step Periodic Table:
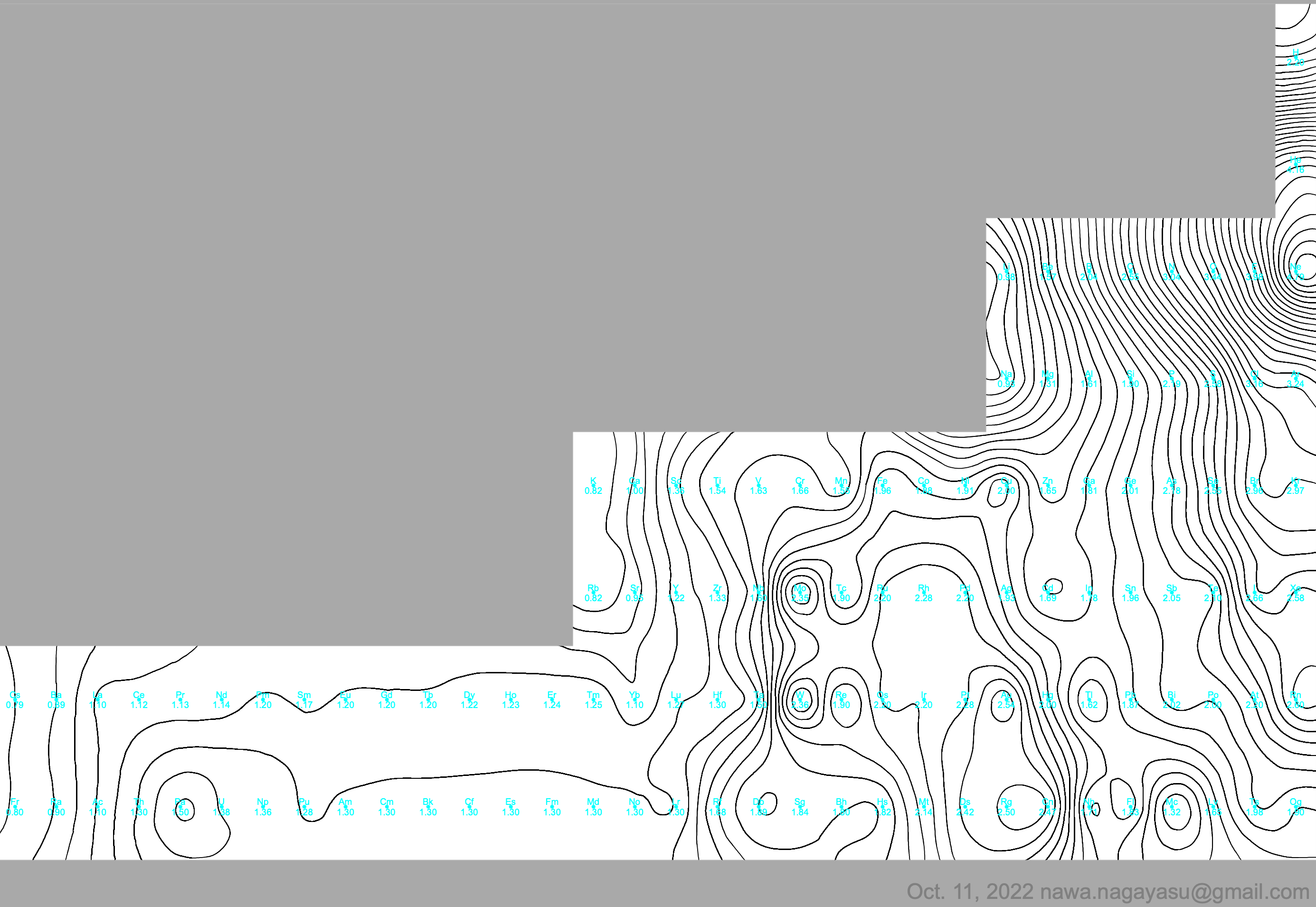
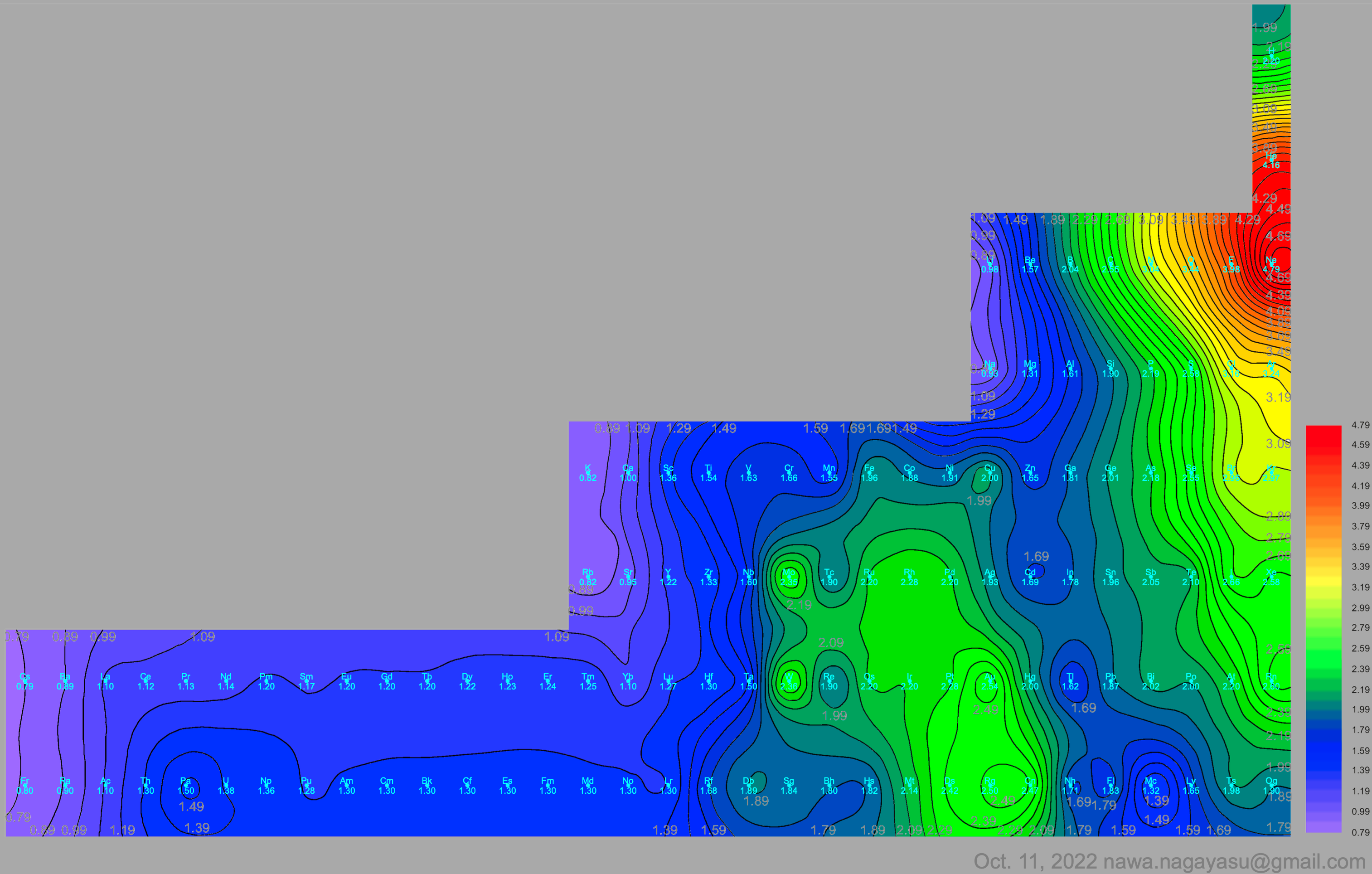
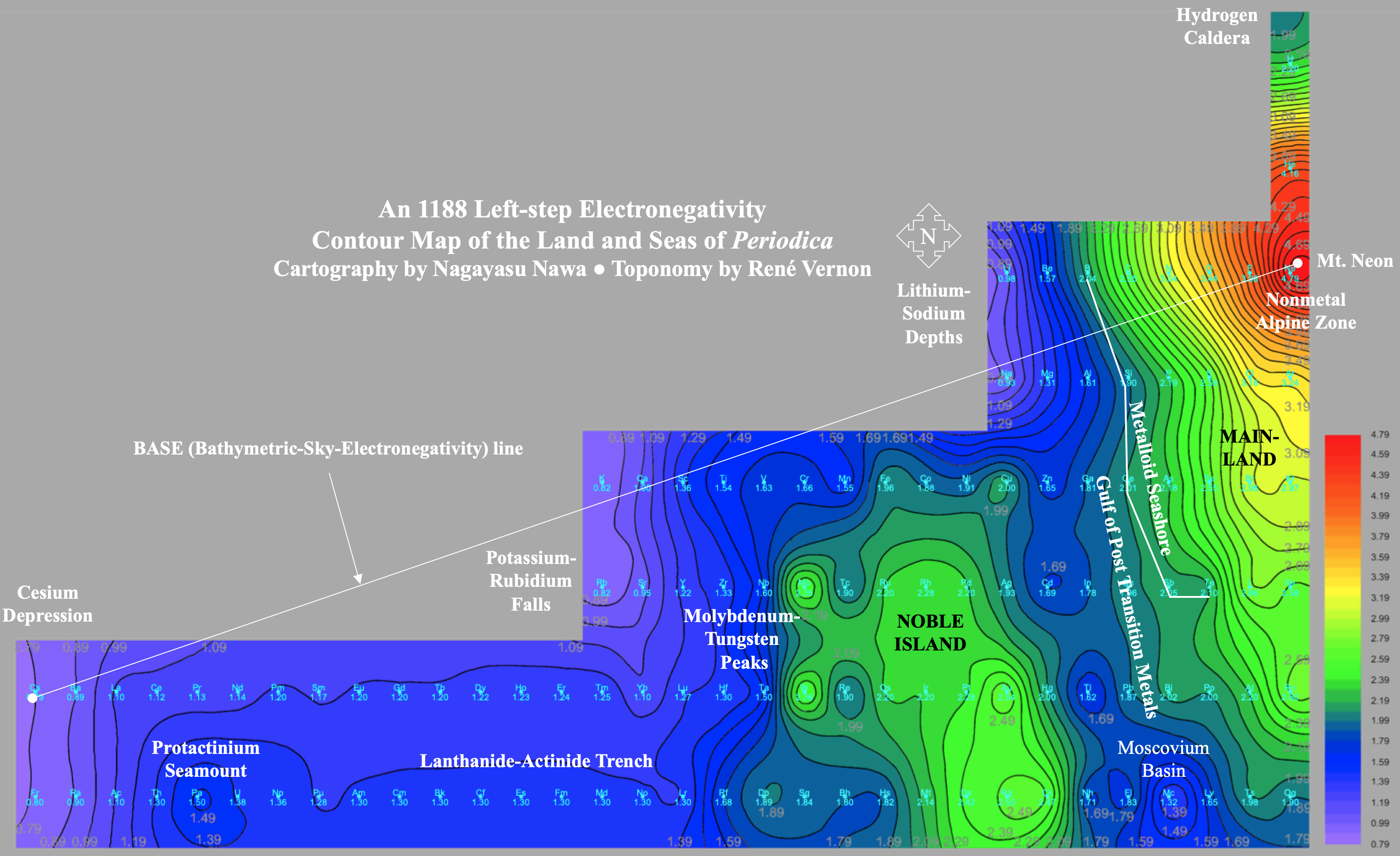
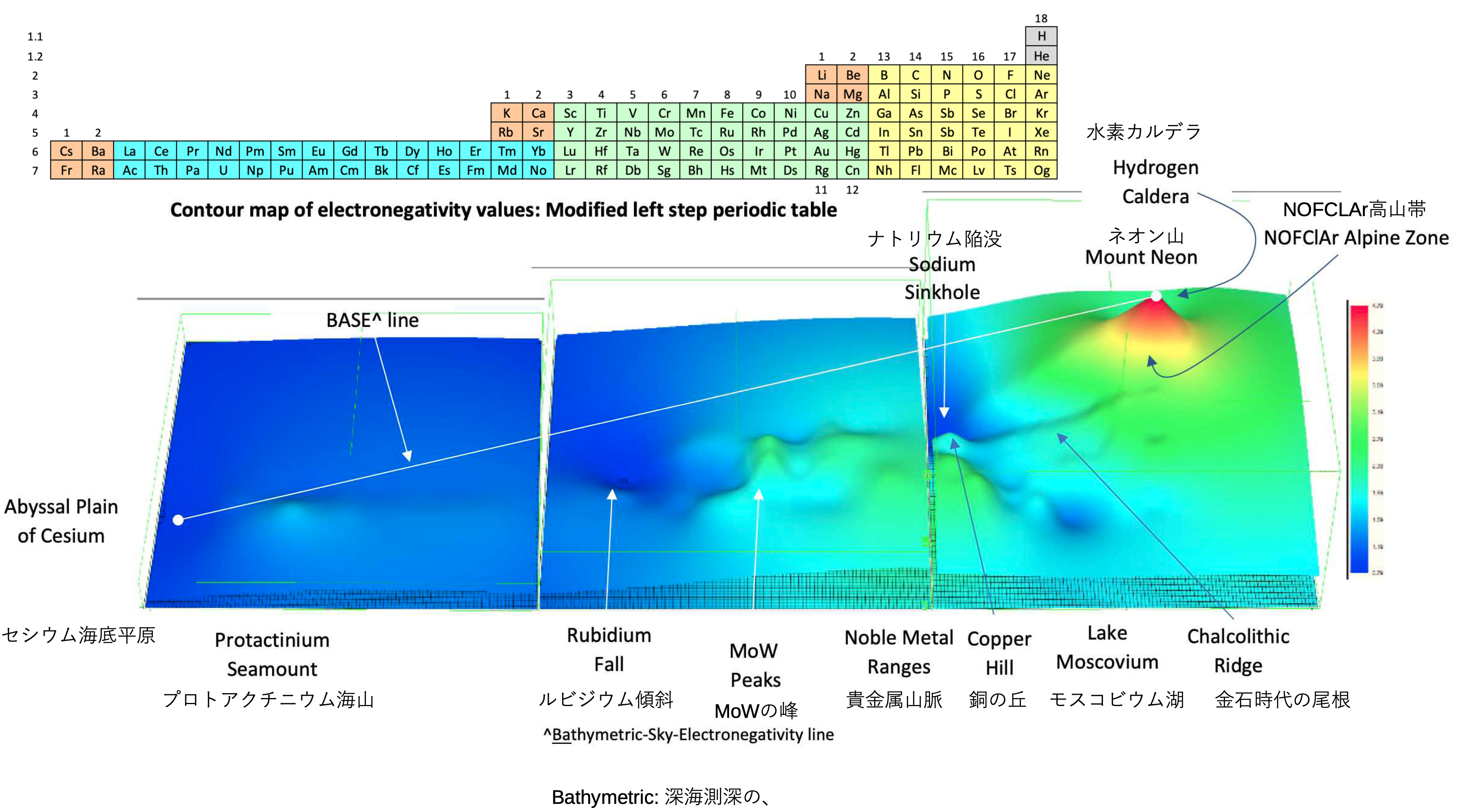
Nawa Nagayasu has mapped electronegativity to Mendeleeve's formulation:
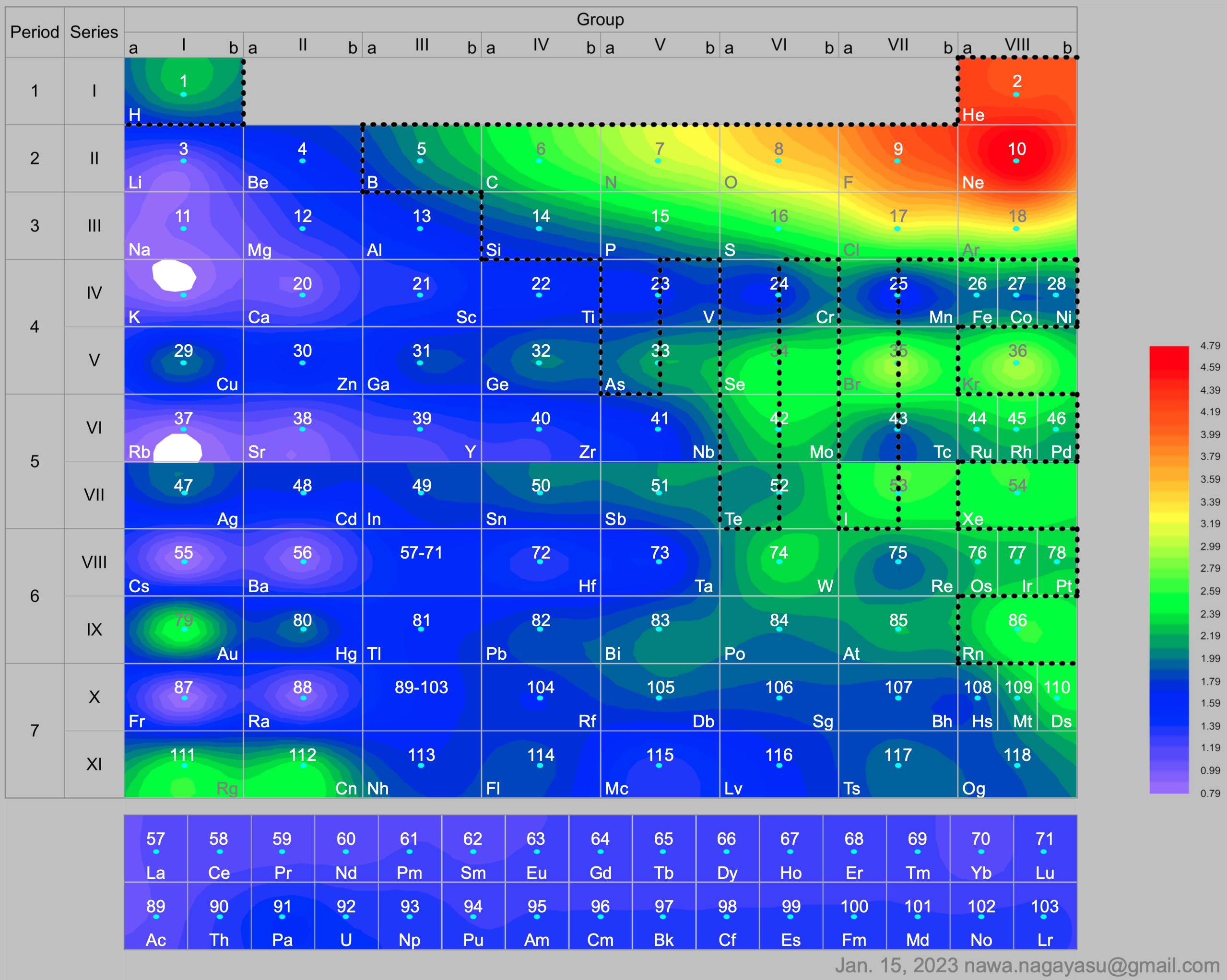
Nawa Nagayasu has mapped electronegativity onto other formulations, Julio's Binode Spiral:
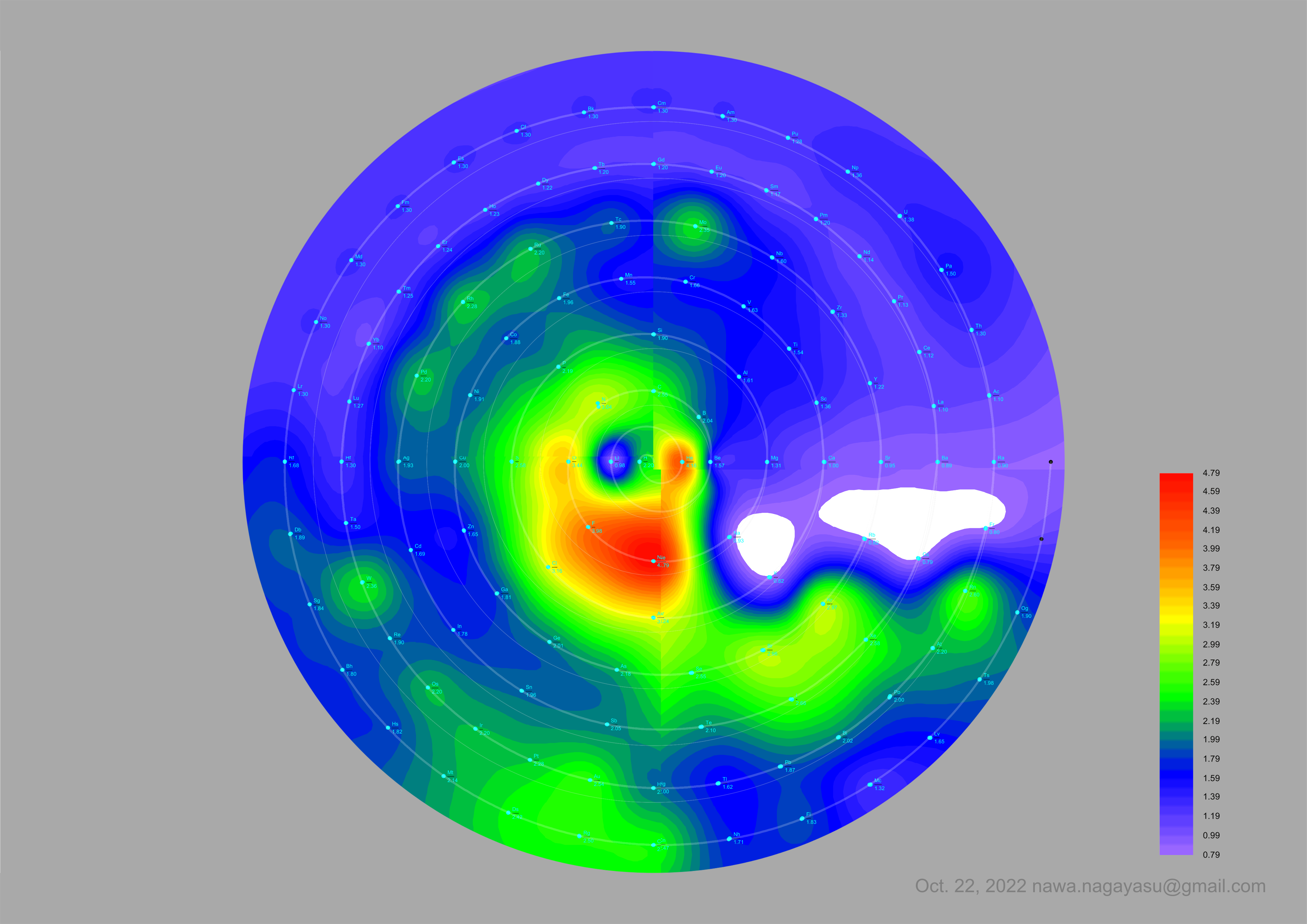
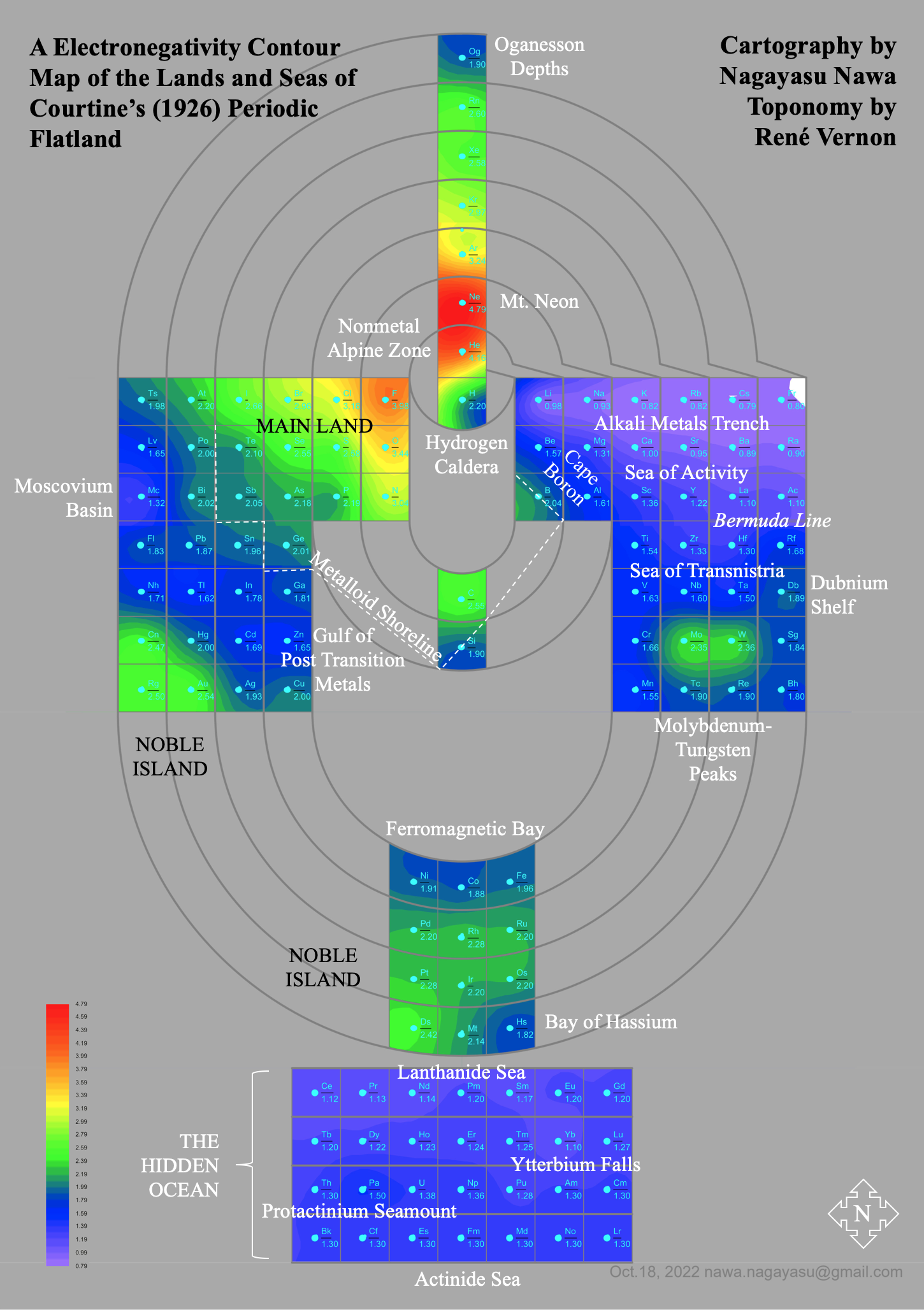
and the "conventional", short, medium and long forms of the periodic table with hydrogen above and between B & C which show the botom-right-to-top-left electronegativity trend:
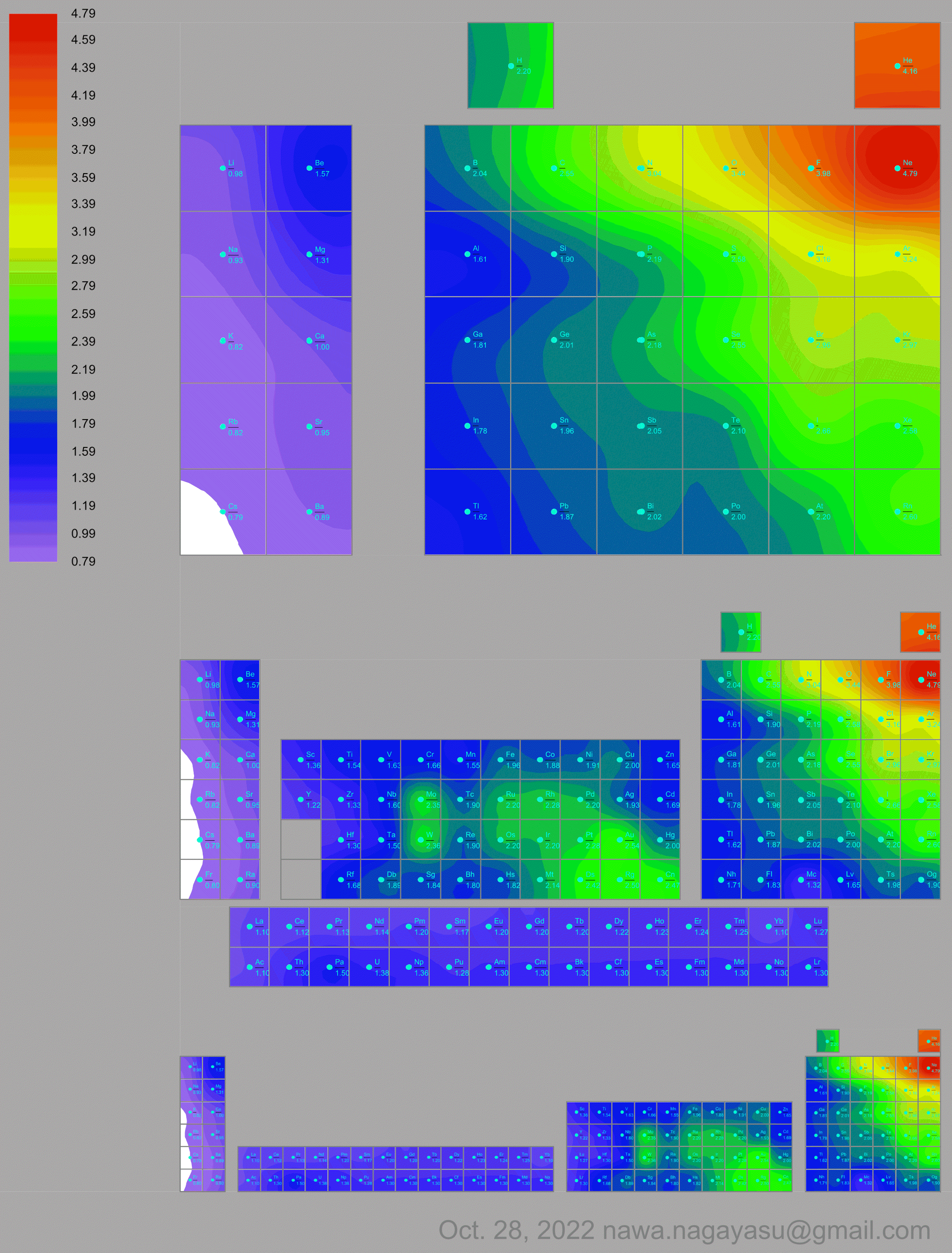
René Vernon's 777 Periodic Wedding Cake:
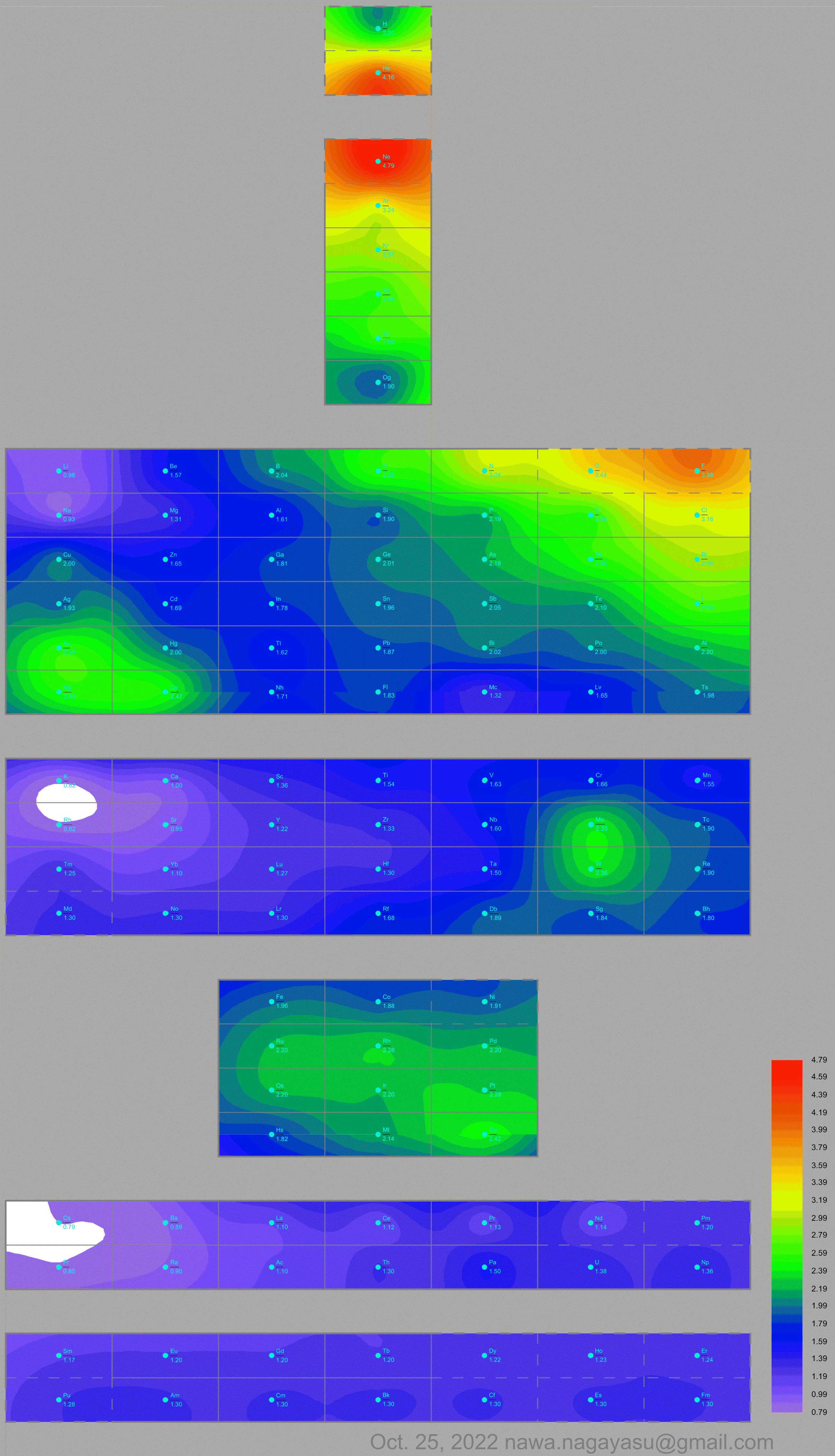
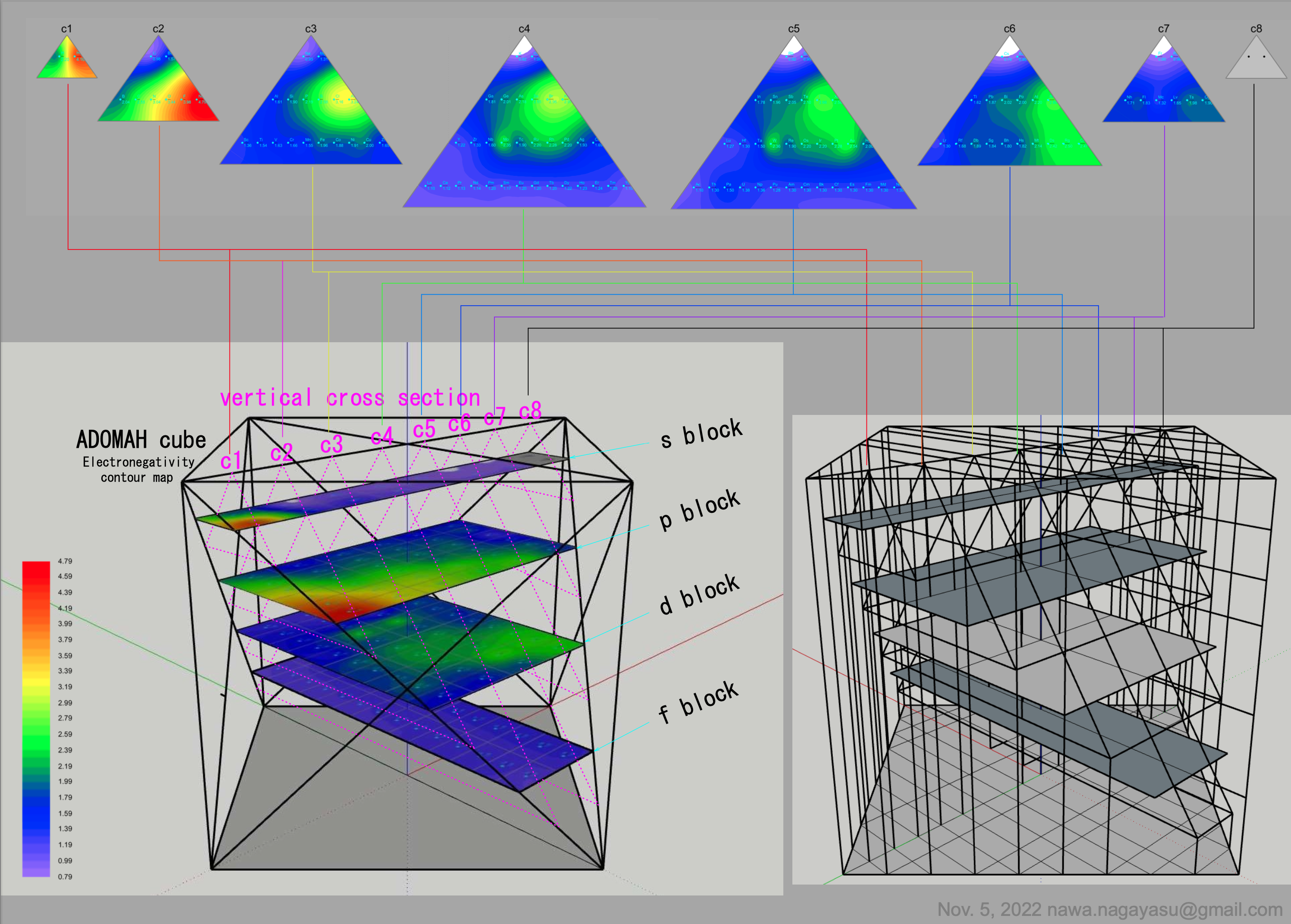
Valery Tsimmerman's ADOMAH formulation:
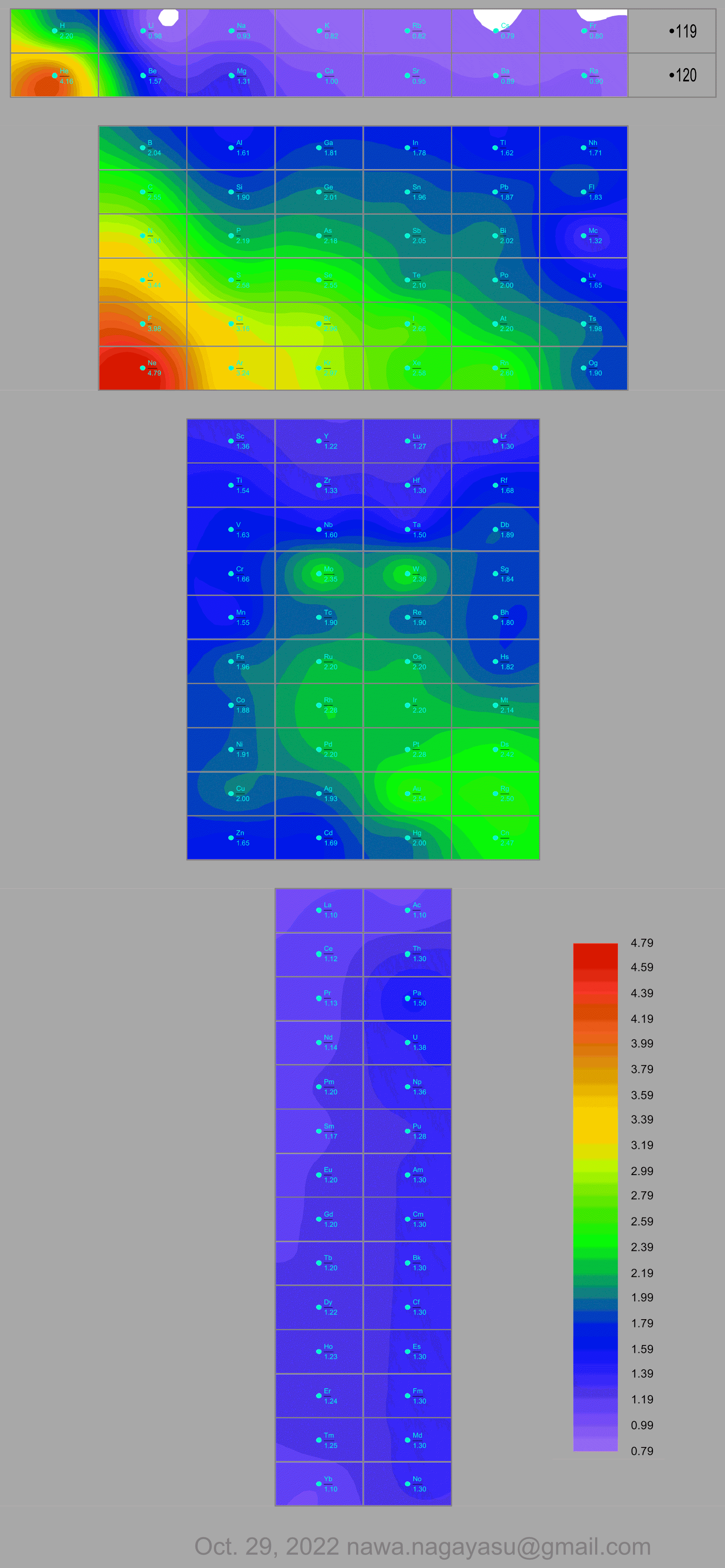
Valery Tsimmerman's ADOMAH tetrahedron (in a glass cube) formulation:
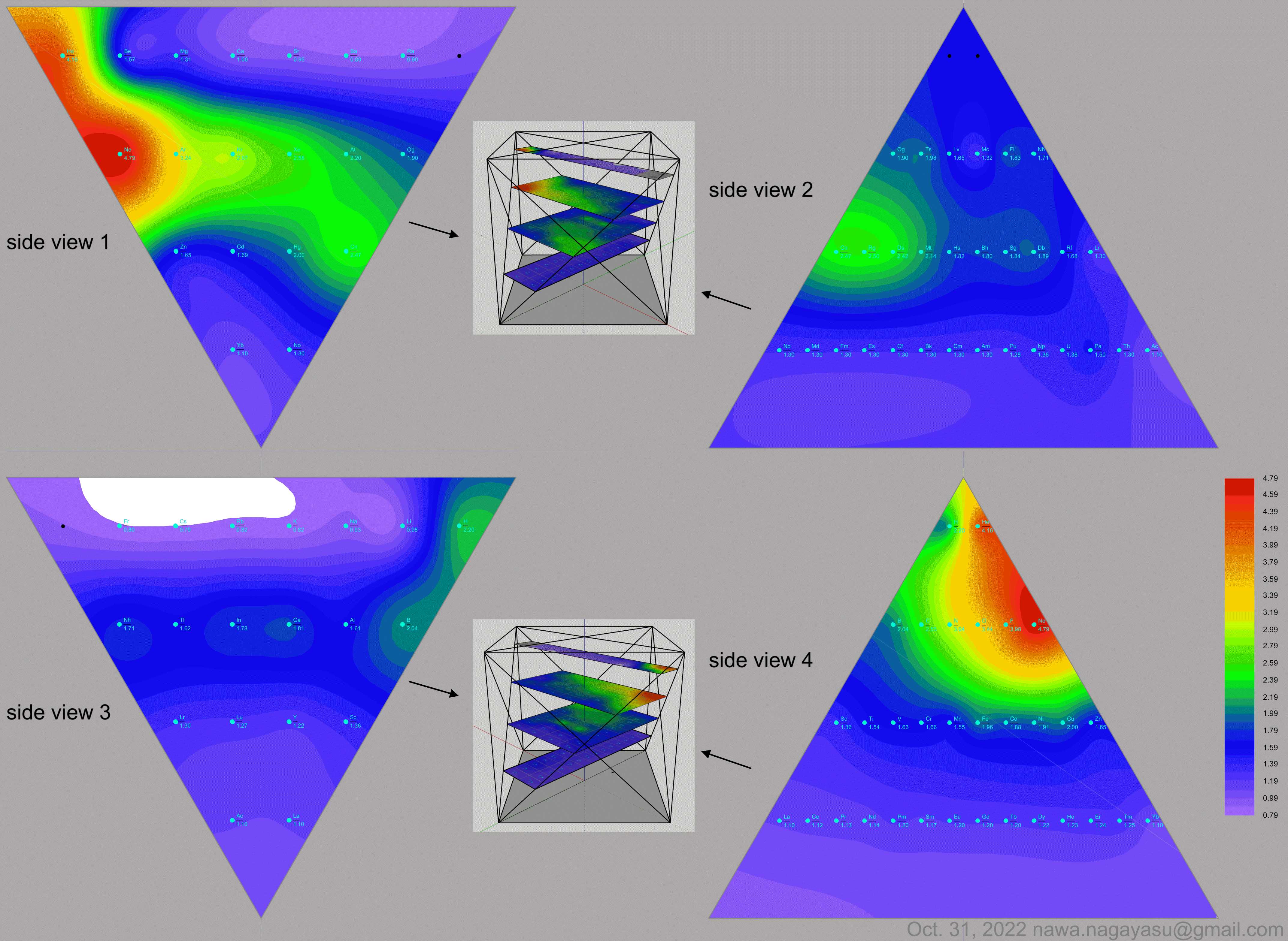
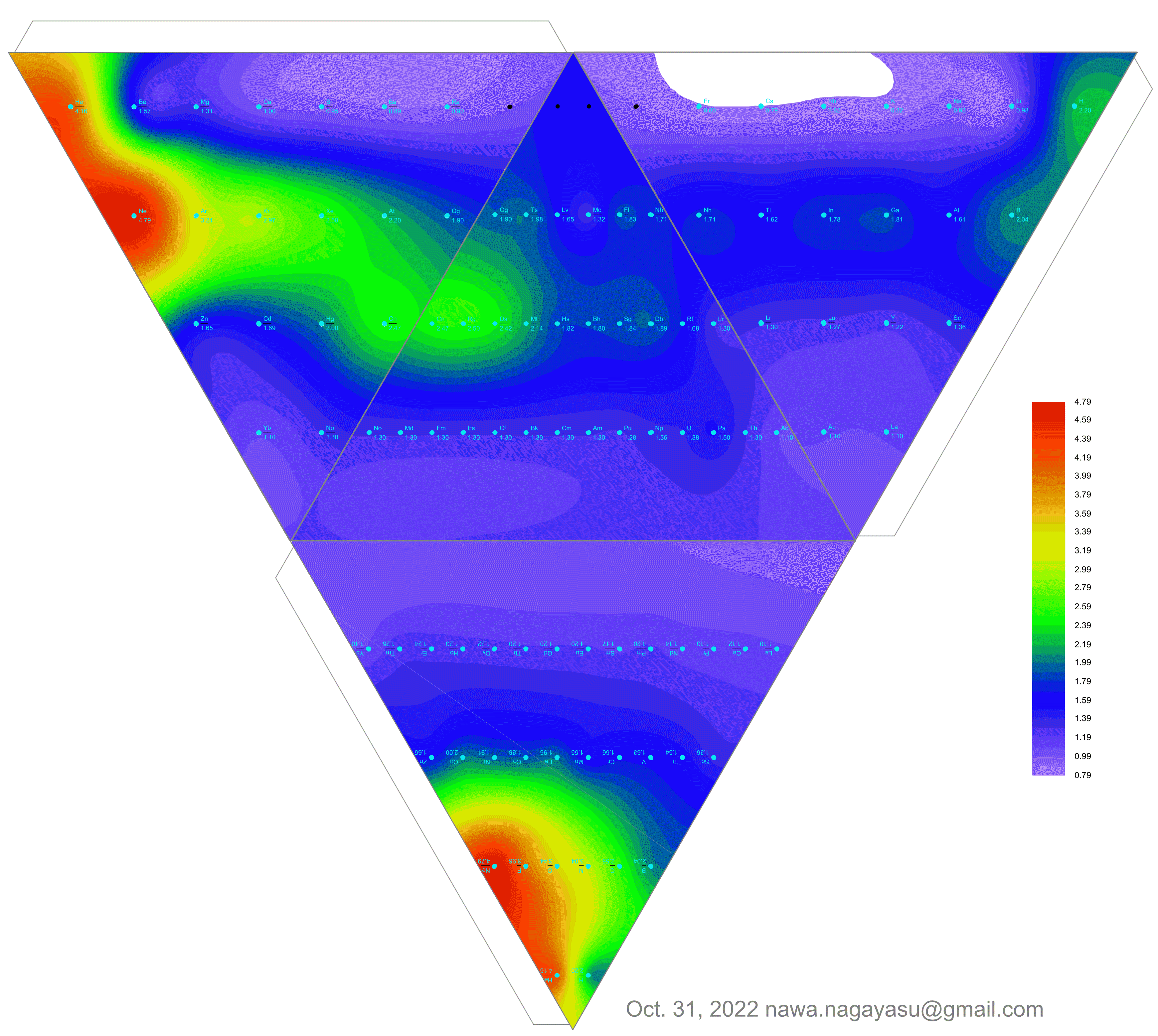
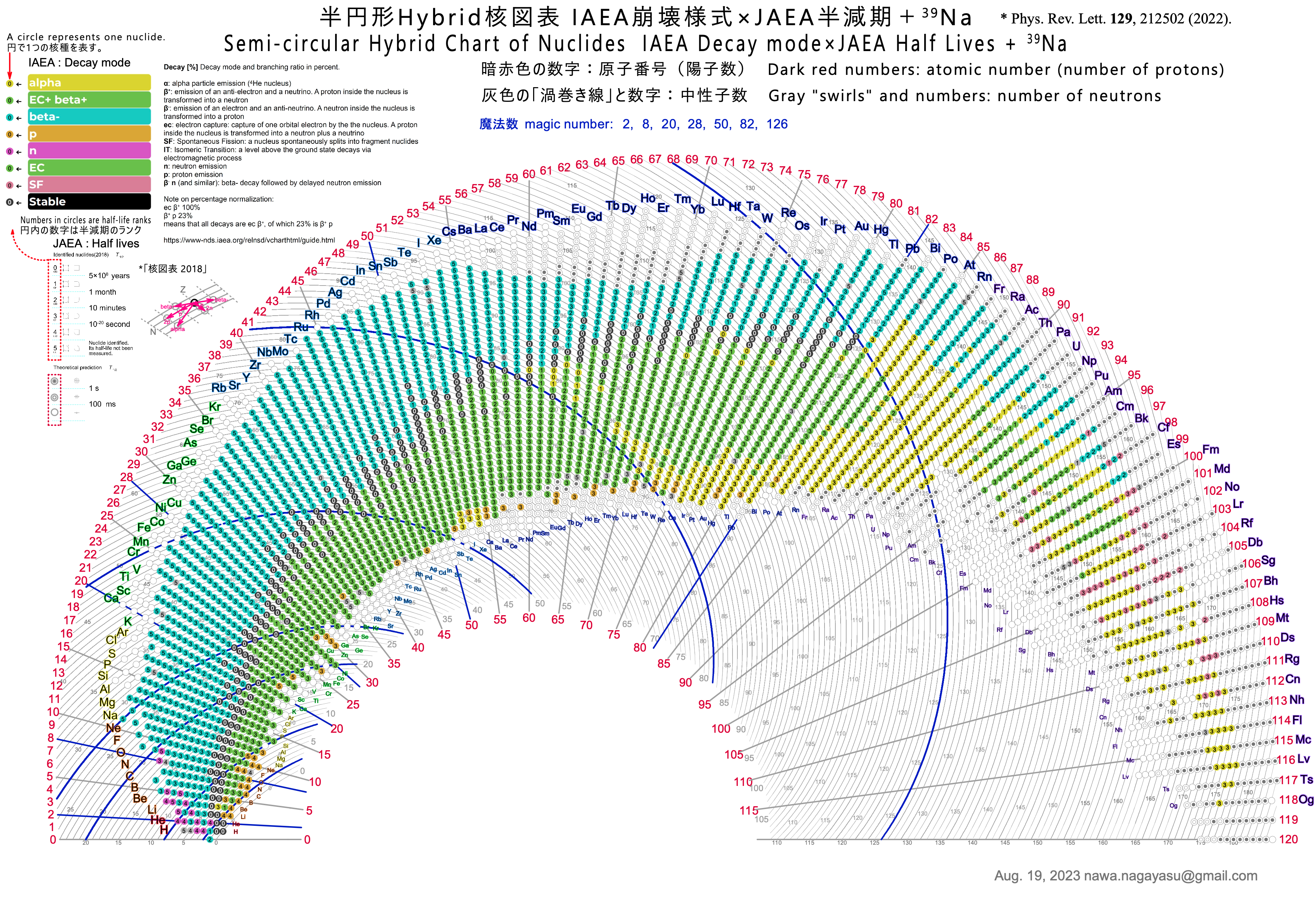
| Year: 2023 | PT id = 1287, Type = formulation element data misc spiral |
Semicircular Hybrid Chart of the Nuclides
Nawa Nagayasu has produced a new version of the Segrè Chart of the Nuclides.
Nawa writes:
"The chart has the number of neutrons on the [curved] horizontal axis and the number of protons (atomic number) on the vertical axis. I used the IAEA colour coding [scheme]. JAEA's half-life ranks are indicated by simple numbers, not rounded frames.
"In order to fit the whole chart into a semicircle, the axis representing the number of neutrons was made a spiral-like curve. For clarity, the number of neutrons is shown in the middle of each curve."
Yuri Oganessian has commented:
"Nawa Nagayasu is an original and talented designer. After all, it is not easy to work with 118 elements, but now also with isotopes, of which there are more than 3000. The fan design looks attractive and this is very important. This will make people, especially school age, guess the numbers that are written there. So they will gradually delve into the content of the Table, a truly brilliant creation."
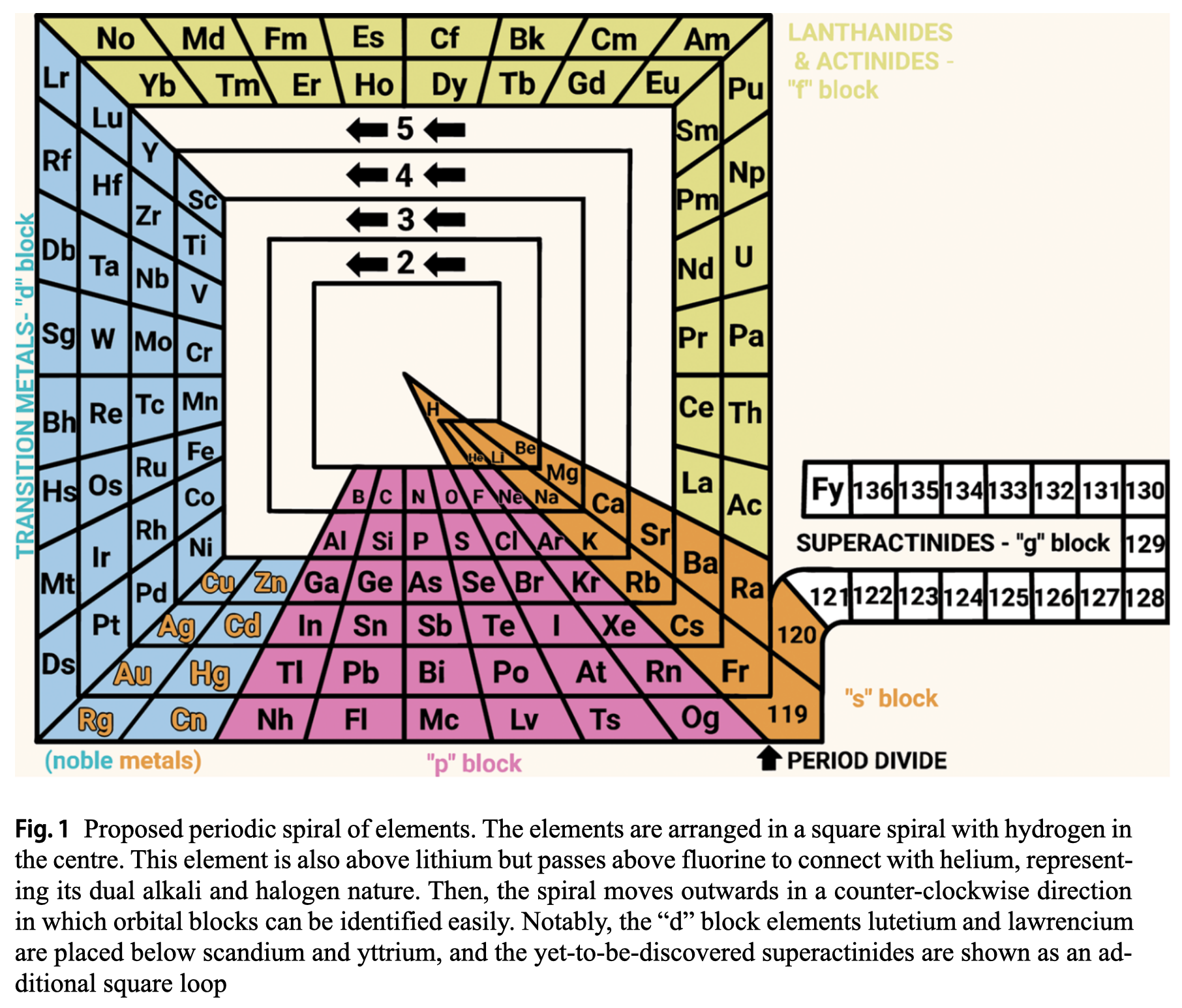
| Year: 2024 | PT id = 1311, Type = formulation spiral |
Rodríguez Peña & García Guerra's Periodic Spiral of The Elements
Rodríguez Peña, M., García Guerra, J.Á. The periodic spiral of elements. Found Chem (2024). https://doi.org/10.1007/s10698-024-09510-4
Abstract There are 2 main problems with the current periodic table: artificial breaks from a given noble gas to the next alkali metal (along with the common protrusion of the "f" block) and hydrogen placed in the alkali group, although this gas also exhibits halogen properties. This paper proposes arranging chemical elements in a square spiral with hydrogen at the centre. This element is also above lithium but passes above fluorine to connect with helium, representing its dual alkali and halogen nature effectively. Then the spiral moves outwards in a counter-clockwise direction, avoiding artificial breaks and following the natural direction of reading for the "s" and "p" blocks elements placed at the bottom of the spiral. Furthermore, this proposed square spiral improves upon previous Janet's and Benfey's representations with a more regular shape to draw, an effective depiction of the dual nature of hydrogen, and easily identifiable orbital blocks without the need for protrusions.
| Year: 2025 | PT id = 1331, Type = formulation misc spiral 3D |
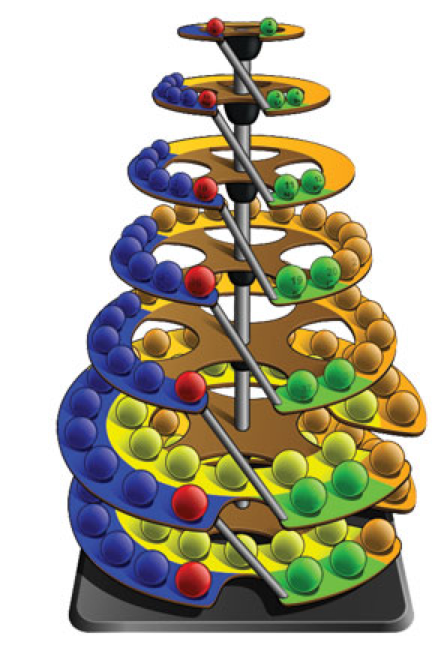
LEGO Periodic Tower of The Elements
A LEGO Periodic Tower of The Elements, a form of Schaltenbrand's 1920 Helical Periodic Table, by Enrique Barrajón, as shown on the LEGO Ideas website.
Enrique writes:
"The Periodic Tower of Elements is a complex LEGO project designed for students with some chemistry knowledge. It serves as an educational tool to help with learning basic chemistry and physics concepts. Building this model enhances skills such as patience, balance, attention to detail, and innovation—qualities essential for mastering abstract scientific ideas. Additionally, the model offers insights into architectural stability concepts like spiral staircases."

| Year: 2025 | PT id = 1334, Type = formulation spiral non-chem misc 3D |
Spiral-Struktur-Periodensystem der Elemente
Martin Röder writes:
https://archive.org/details/spiral-struktur-periodensystem
This is a new periodic table of the elements, which classifies the elements as follows:
1. according to the structural groups of occult chemistry
2. on the basis of positive charges (proton number)
3. according to the number of primary atoms (mass)Detailed description under "PDF" in "Article - Spiral-Structure-Periodic System 250621.pdf"
Overview chart to print out: "Spiral-Structure-Periodic System of the Elements (with structure groups of Occult Chemistry) 250621.pdf".
2D and 3D animations under: "ANIMATED GIF" Graphics and diagrams under: "PNG" video with 3D animation



 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.

.SVG-1.png)