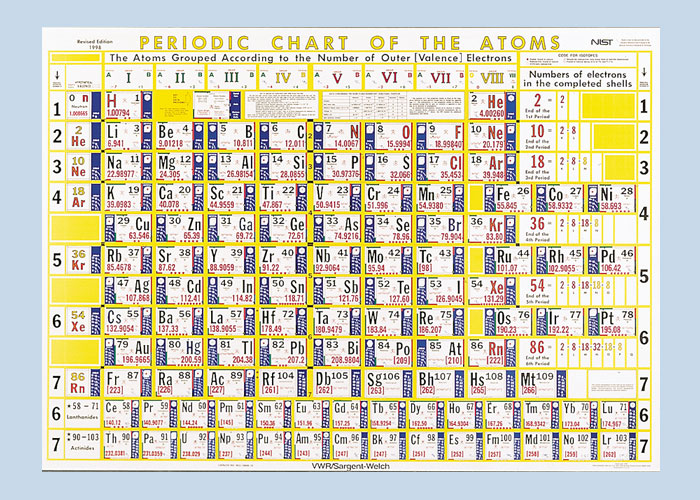
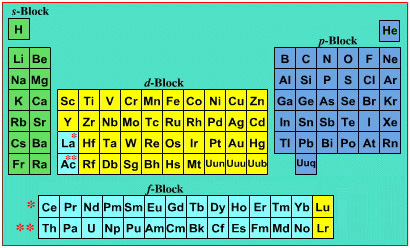
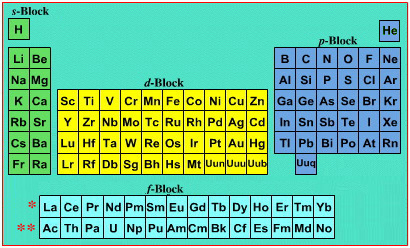
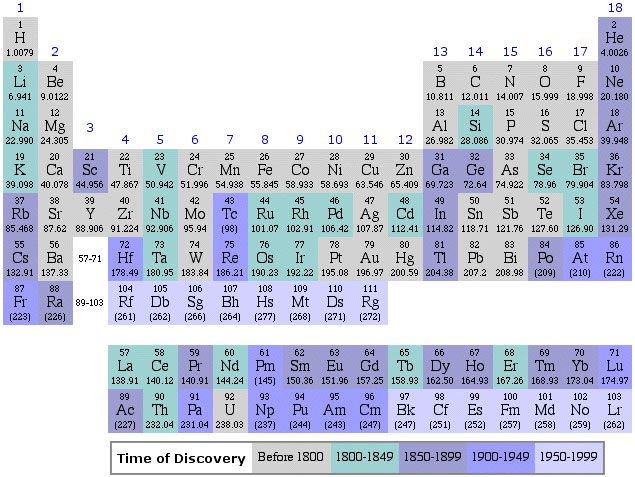
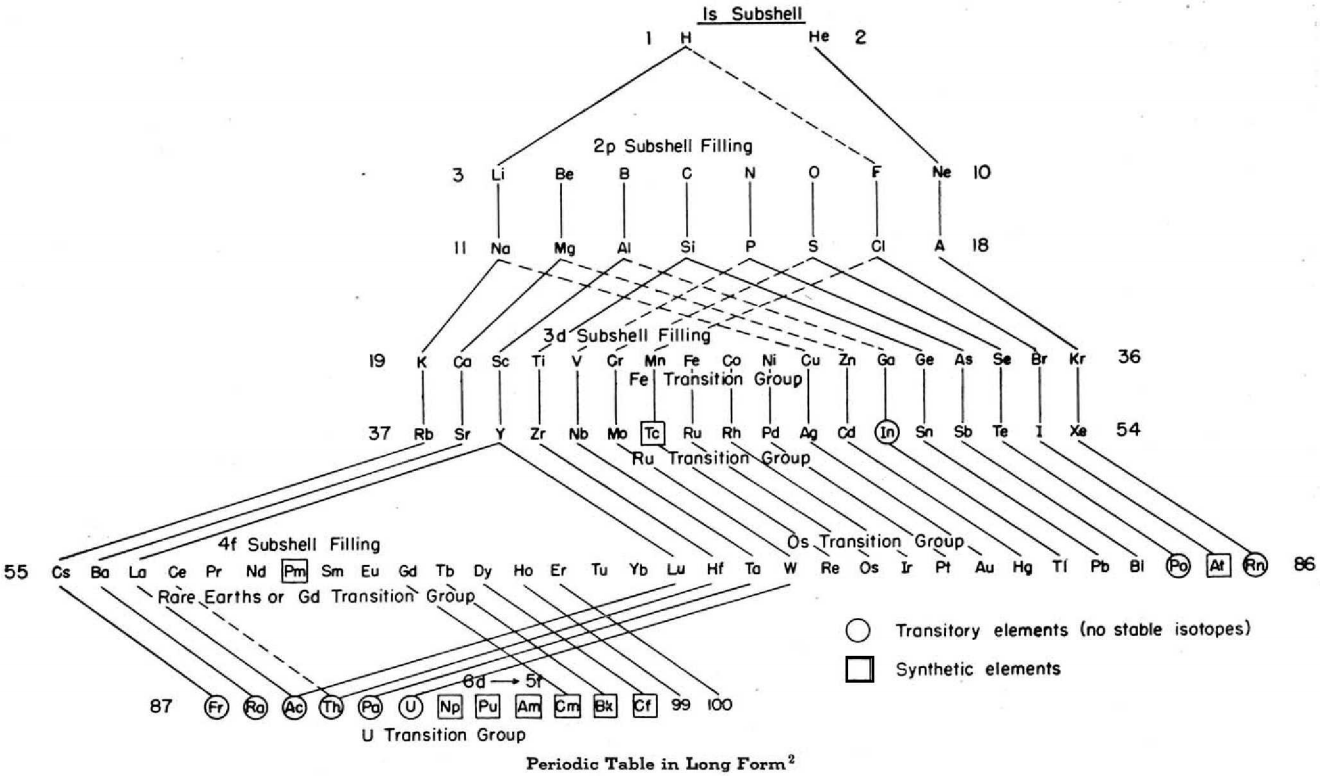
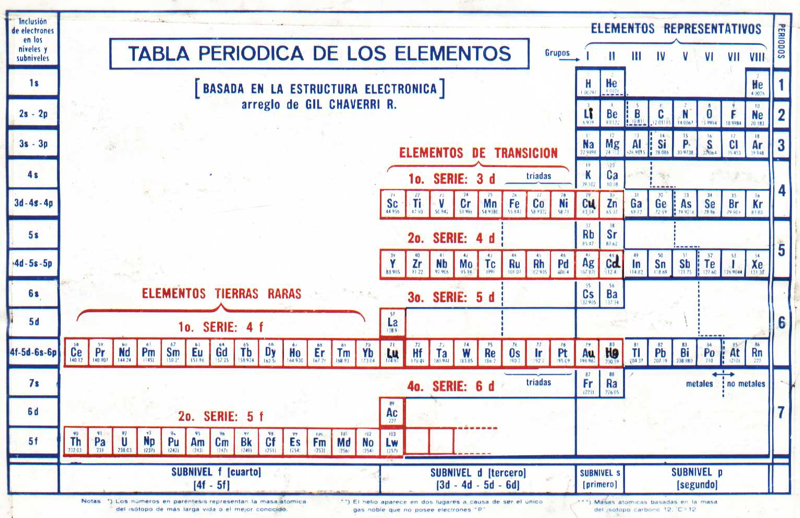
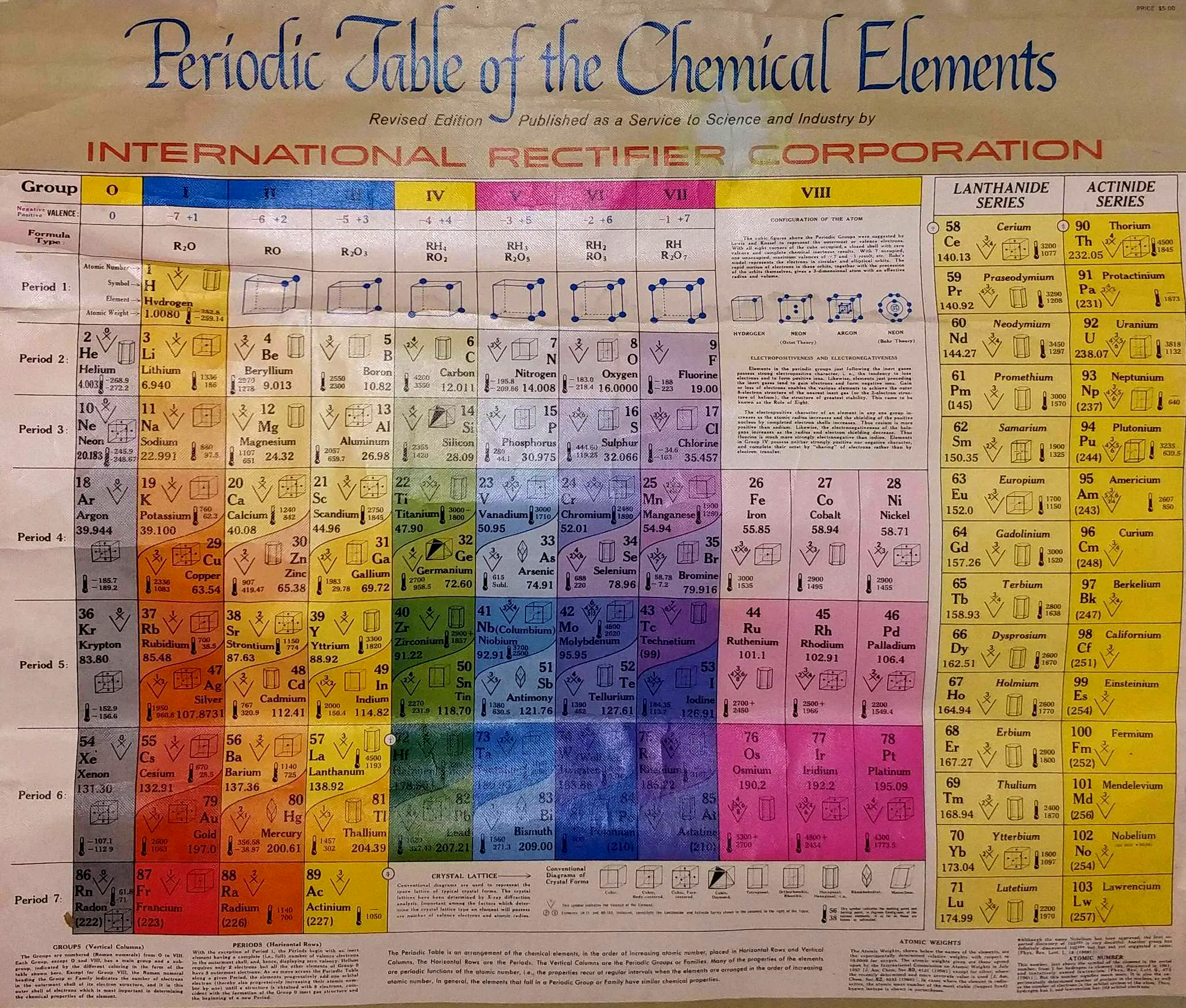
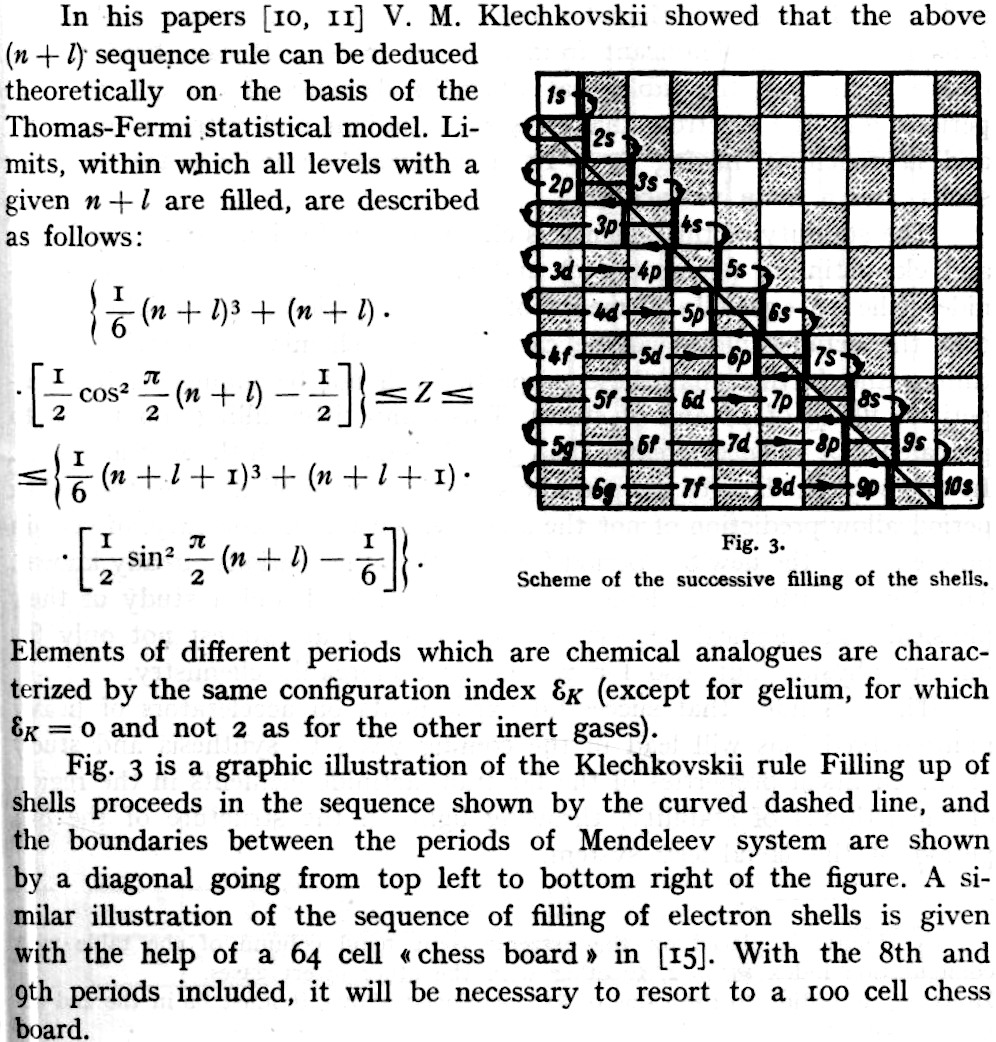
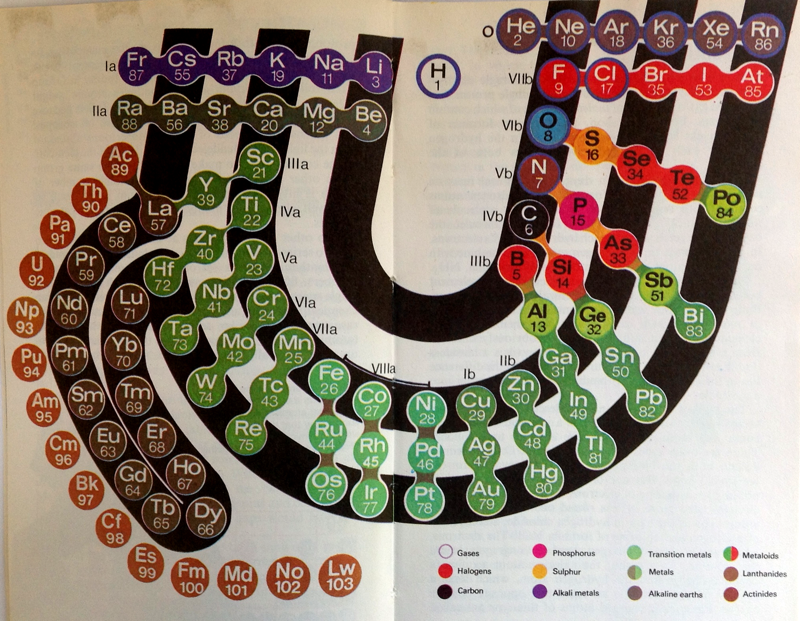
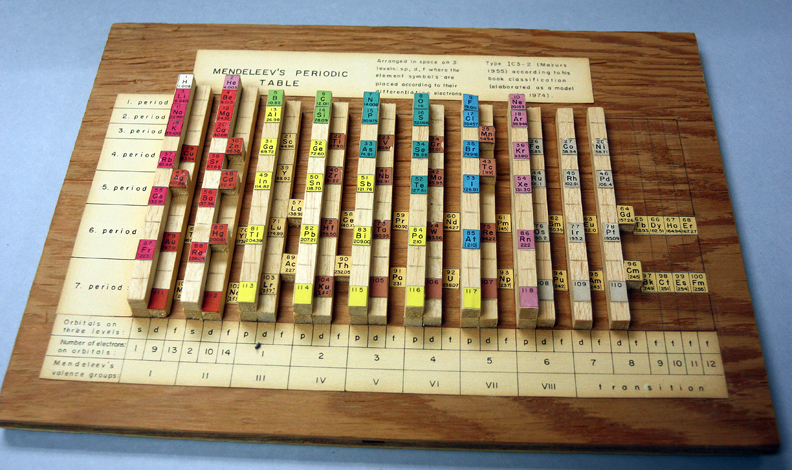
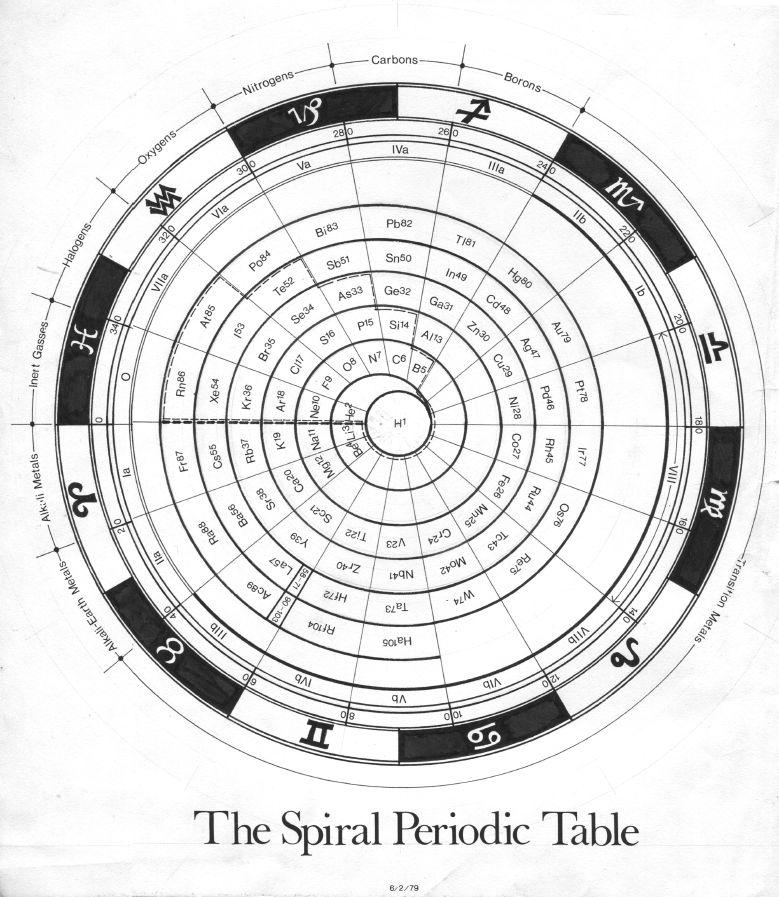
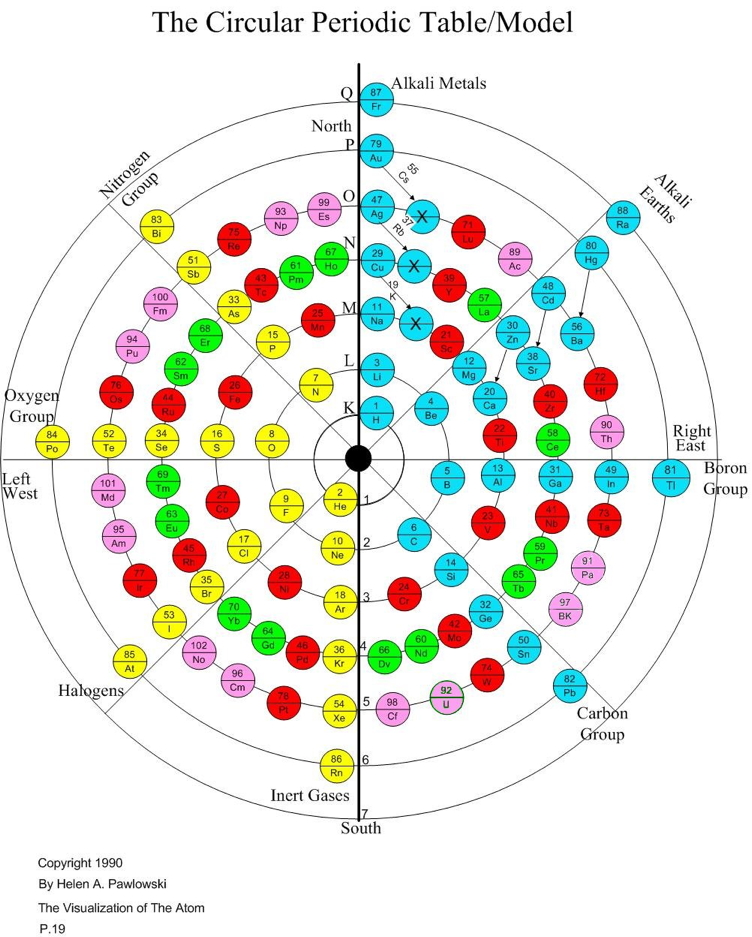
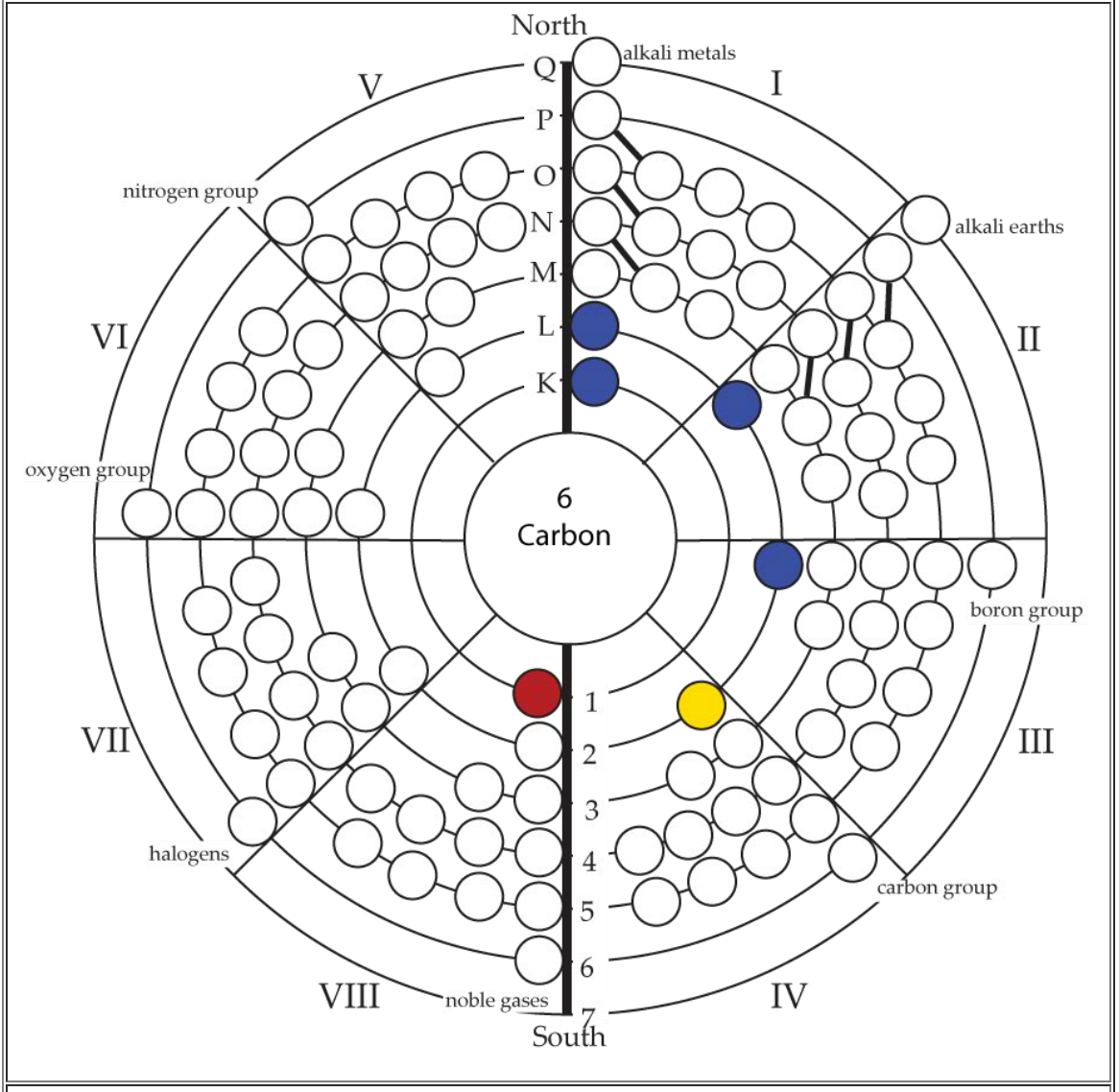
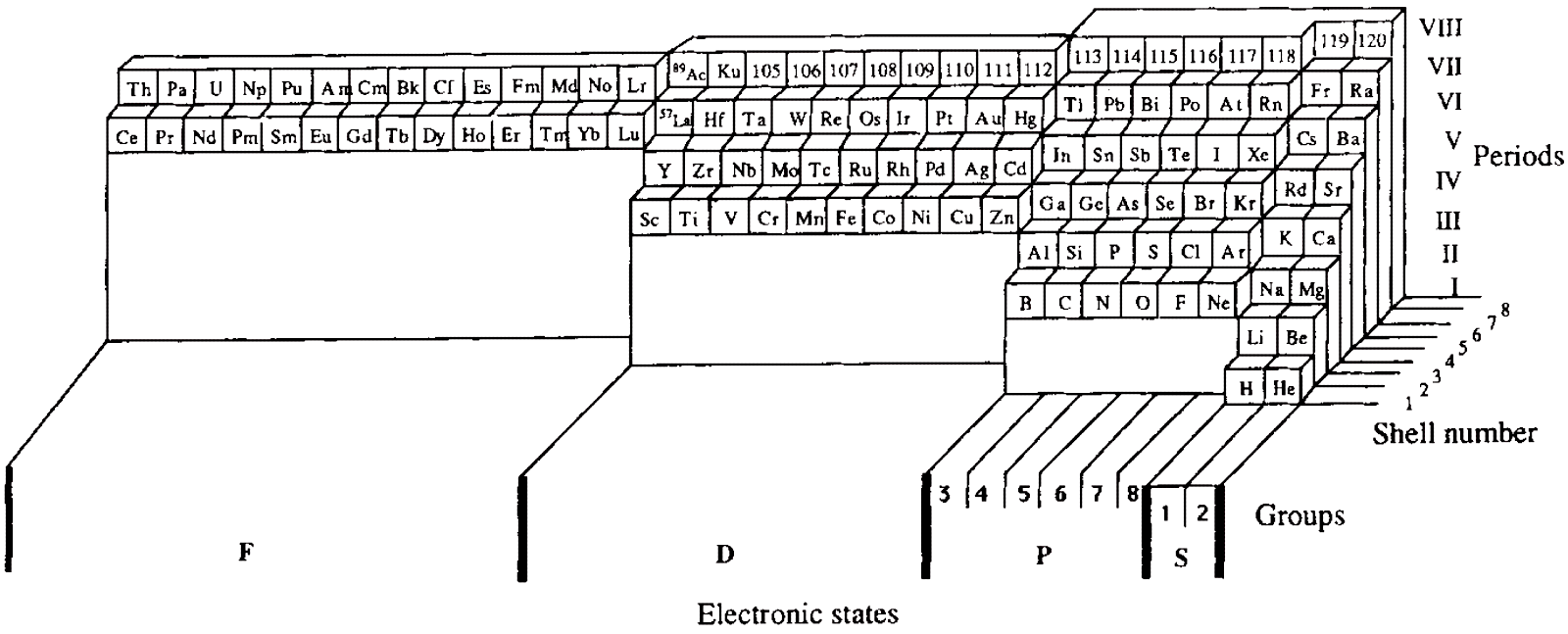
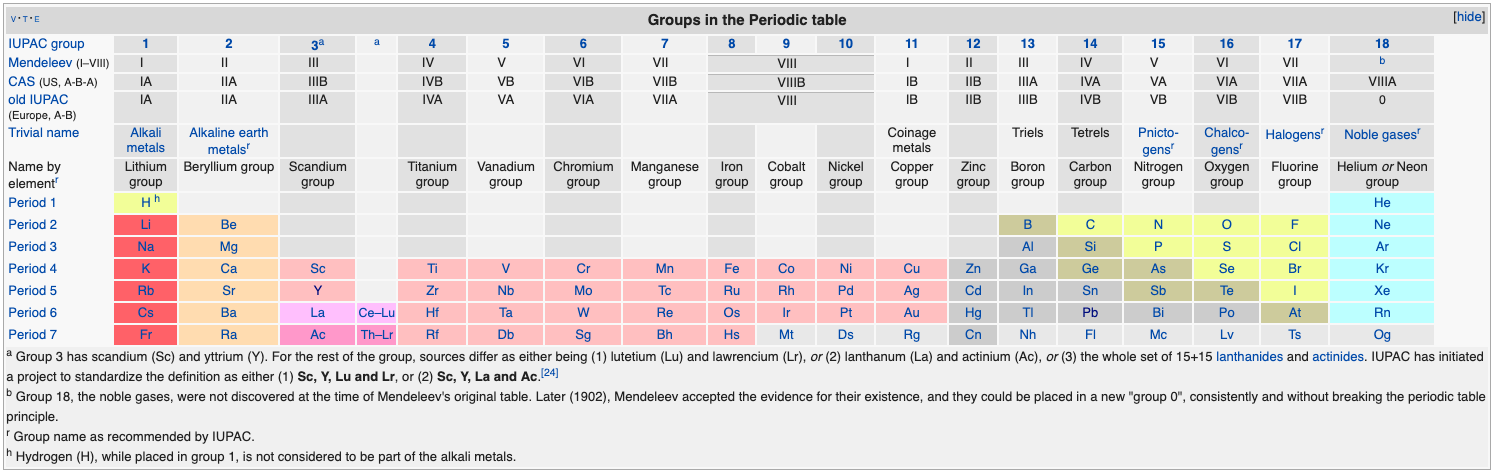
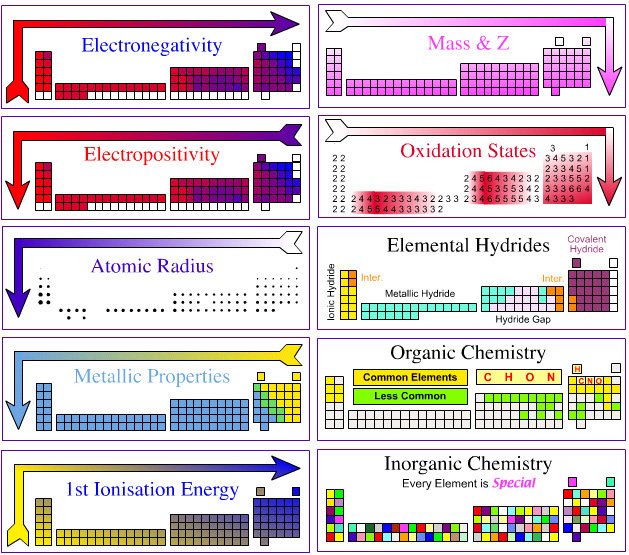
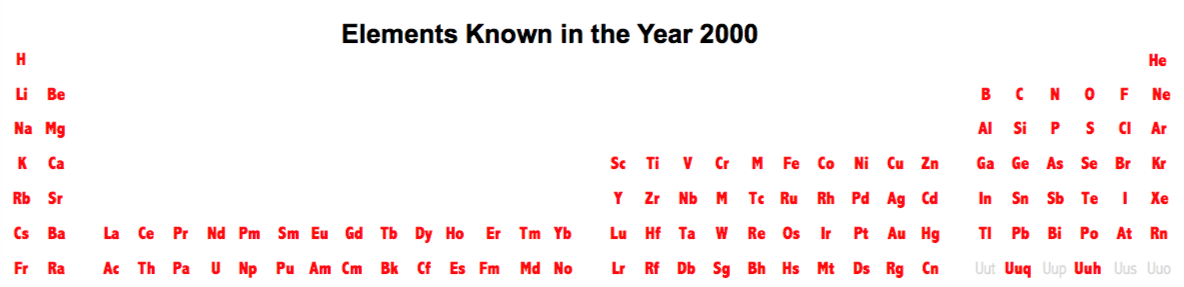
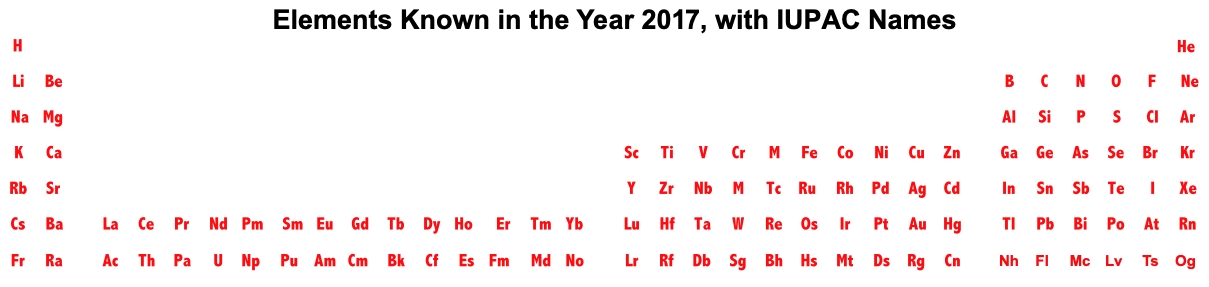
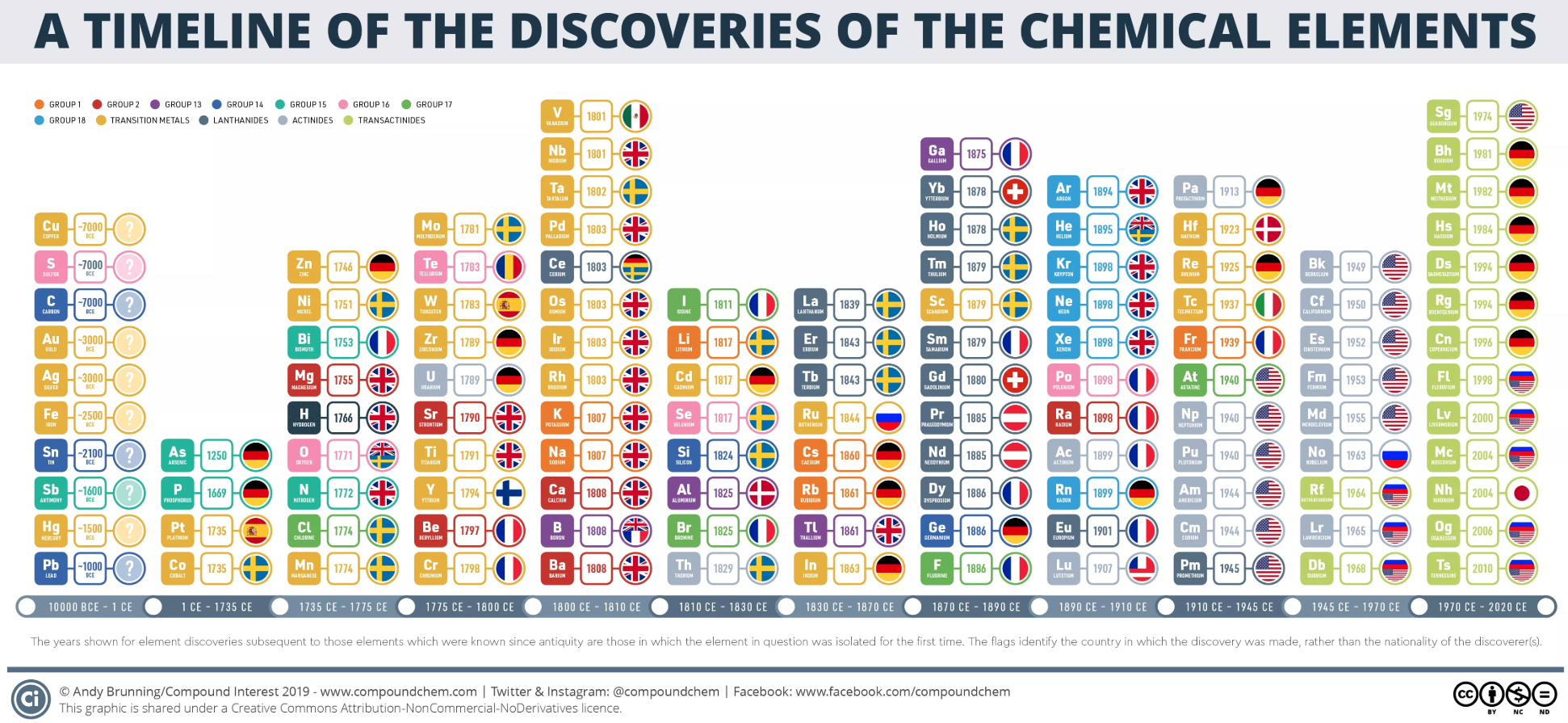
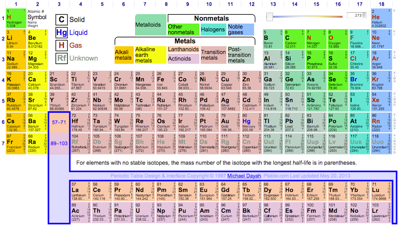
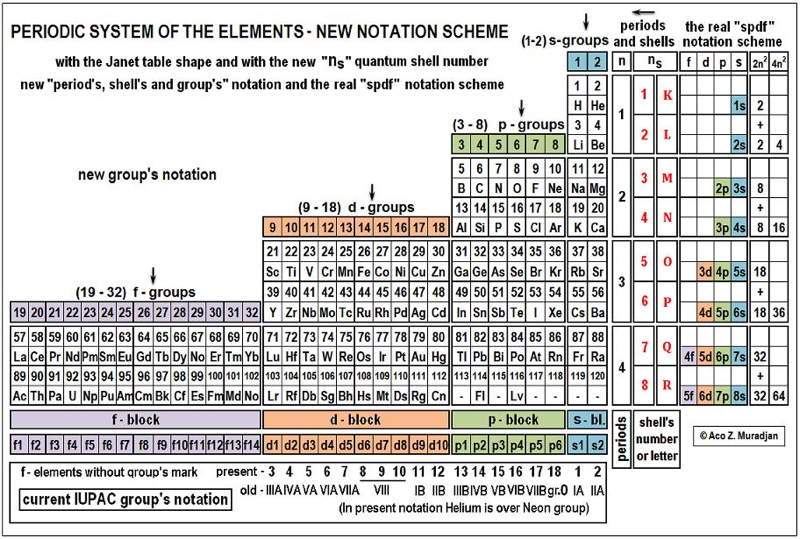
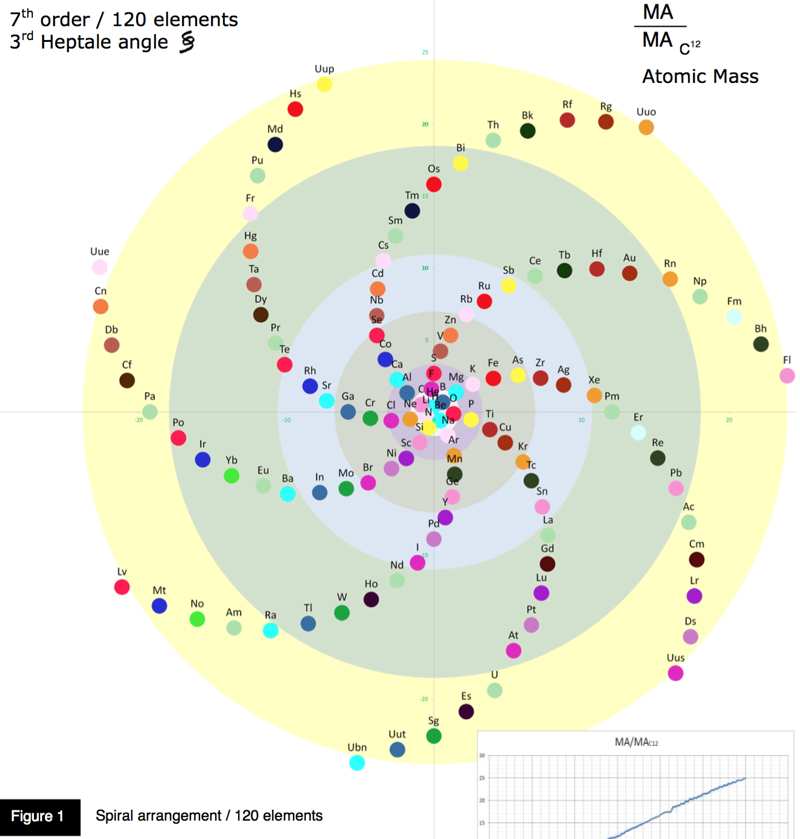
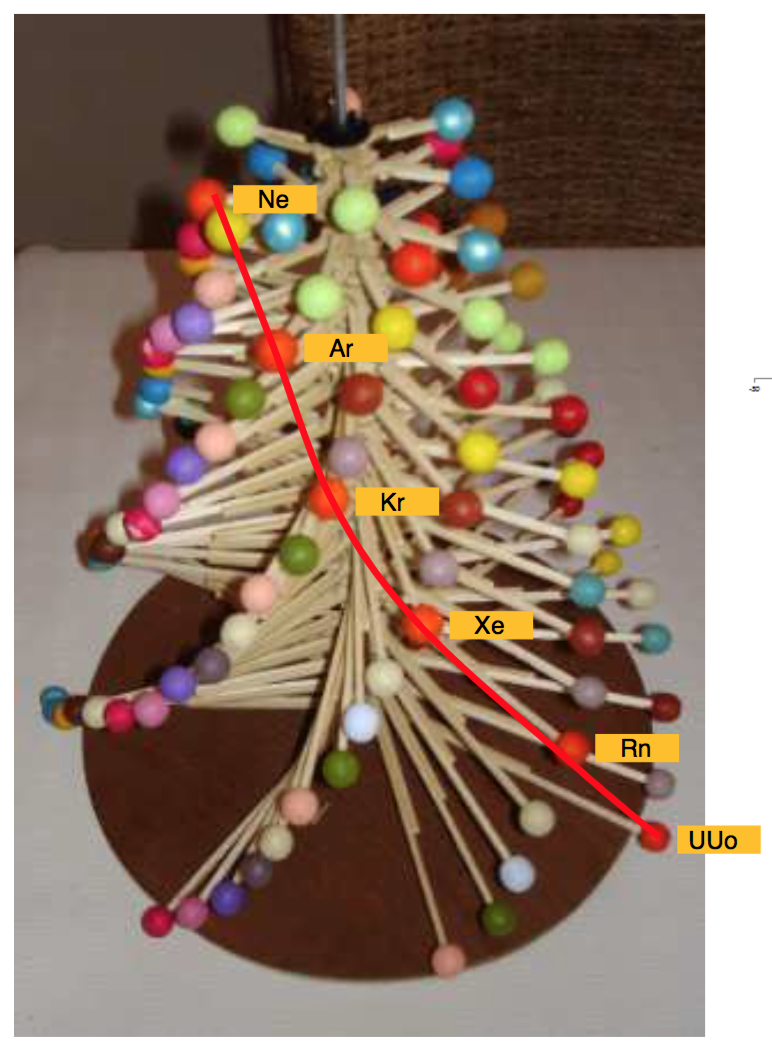
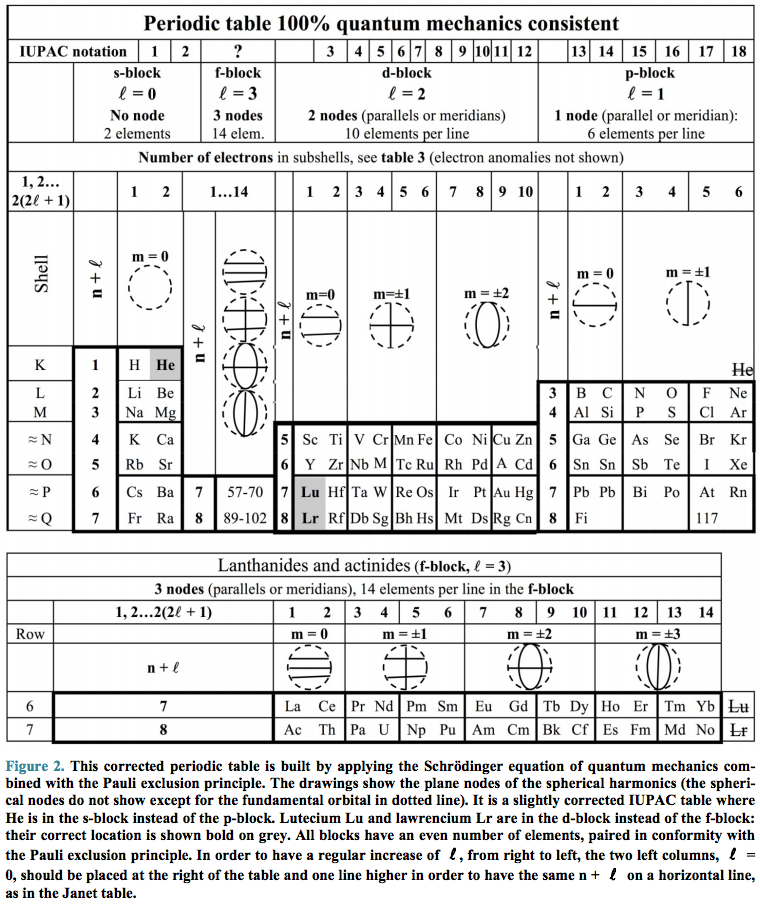
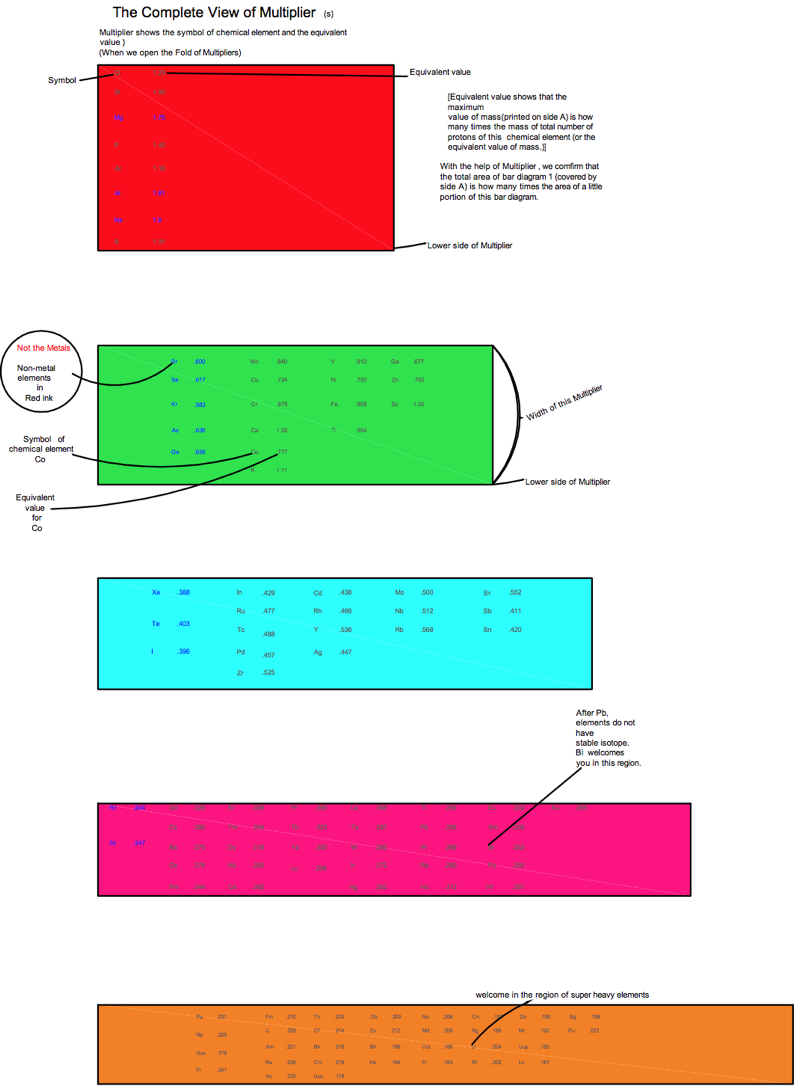
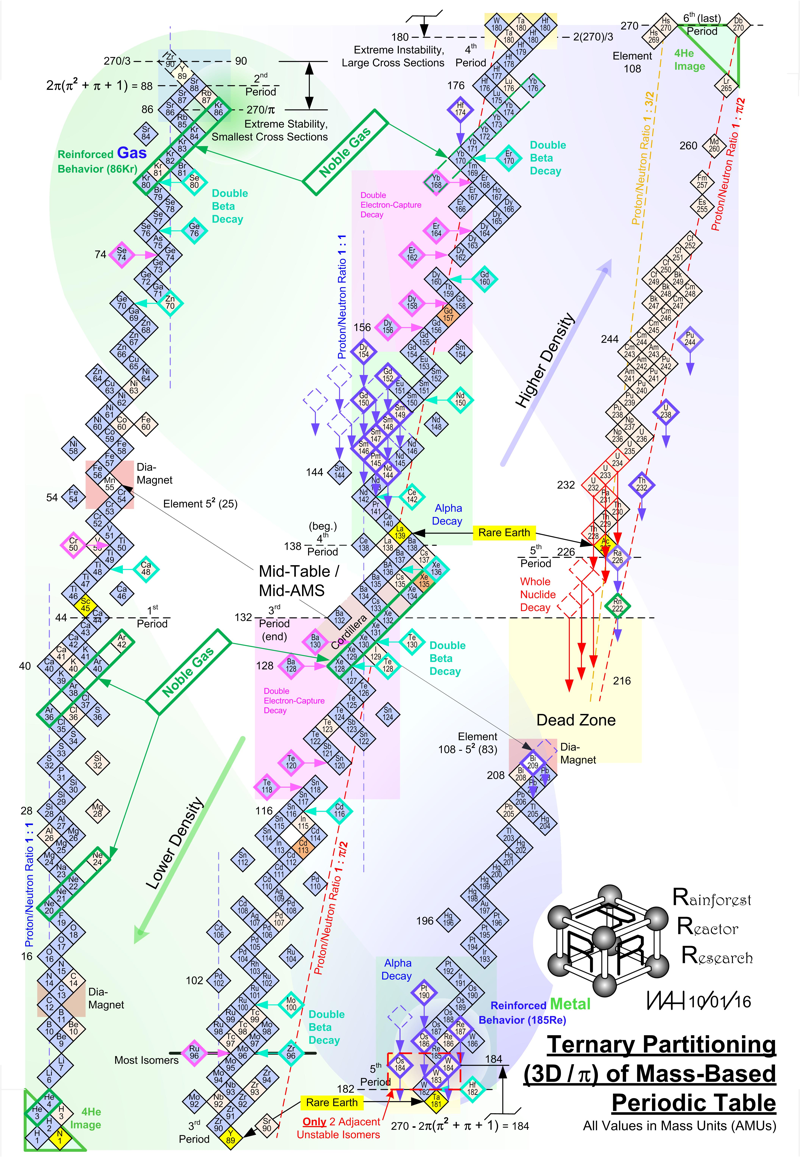
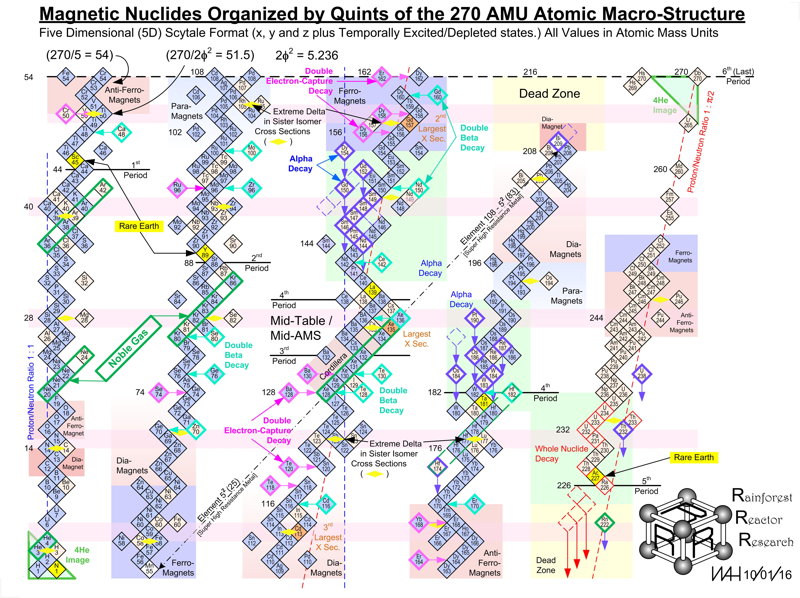
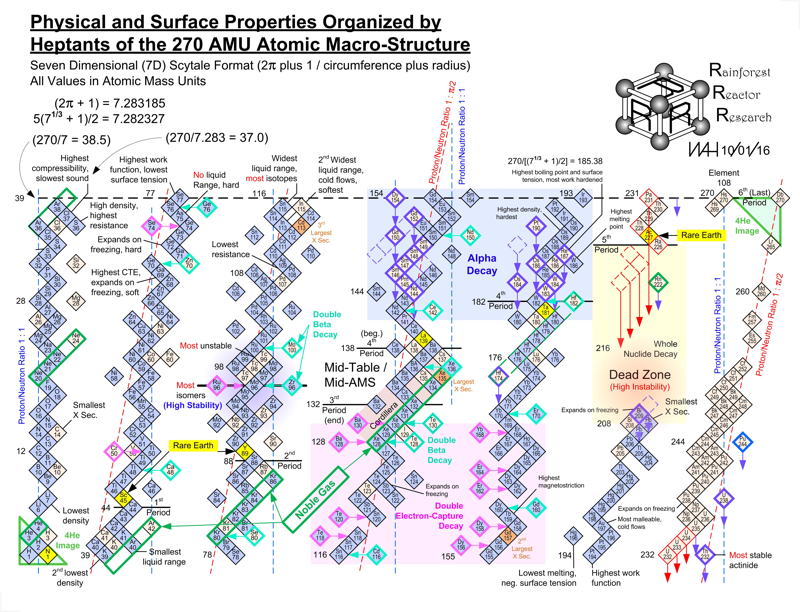
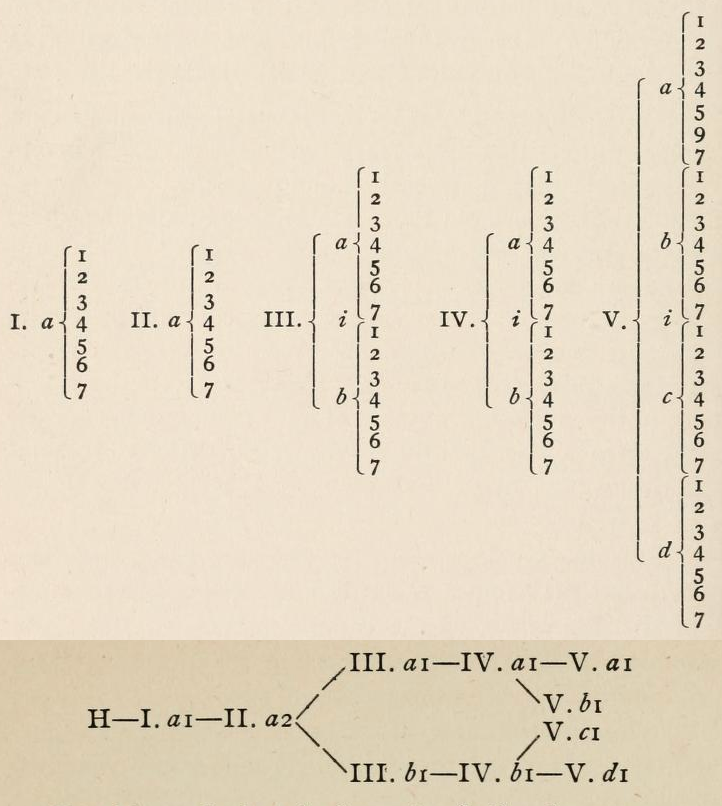
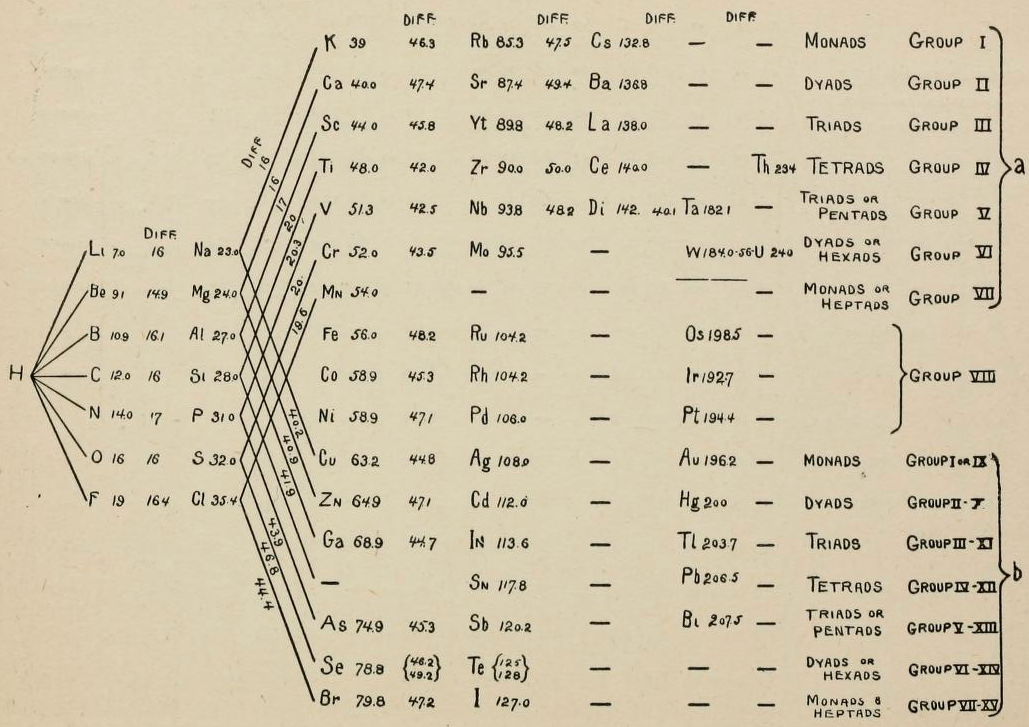
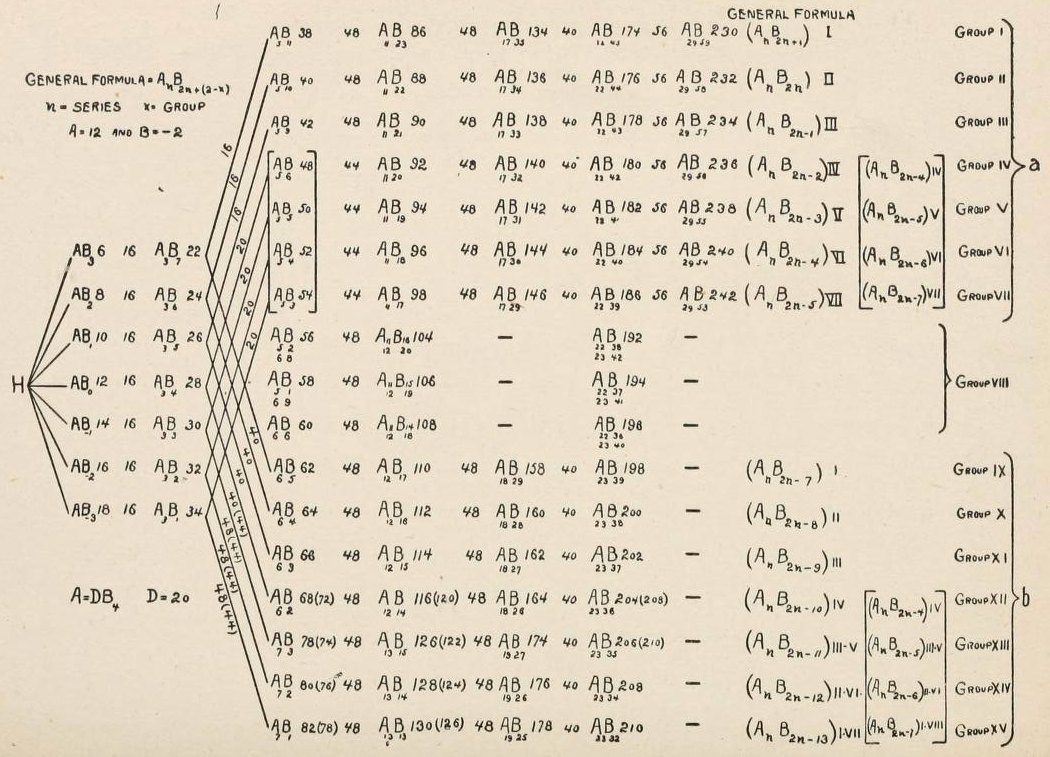
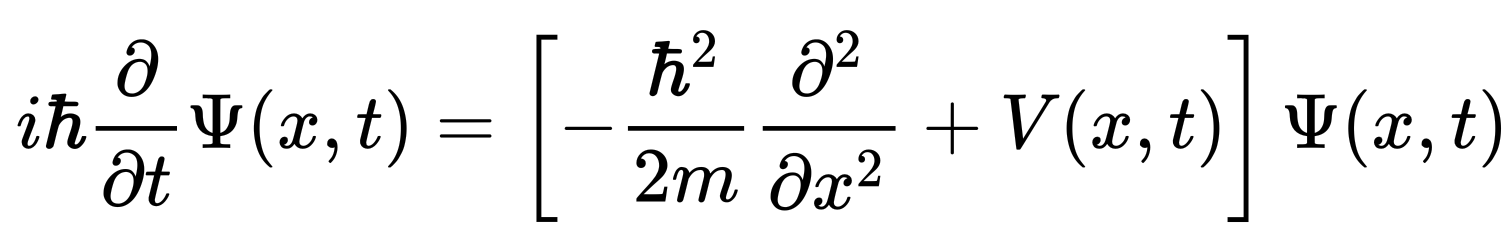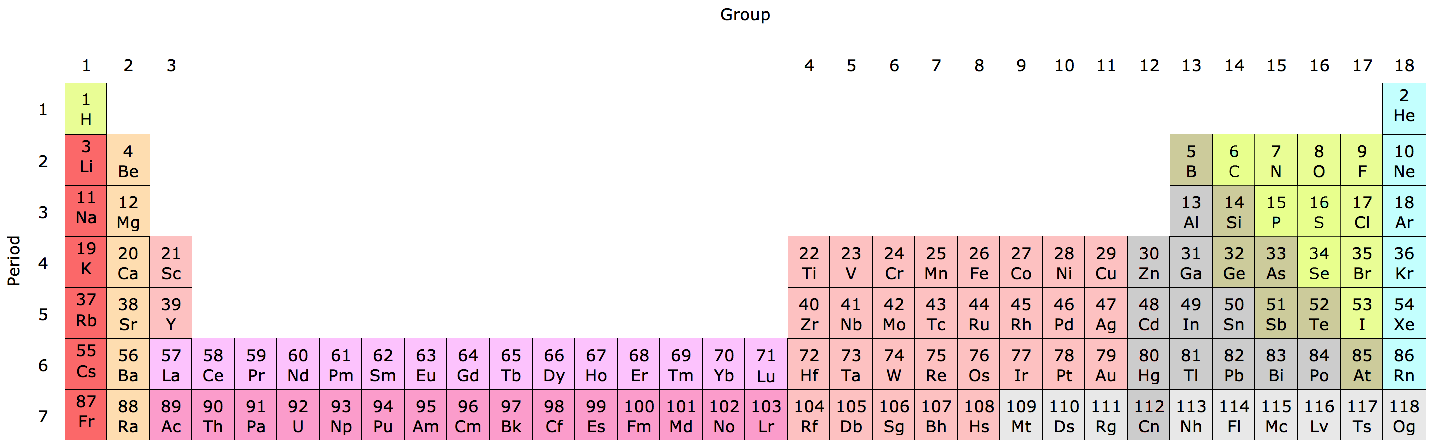Periodic Table |
 |
 |
 |
 |
 |
 |
 |
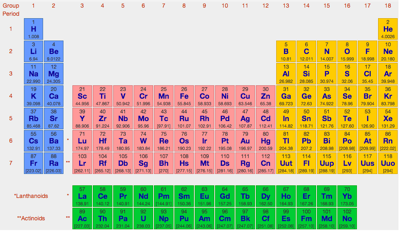
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
Periodic Tables referencing the text string "Ren", listed by date:
| Year: 450 BCE | PT id = 229, Type = formulation |
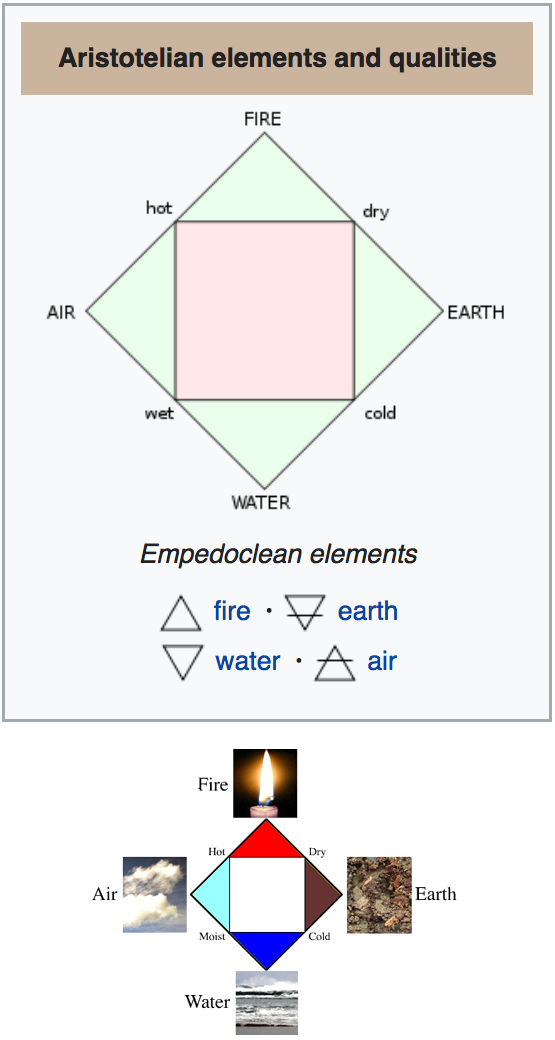
Classical Elements: Earth, Water, Air & Fire
The Greek Classical Elements — Earth, Water, Air, Fire [& Aether] — date from 450 BC or so, and persisted throughout the Middle Ages and into the Renaissance, deeply influencing European thought and culture.
A Greek text Kore Kosmou ("Virgin of the World" - associated with the Egyptian god Thoth - names the four elements fire, water, air, and earth:
And Isis answer made: Of living things, my son, some are made friends with fire, and some with water, some with air, and some with earth, and some with two or three of these, and some with all. And, on the contrary, again some are made enemies of fire, and some of water, some of earth, and some of air, and some of two of them, and some of three, and some of all. For instance, son, the locust and all flies flee fire; the eagle and the hawk and all high-flying birds flee water; fish, air and earth; the snake avoids the open air. Whereas snakes and all creeping things love earth; all swimming things love water; winged things, air, of which they are the citizens; while those that fly still higher love the fire and have the habitat near it. Not that some of the animals as well do not love fire; for instance salamanders, for they even have their homes in it. It is because one or another of the elements doth form their bodies' outer envelope. Each soul, accordingly, while it is in its body is weighted and constricted by these four.
The four elements were used by Hippocrates in describing the human body with an association with the four humours:
- yellow bile (fire)
- black bile (earth)
- blood (air)
- phlegm (water)
Plato characterizes the elements from a list created by the Sicilian philosopher Empedocles called these the four "roots." Plato seems to have been the first to use the term element:
| Year: 1787 | PT id = 964, Type = formulation data |
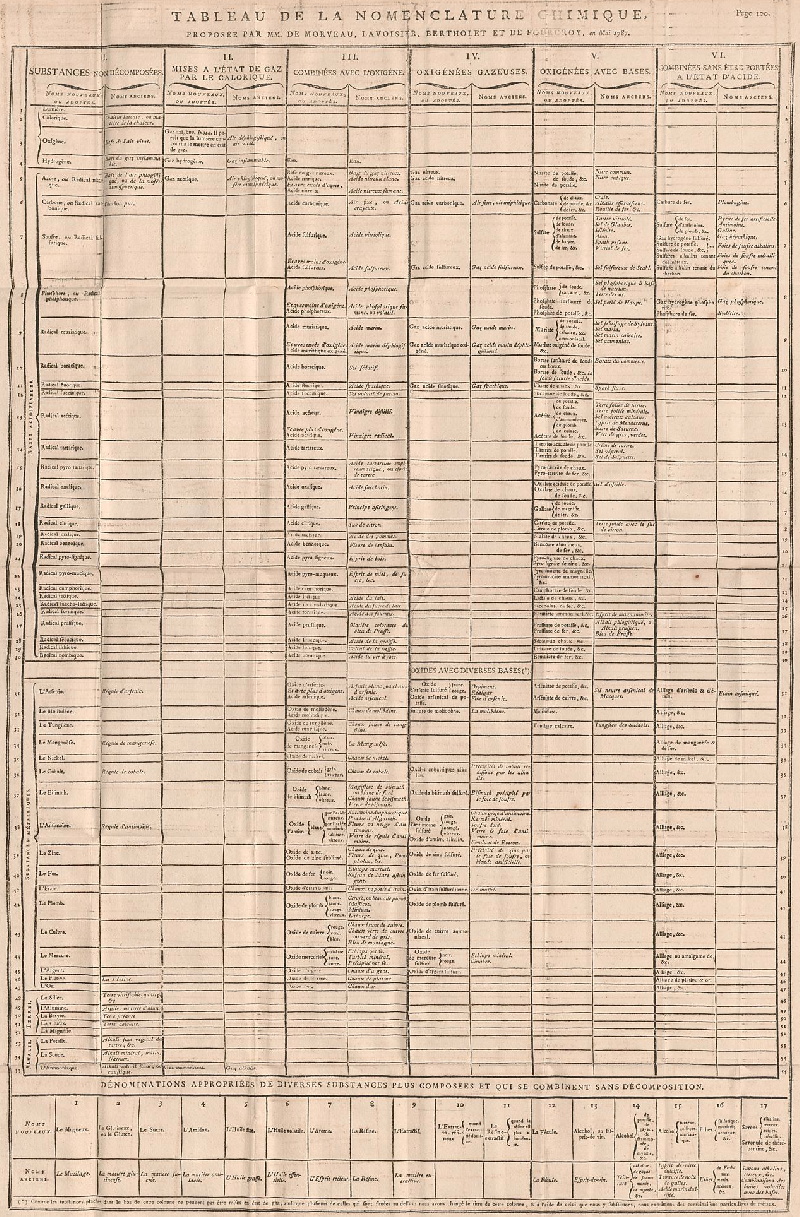
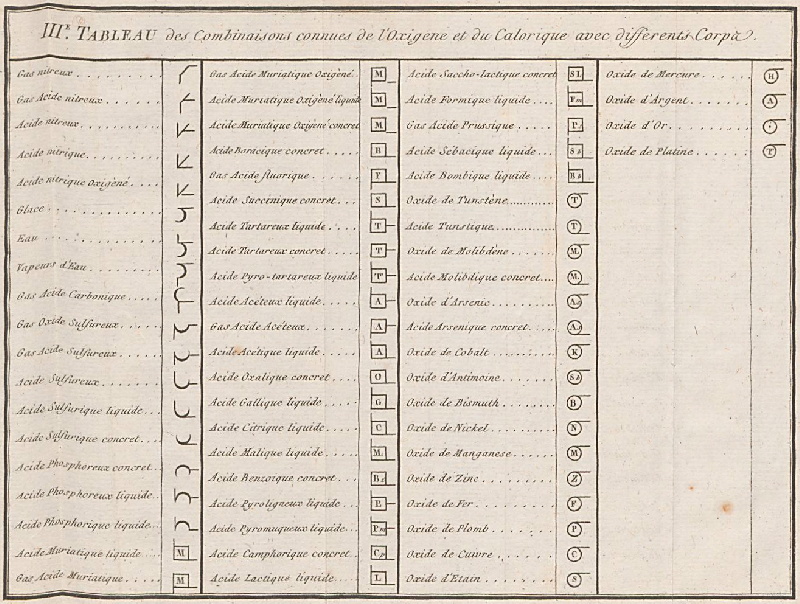
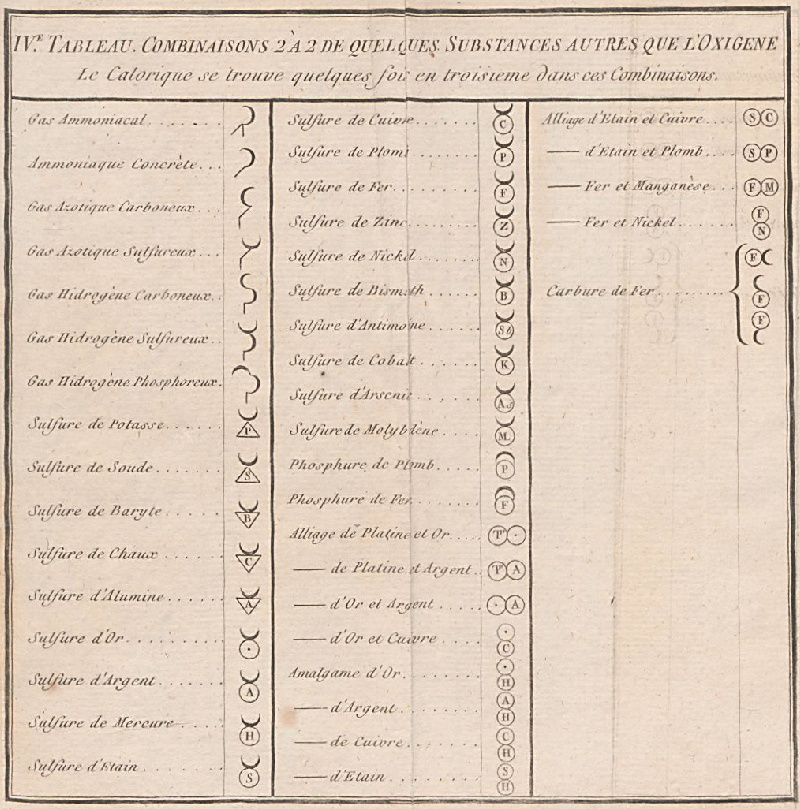
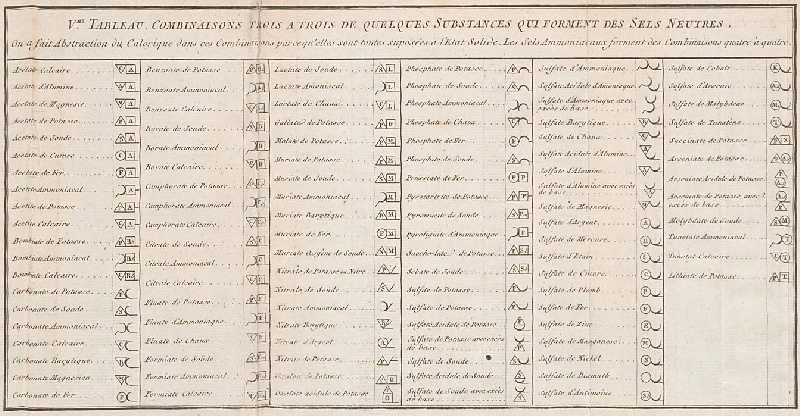
Méthode de Nomeclature Chimique
By Louis Bernard Guyton de Morveau (1737-1816), Antoine Laurent Lavoisier (1743-1794) , Claude-Louis Berthollet (1748-1822) & Antoine-François de Fourcroy (1755-1809) a book: Méthode de Nomeclature Chimique.
The complete scanned book is available. (Click the 'page view' button, or here.)
The book lists the several hundred chemicals known at the time, including chemical elements, and it discusses the nomenclature (naming). Although not a periodic table as such, the information contained in this book was state of the art for 1787.
Click on an image below to enlarge.
| Year: 1789 | PT id = 3, Type = formulation early |
Antoine Lavoisier
Antoine Lavoisier produced a list chemical substances, that included the 23 known elements. He also refined the concept as before this time, metals - with the exception of mercury - were not considered to be elements. Wikipedia.
A list of 33 simple substances compiled by Lavoisier, from Traité Élémentaire de Chimie, Cuchet, Paris, 1789, p. 192:

From Peter van der Krogt's Elementymology & Elements Multidict web site:
| Lavoisier's Table of Simple Substances (1789) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Year: 1803 | PT id = 4, Type = formulation element weight structure |
Dalton's Postulates About The Elements
Around the year 1803 in Manchester, John Dalton gave a series of lectures in which he presented his postulates:
- Elements are made of tiny particles called atoms.
- The atoms of a given element are different from those of any other element, and the atoms of different elements can be distinguished from one another by their respective relative atomic weigh/mass.
- All atoms of a given element are identical.
- Atoms of one element can combine with atoms of other elements to form chemical compounds, and a given compound always has the same relative numbers of types of atoms.
- Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process, and a chemical reaction simply changes the way atoms are grouped together.
From a very early notebook from around this time:

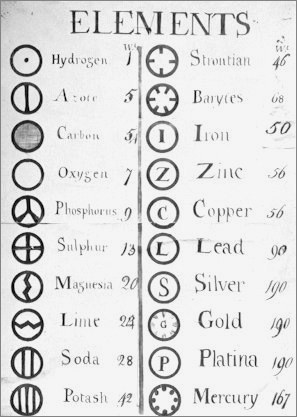
| Year: 1813 | PT id = 1043, Type = formulation |
Wollaston's Slide Rule of Chemical Equivalents
Philosophical Transactions: A Synoptic Scale of Chemical Equivalents by William Hyde Wollaston, M.D. Sec. R.S. – or from here – has a diagram for a slide rule of chemical equivalents:
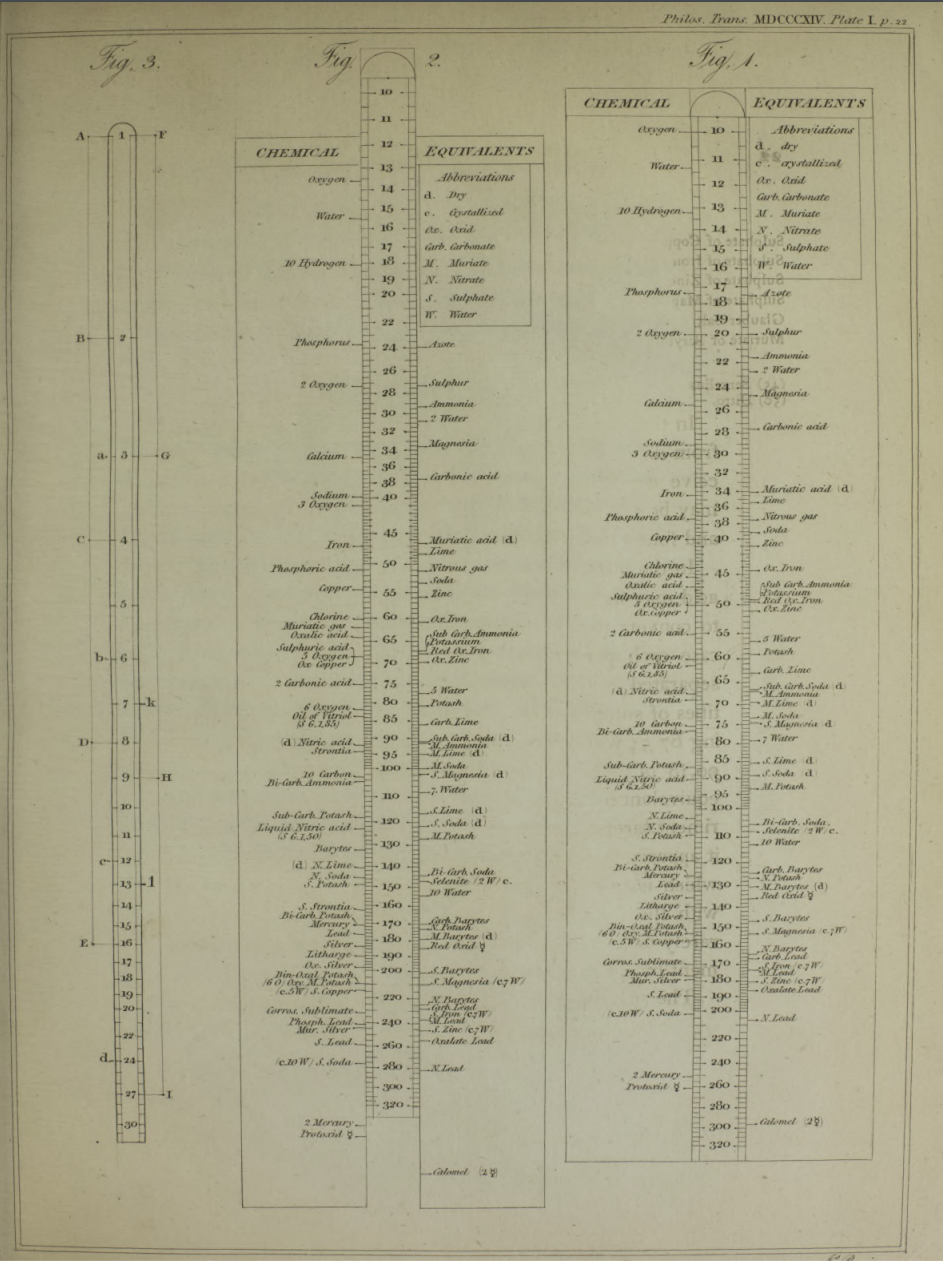
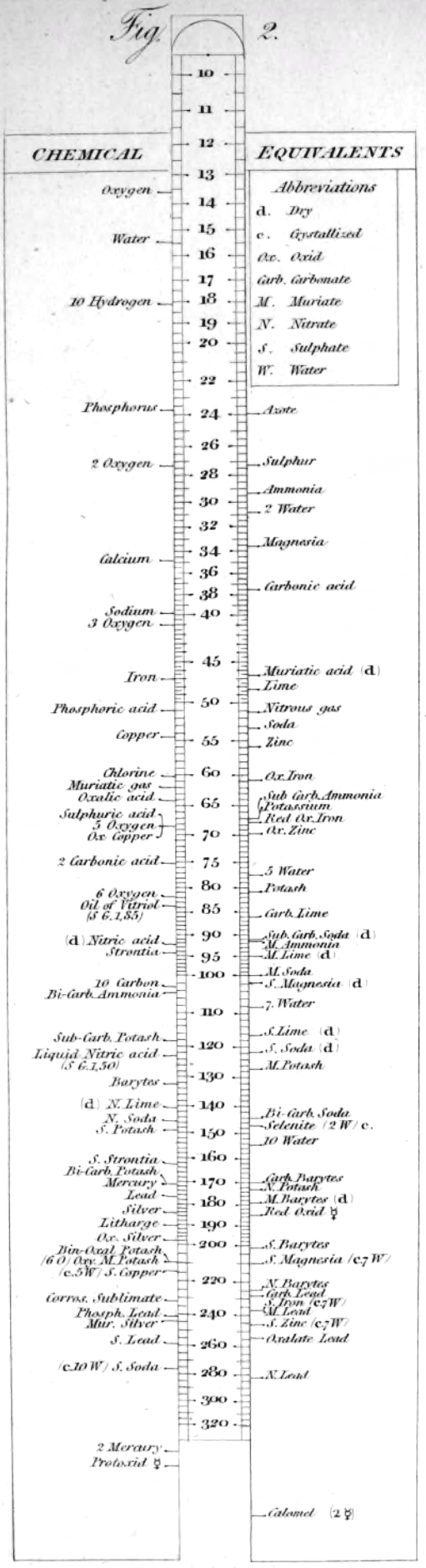
Wollaston writes:
"In order to shew more clearly the use of this scale, the Plate [diagram of the chemical slide rule] exhibits two different situations of the slider, in one of which oxygen is 10 [oxygen is defined as having an atomic weight/mass of 10.00], and other bodies are in their due proportion to it, so that carbonic acid being 27,54, and lime 35,46, carbonate of lime is placed at 63.
"In the second figure, the slider is represented drawn upwards till 100 corresponds to muriate of soda [sodium chloride, NaCl]; and accordingly the scale then shews how much of each substance contained in the table is equivalent to 100 of common salt. It shews, with regard to the different views of the analysis of this salt, that it contains 46,6 dry muriatic acid [hydrogen chloride], and 53,4 of soda, or 39,8 sodium, and 13,6 oxygen; or if viewed as chlorid of sodium, that it contains 60,2 chlorine, and 39,8 sodium."
Read more in an entry concerning chemical slide rules.
Thanks to Nawa for the tip!
| Year: 1813 | PT id = 1044, Type = formulation element weight |
Wollaston's Synoptic Scale of Chemical Equivalents
Philosophical Transactions: A Synoptic Scale of Chemical Equivalents by William Hyde Wollaston, M.D. Sec. R.S., or from here.
It is apparent that chemistry the years 1810 to 1850 was largely concerned with discovering the whole number stoichiometric ratios of atoms in chemical compounds.
Wollaston writes in the text above:
"It is impossible in several instances, where only two combinations of the same ingredients are known, to discover which of the compounds is to be regarded as consisting of a pair of single atoms, and since the decision of these questions is purely theoretical, and by no means necessary to the formation of a table adapted to most practical purposes, I have not been desirous of warping my numbers according to an atomic theory, but have endeavored to make practical convenience my sole guide, and have considered the doctrine of simple multiples, on which that of atoms is founded, merely as a valuable assistant in determining, by simple division, the amount of those quantities that are liable to such definite deviations from the original law of Richter."
"Mr. Dalton in his atomic views of chemical combination appears not to have taken much pains to ascertain the actual prevalence of that law of multiple proportions by which the atomic theory is best supported [however] it is in fact to Mr. Dalton that we are indebted for the first correct observation of such an instance of a simple multiple in the union of nitrous gas with oxygen."
"[I have] computed a series of supposed atoms, I [have] assumed oxygen as the decimal unit of my scale [ie. oxygen = 10], in order to facilitate the estimation of those numerous combinations which it forms with other bodies. Though the present table of Equivalents, I have taken care to make oxygen equally prominent on account of the important part it performs in determining the affinities of bodies by the different proportions in which it is united to them.."
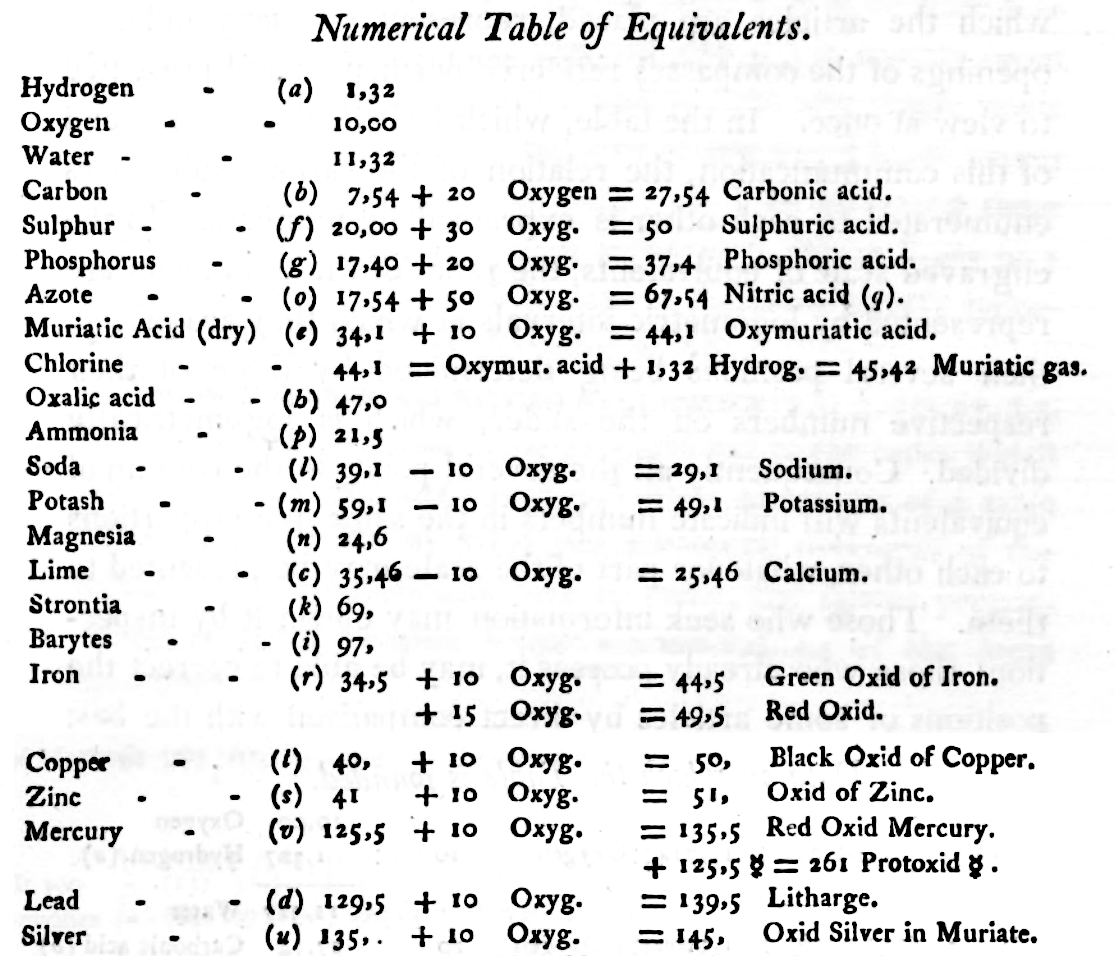
Mark Leach writes:
"When Wollaston's equivalent weights are converted from O = 10.00 to the modern value of O = 15.999, the atomic weight values can be seen to be astonishingly accurate.
"However, the language of the article is quite difficult as the meaning of many of the terms is unclear (to me, at least). For example, in modern usage adding 'ia' to a metal implies the oxide: 'magnesia' is magnesium oxide, MgO. I am not clear if this historical usage is consistent. 'Azote' is nitrogen and 'muriatic acid (dry)' is hydrogen chloride gas. I have only analyses/re-calculated the elements and a couple of common/obvious compounds:"
| Wollaston's data | Scaled to O = 15.999 | Modern Values | % error | |
| H (as H2) | 1.32 | 2.112 | 2.016 | 5% |
| O | 10.00 | 15.999 | 15.999 | ref. value |
| H2O | 11.32 | 18.111 | 18.015 | 1% |
| C | 7.74 | 12.383 | 12.011 | 3% |
| S | 20.00 | 31.998 | 32.060 | 0% |
| P | 17.40 | 27.838 | 30.974 | -11% |
| N (as N2) | 17.54 | 28.062 | 28.014 | 0% |
| Cl (as Cl2) | 44.10 | 70.556 | 70.900 | 0% |
| Fe | 34.50 | 55.197 | 55.845 | -1% |
| Cu | 40.00 | 63.996 | 63.546 | 1% |
| Zn | 41.00 | 65.596 | 65.380 | 0% |
| Hg | 125.50 | 200.787 | 200.590 | 0% |
| Pb | 129.50 | 207.187 | 207.980 | 0% |
| Ag | 135.00 | 215.987 | 107.870 | 50% |
- The elements hydrogen, nitrogen (azote) and chlorine have clearly been measured as the diatomic molecules, even if this was unknown to Wollaston in 1813.
- Phosphorus is out by 11%... [fair enough].
- Only silver is clearly wrong, but it is out by 50% so it looks like a simple stoichiometry error: Perhaps the oxide was assumed to be AgO was instead of the correct Ag2O.
Interestingly, Wollaston's analysis is far better than Daubeny's 1831 data seen in Oxford.
Read more in an entry concerning chemical slide rules.
Thanks to Nawa for the tip!
| Year: 1829 | PT id = 6, Type = formulation element |
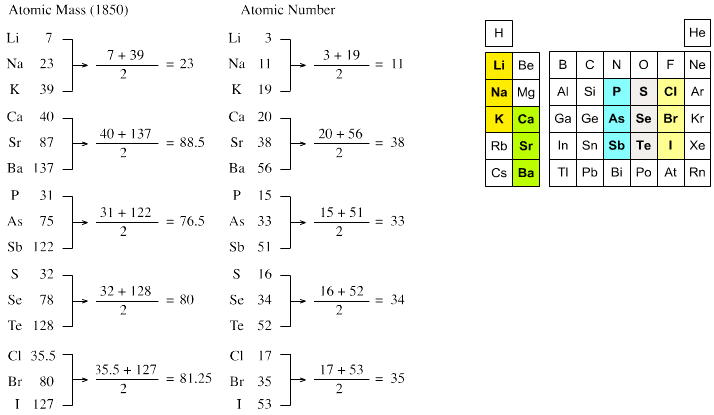
Döbereiner's Triads
Johann Döbereiner found triads: a sequence of three similar elements, where the middle element has a mass equal to the average of the least and most massive.
A brief biography can be found on the Nature website.
Döbereiner writes in An Attempt to Group Elementary Substances according to Their Analogies (in English)
From Poggendorf's Annalen der Physik und Chemie 15, 301-7 (1829) (in German) [from Henry M. Leicester & Herbert S. Klickstein, eds., A Source Book in Chemistry, 1400-1900 (Cambridge, MA: Harvard, 1952)]:
"The work of Berzelius on the determination of the atomic weights of bromine and iodine has interested me greatly, since it has established the idea, which I expressed earlier in my lectures, that perhaps the atomic weight of bromine might be the arithmetical mean of the atomic weights of chlorine and iodine. This mean is (35.470+126.470)/2 = 80.470. This number is not much greater than that found by Berzelius (78.383); however, it comes so close that it may almost be hoped that the difference will vanish entirely after repeated careful and exact determinations of the atomic weights of these three salt-forming elements. This idea was the motive for an attempt which I made twelve years ago to group substances by their analogies."
[Note: L&K noticed an error in the above math: (35.47 + 126.47)/2 = 80.97 not 80.47. Whoops...]

The diagram below uses mid-nineteenth century atomic mass information rather than modern data. If atomic numbers (Z) are used (a property unknown in 1850), the triads are exact:
| Year: 1858 | PT id = 1047, Type = formulation review element weight structure |
Cannizzaro's Letter or Sunto
Letter of Professor Stanislao Cannizzaro to Professor S. De Luca: Sunto di un corso di filosofia chimica (Sketch of a Course of Chemical Philosophy) given in the Royal University of Genoa, Il Nuovo Cimento, vol. vii. (1858), pp. 321-366.

Many thanks to Carmen Giunta, Professor of Chemistry Emeritus, Le Moyne College who provided the information about, and link to, Cannizzaro's Letter. See a list of other classic chemistry papers.
Read the full letter/paper, in English translation, here. (The Italian version is here.)
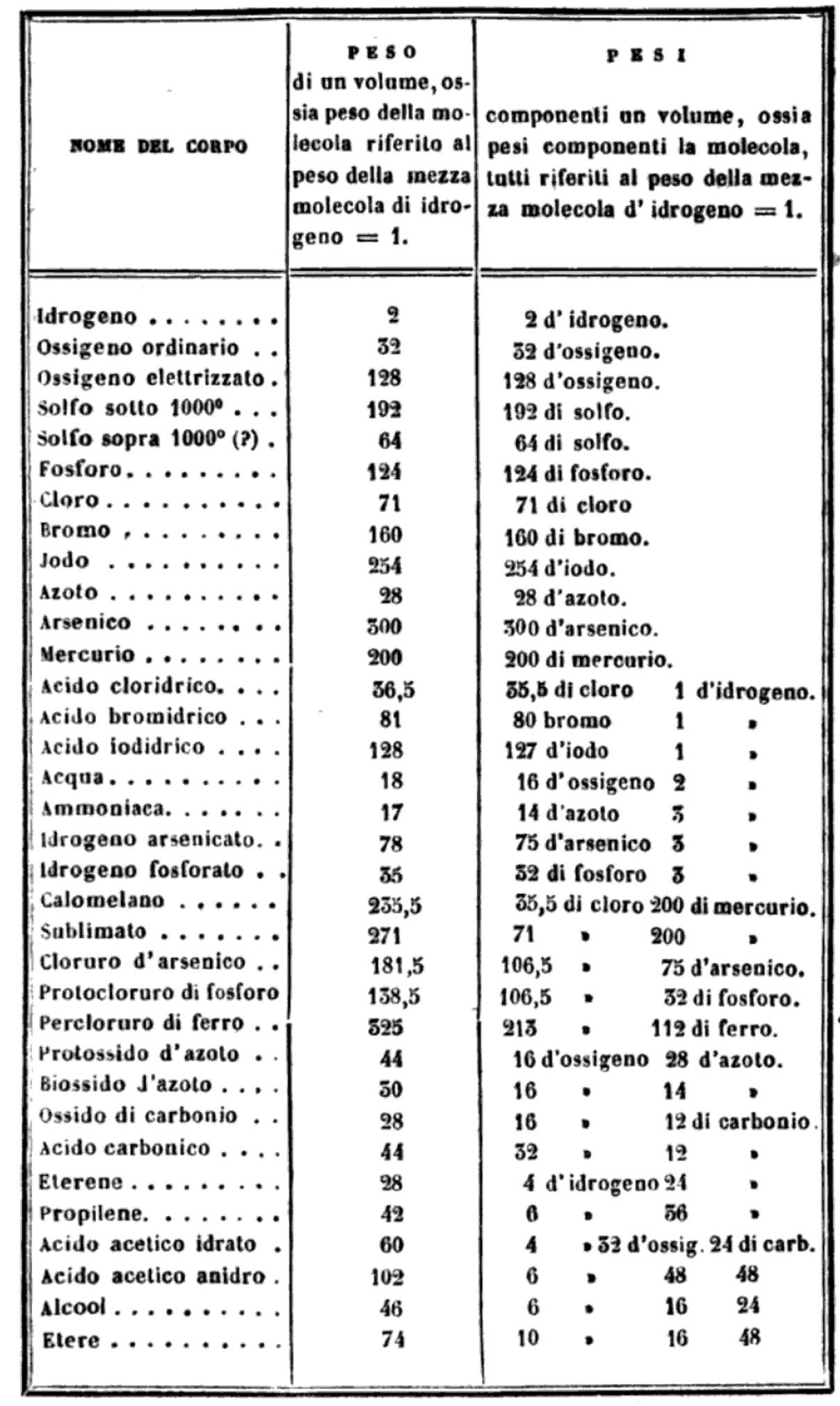
"I believe that the progress of science made in these last years has confirmed the hypothesis of Avogadro, of Ampère, and of Dumas on the similar constitution of substances in the gaseous state; that is, that equal volumes of these substances, whether simple or compound, contain an equal number of molecules: not however an equal number of atoms, since the molecules of the different substances, or those of the same substance in its different states, may contain a different number of atoms, whether of the same or of diverse nature."
From the Science History of Science Institute:
"In 1858 Cannizzaro outlined a course in theoretical chemistry for students at the University of Genoa,where he had to teach without benefit of a laboratory. He used the hypothesis of a fellow Italian, Amedeo Avogadro, who had died just two years earlier, as a pathway out of the confusion rampant among chemists about atomic weights and the fundamental structure of chemical compounds."
Mark Leach writes:
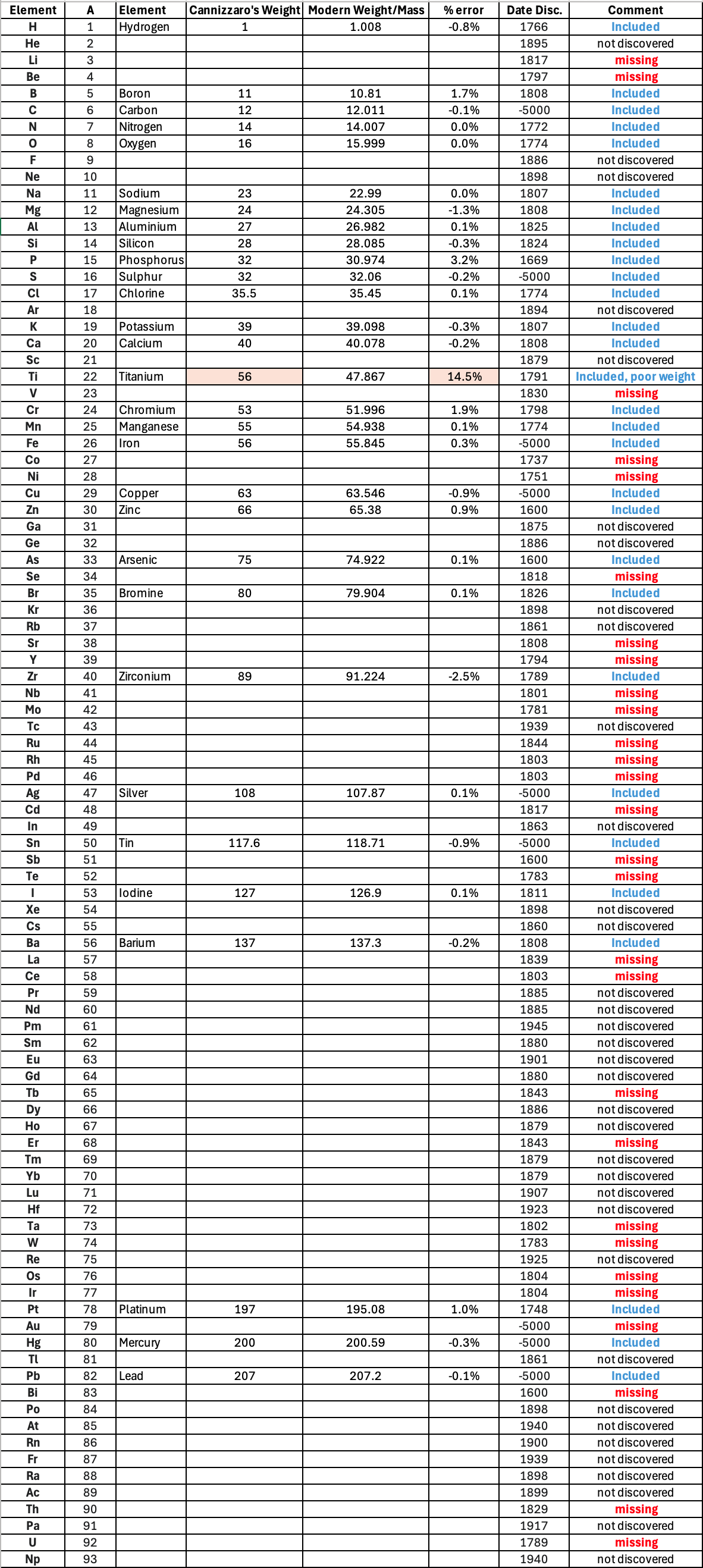
"Before a periodic table of the chemical elements – which orders the elements by atomic weight and then groups them by property – could be developed it was necessary to know the atomic weight values. However, to deduce the atomic weights was a problem as it was necessary to know the ratios of how the elements combined, the stoichiometry.
"Tables of atomic weight data by Dalton (1808), Wollaston (1813), Daubeny (1831) and Kopp & Will (1858) show progress, but the 1858 Cannizzaro letter was the first where the atomic weight data is more or less both complete and accurate, thus removing stiochiometric errors.
"I have extracted the element atomic weight data from the paper, and given the % error with respect to modern atomic weight/mass data. Only titanium is significantly out! It is clear that Cannizzaron knew that hydrogen, nitrogen, oxygen, chlorine, bromine & iodine existed as diatomic molecules."
| Element | Symbol | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H | 1 | 1.008 | -0.8% |
| Boron | B | 11 | 10.81 | 1.7% |
| Carbon | C | 12 | 12.011 | -0.1% |
| Nitrogen | N | 14 | 14.007 | 0.0% |
| Oxygen | O | 16 | 15.999 | 0.0% |
| Sodium | Na | 23 | 22.99 | 0.0% |
| Magnesium | Mg | 24 | 24.305 | -1.3% |
| Aluminium | Al | 27 | 26.982 | 0.1% |
| Silicon | Si | 28 | 28.085 | -0.3% |
| Sulphur | S | 32 | 32.06 | -0.2% |
| Phosphorus | P | 32 | 30.974 | 3.2% |
| Chlorine | Cl | 35.5 | 35.45 | 0.1% |
| Potassium | K | 39 | 39.098 | -0.3% |
| Calcium | Ca | 40 | 40.078 | -0.2% |
| Chromium | Cr | 53 | 51.996 | 1.9% |
| Manganese | Mn | 55 | 54.938 | 0.1% |
| Iron | Fe | 56 | 55.845 | 0.3% |
| Titanium | Ti | 56 | 47.867 | 14.5% |
| Copper | Cu | 63 | 63.546 | -0.9% |
| Zinc | Zn | 66 | 65.38 | 0.9% |
| Arsenic | As | 75 | 74.922 | 0.1% |
| Bromine | Br | 80 | 79.904 | 0.1% |
| Zirconium | Zr | 89 | 91.224 | -2.5% |
| Silver | Ag | 108 | 107.87 | 0.1% |
| Tin | Sn | 117.6 | 118.71 | -0.9% |
| Iodine | I | 127 | 126.9 | 0.1% |
| Barium | Ba | 137 | 137.3 | -0.2% |
| Platinum | Pt | 197 | 195.08 | 1.0% |
| Mercury | Hg | 200 | 200.59 | -0.3% |
| Lead | Pb | 207 | 207.2 | -0.1% |
| Diatomic Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H2 | 2 | 2.016 | -0.8% |
| Oxygen | O2 | 32 | 31.998 | 0.0% |
| Sulphur | S2 | 64 | 64.12 | -0.2% |
| Chlorine | Cl2 | 71 | 70.9 | 0.1% |
| Bromine | Br2 | 160 | 159.808 | 0.1% |
| Iodine | I2 | 254 | 253.8 | 0.1% |
| Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Water | H2O | 18 | 18.015 | -0.1% |
| Hydrochloric Acid | HCl | 36.5 | 36.458 | 0.1% |
| Methane | CH4 | 16 | 16.043 | -0.3% |
| Hydrogen sulphide | H2S | 34 | 34.076 | -0.2% |
| Diethyl ether | CH3CH2OCH2CH3 | 74 | 74.123 | -0.2% |
| Carbon disulphide | CS2 | 76 | 76.131 | -0.2% |
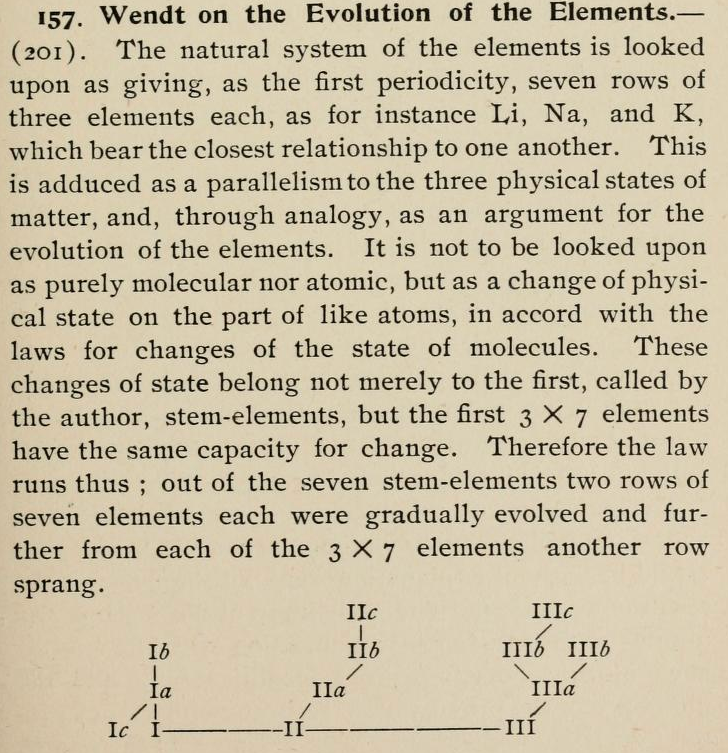
| Chloroethane | CH3CH2Cl | 64.5 | 64.512 | 0.0% |
Below is a list of the elements showing which ones were included by Cannizzaro and which one were ommitted (because they had not been discovered) or are strangely missing. Odd ommissions (to the modern eye) include: Lithium, Beryllium, Cobalt, Nickel, Palladium, Tungsten and Gold.
| Year: 1858 | PT id = 1348, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1858
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1858 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6
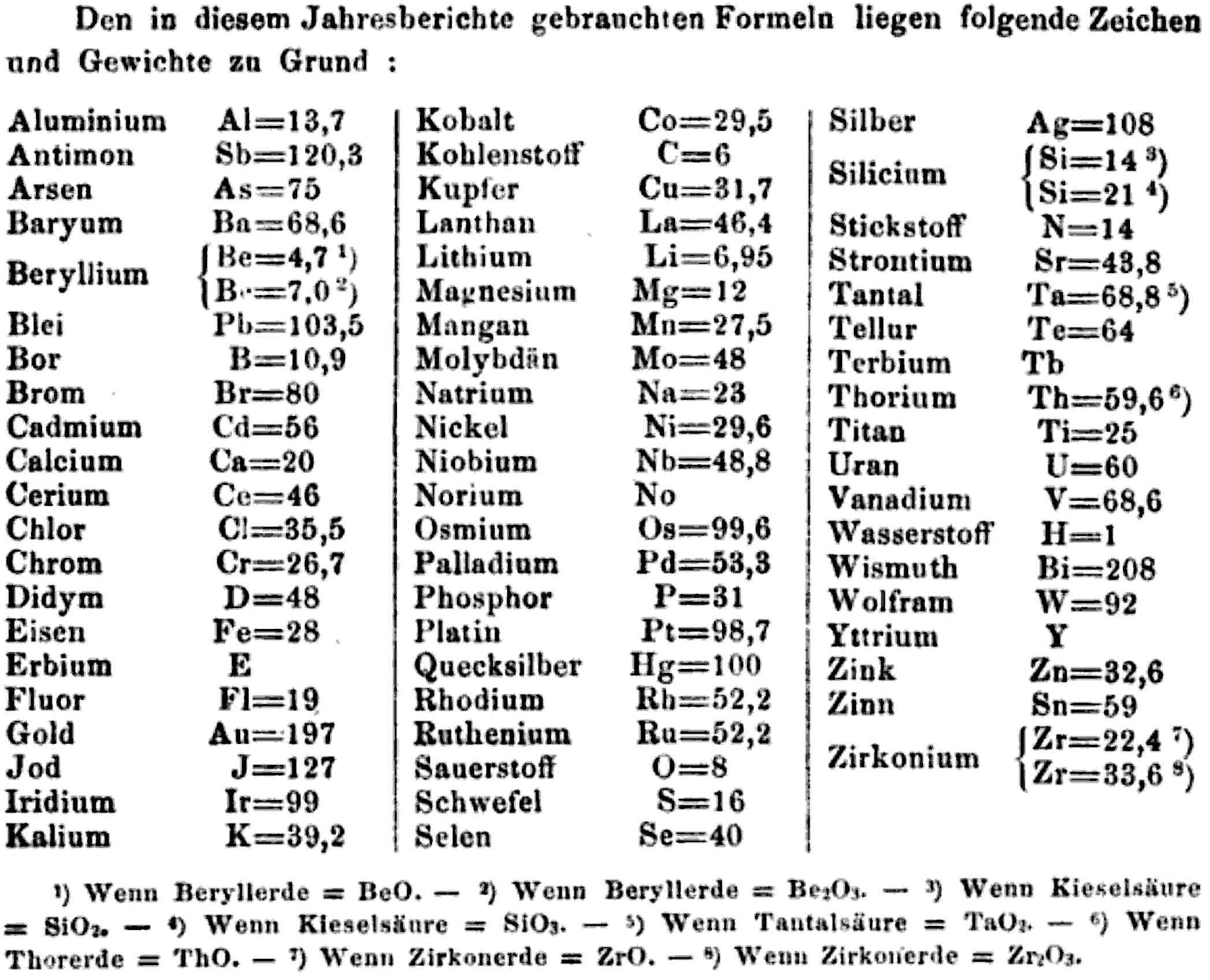
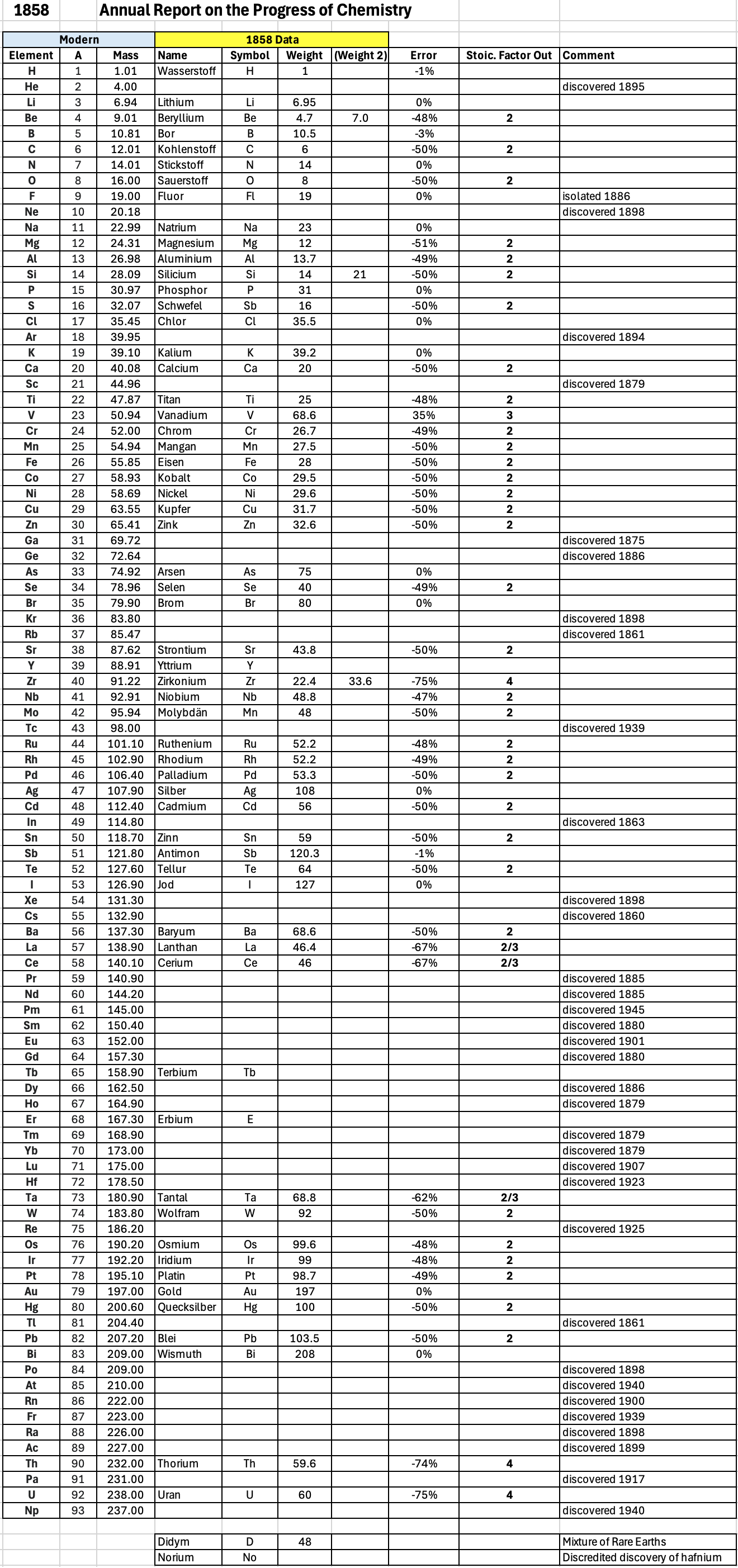
Thanks to René and Mario Rodriguez for the tip!
| Year: 1859 | PT id = 1349, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1859
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1859 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6

Thanks to René and Mario Rodriguez for the tip!
| Year: 1860 | PT id = 1350, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1860
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1860 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si =14 and 21
- Zr = 22.4, 33.6 and 44.8
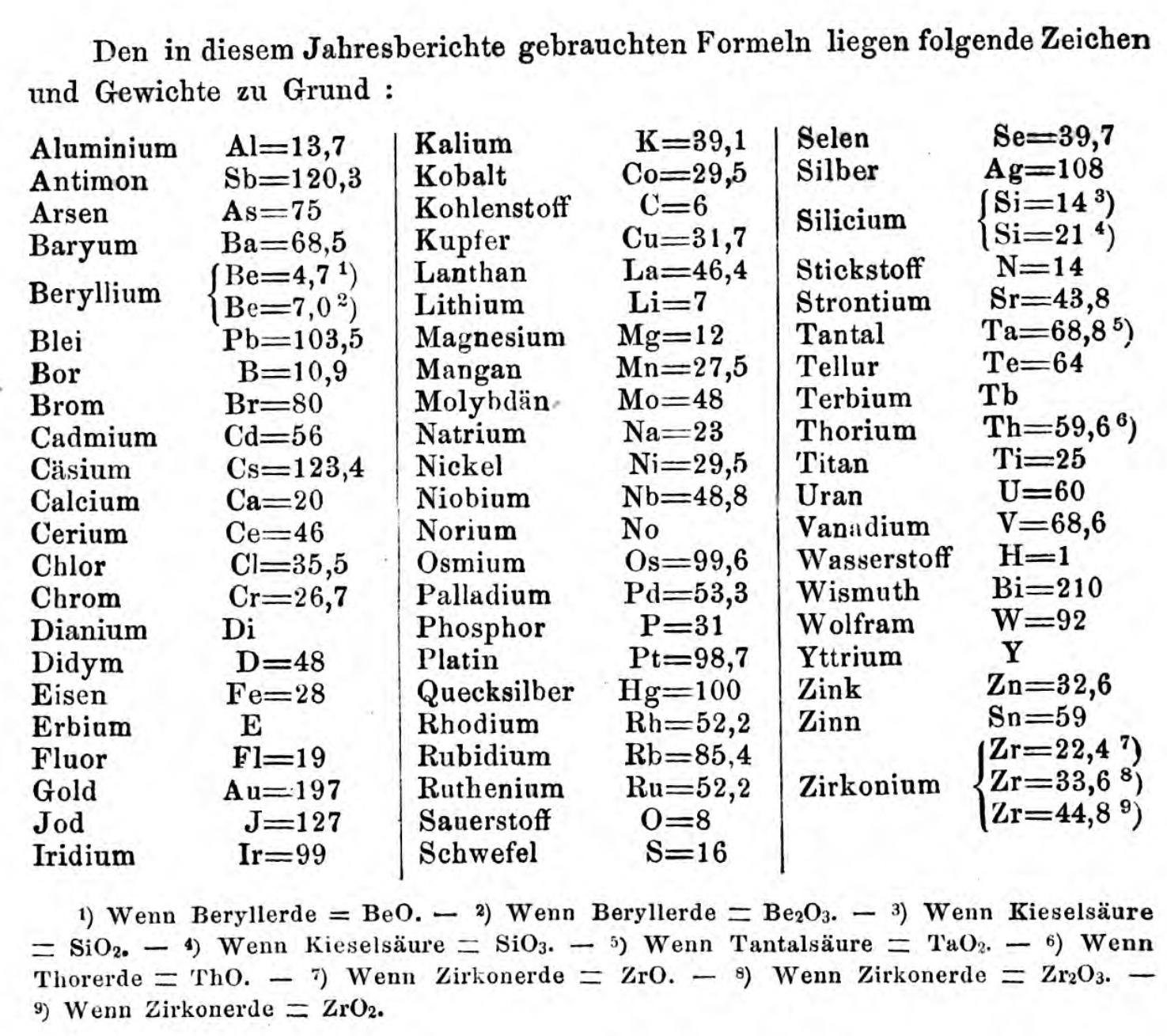
Thanks to René and Mario Rodriguez for the tip!
| Year: 1861 | PT id = 1351, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1861
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1861 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8
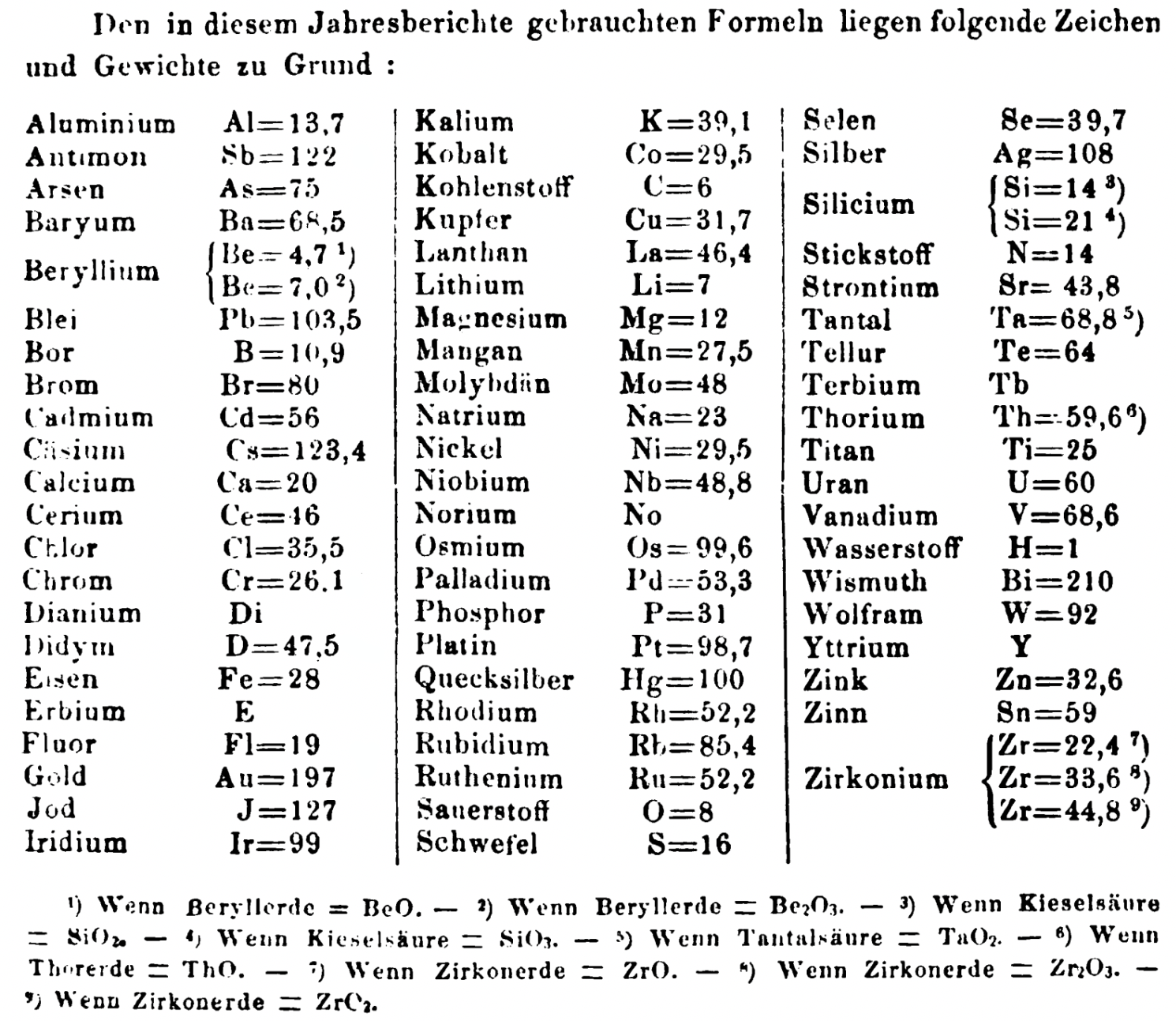
Thanks to René and Mario Rodriguez for the tip!
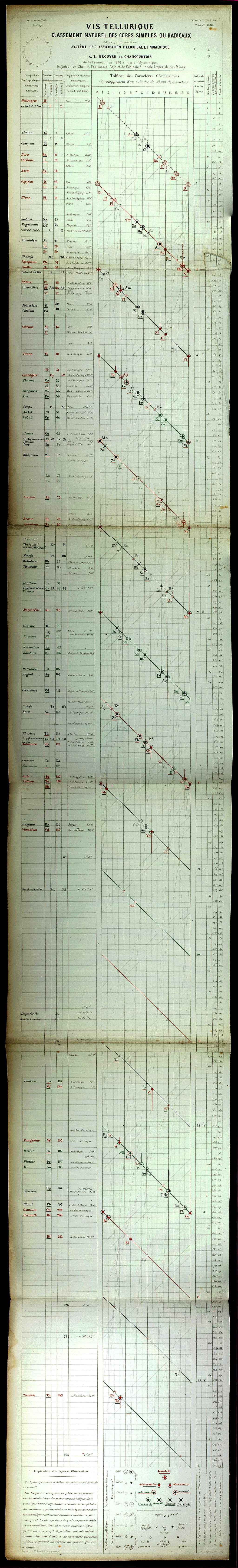
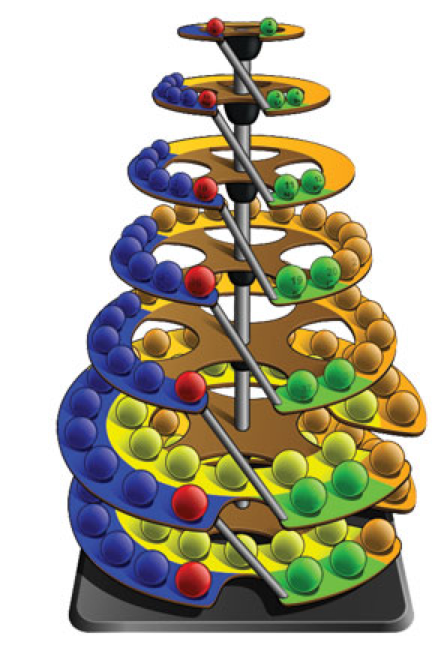
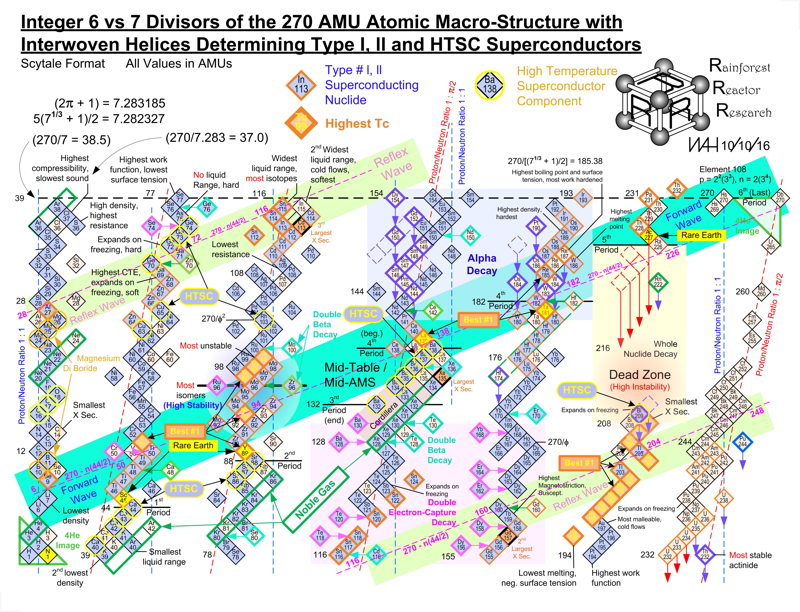
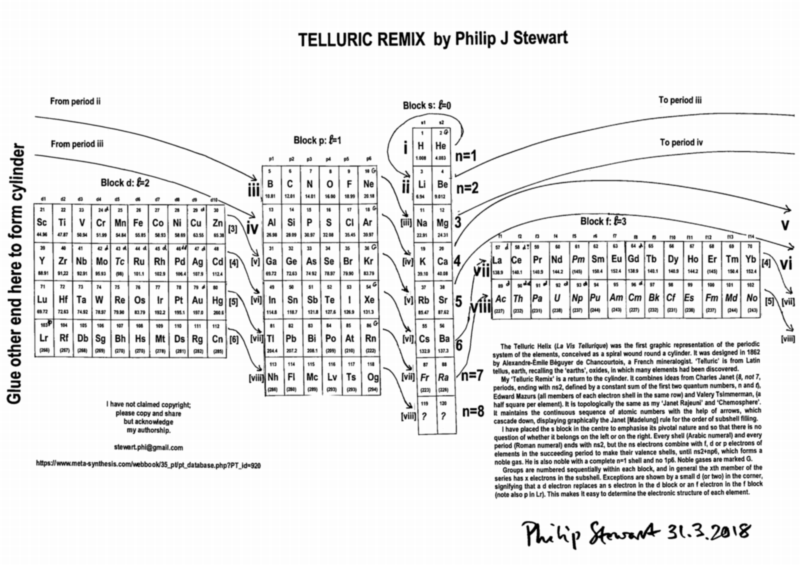
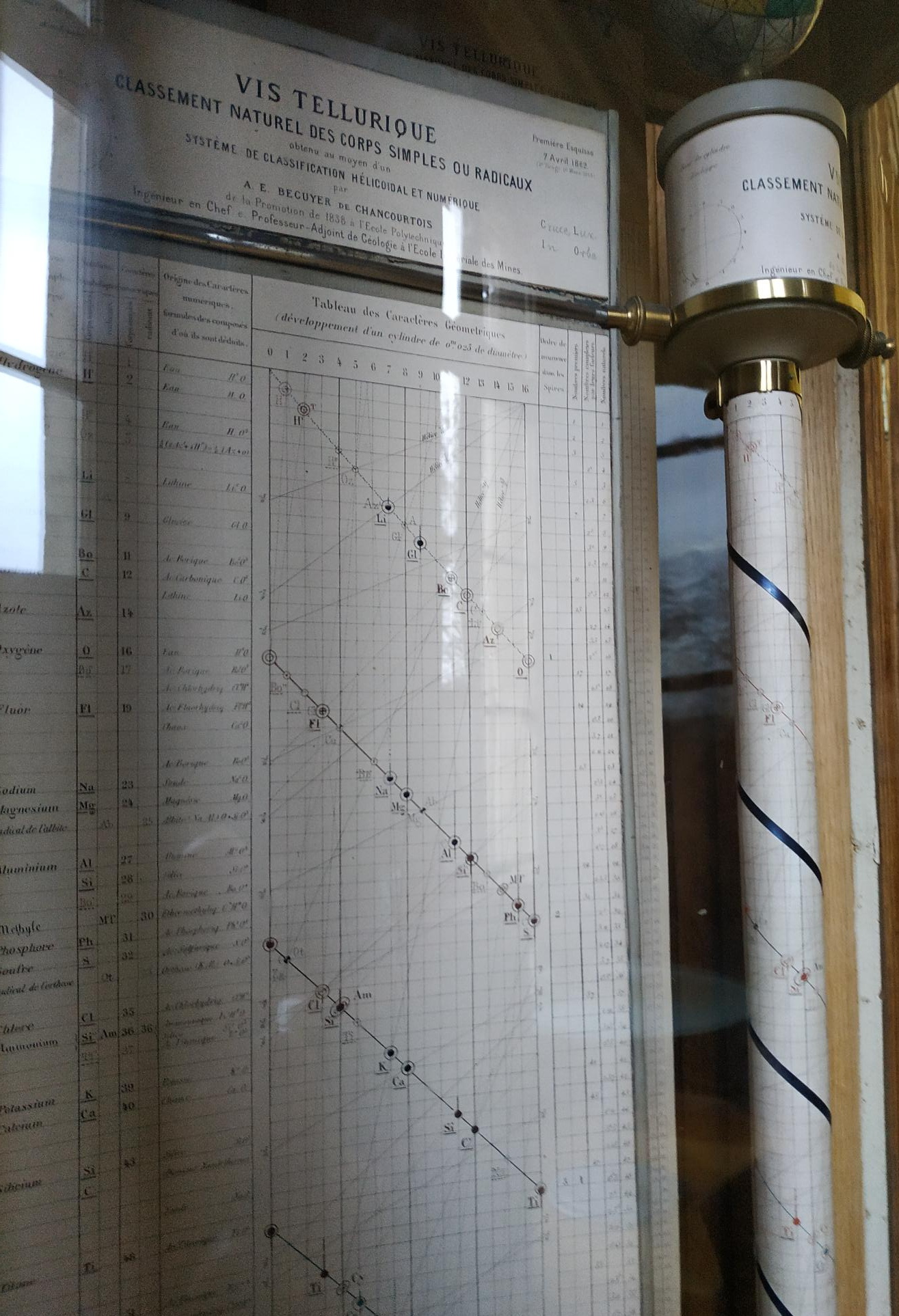
| Year: 1862 | PT id = 7, Type = formulation spiral 3D |
Béguyer de Chancourtois' Vis Tellurique
The French geologist , Alexandre-Émile Béguyer de Chancourtois was the first person to make use of atomic weights to produce a classification of periodicity. He drew the elements as a continuous spiral around a metal cylinder divided into 16 parts. The atomic weight of oxygen was taken as 16 and was used as the standard against which all the other elements were compared. Tellurium was situated at the centre, prompting vis tellurique, or telluric screw.
Many thanks to Peter Wothers – and courtesy of the Master and Fellows of St Catharine's College, Cambridge – comes a high quality image of the original 1862 formulation. Click here, or on the image to enlarge:
Watch Peter Wothers 'unravel' and show Prof. Martyn Poliakoff this first periodic table at 17min 50sec into the YouTube video below:
Some more information:
Chancourtois' original formulation includes elements in their correct places, selected compounds and some elements in more than one place. The helix was an important advance in that it introduced the concept of periodicity, but it was flawed.
It has been suggested that Chancourtois called his formulation a telluric helix because tellurium is found in the middle. However, most elements are found as there their 'earths' – tellus, telluris – or oxides, which for a mineralogist would have been highly significant.
The formulation was rediscovered in the 1889 (P. J. Hartog, "A First Foreshadowing of the Periodic Law" Nature 41, 186-8 (1889)), and since then it has appeared most often in a simplified form that emphasizes the virtues and eliminates its flaws. [Thanks to CG for this info.]
See also:
- Dutch Wikipedia
- ScienceWorld
- Science & Society Picture Library
- Roy Alexander's All Periodic Tables site
A three dimensional models of the telluric helix:
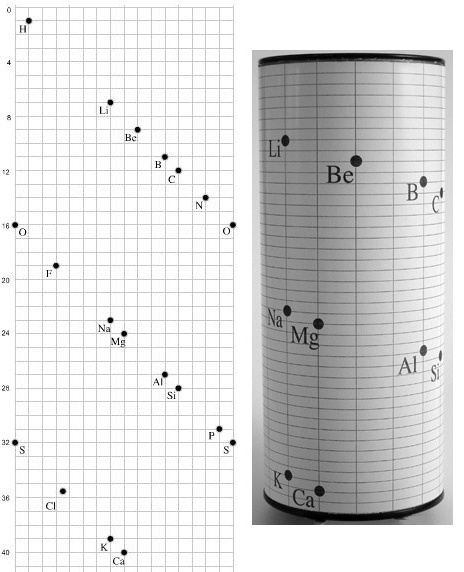
There are representations of the 1862 formulation at the School of Mines at ParisTech:

| Year: 1862 | PT id = 1352, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1862
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1862 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8

Thanks to René and Mario Rodriguez for the tip!
| Year: 1863 | PT id = 1353, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1863
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1863 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- C = 6 and 12
- Hg = 100 and 200
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Zr = 22.4 and 33.6 and 44.8
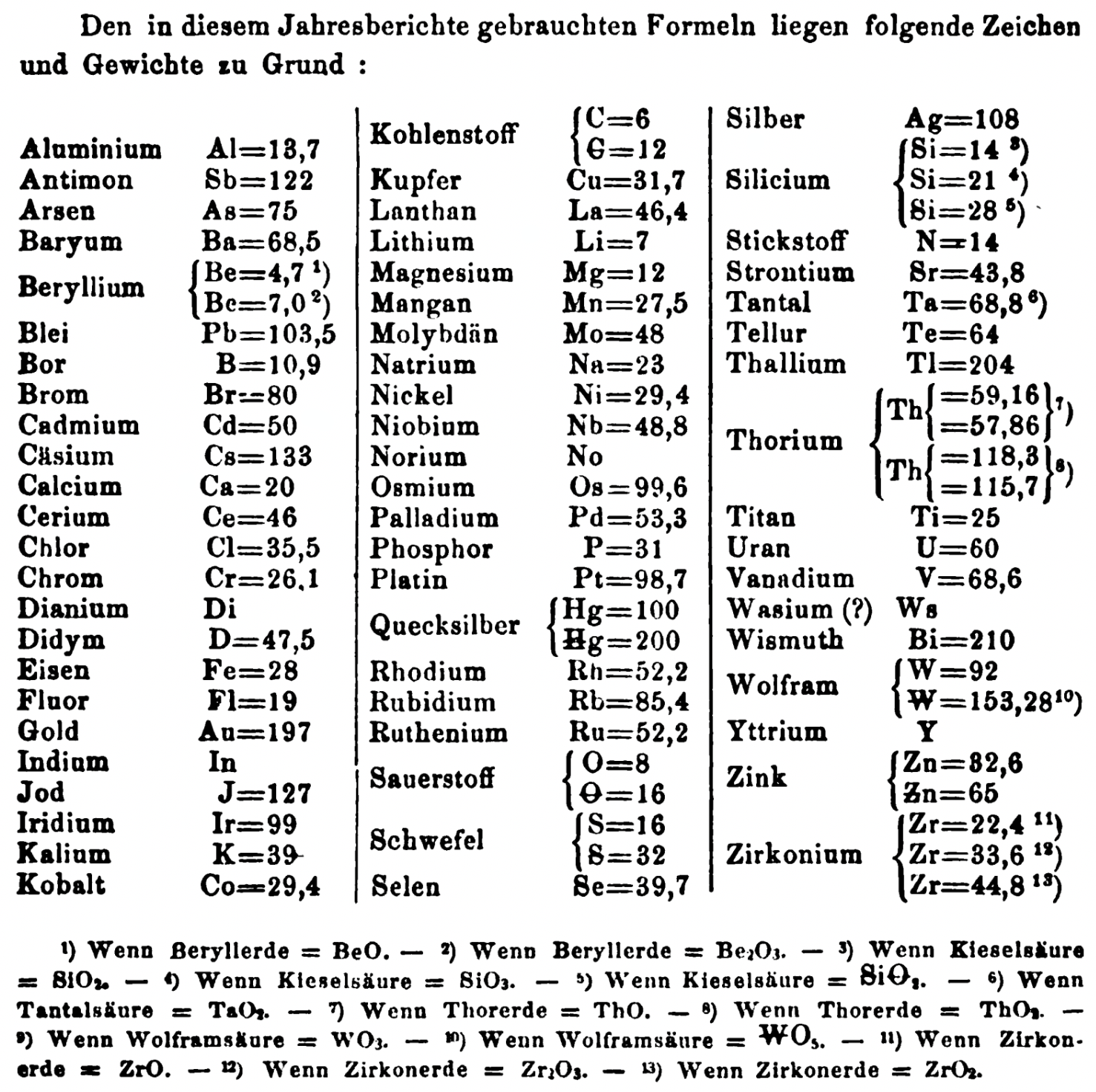
Thanks to René and Mario Rodriguez for the tip!
| Year: 1864 | PT id = 8, Type = formulation |
Newlands' Octaves
One of the first attempts at a periodic table that arranged the known elements by atomic weight and chemical property, was by John Newlands and is known as "Newlands Octaves".
Newland noticed that if he broke up his list of elements into groups of seven – starting a new row with the eighth element – the first element in each of those groups had similar chemistry.
Note: In the tables below, Newlands Octaves go downwards: H to O, F to S, Cl to Fe, etc.
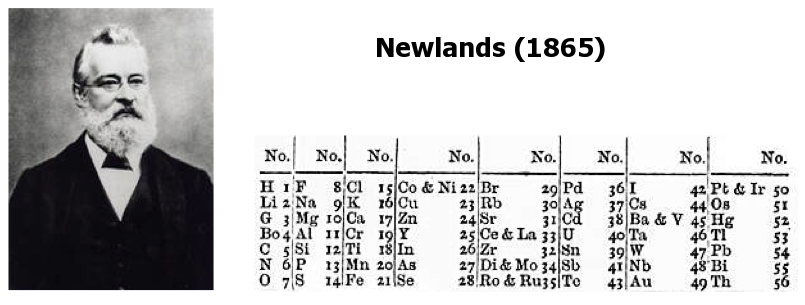
|
H 1
|
F 8
|
Cl 15
|
Co & Ni 22
|
Br 29
|
Pd 36
|
I 42
|
Pt & Ir 50
|
|
Li 2
|
Na 9
|
K 16
|
Cu 23
|
Rb 30
|
Ag 37
|
Cs 44
|
Os 51
|
|
G 3
|
Mg 10
|
Ca 17
|
Zn 24
|
Sr 31
|
Cd 38
|
Ba & V 45
|
Hg 52
|
|
Bo 4
|
Al 11
|
Cr 19
|
Y 25
|
Ce & La 33
|
U 40
|
Ta 46
|
Tl 53
|
|
C 5
|
Si 12
|
Ti 18
|
In 26
|
Zr 32
|
Sn 39
|
W 47
|
Pb 54
|
|
N 6
|
P 13
|
Mn 20
|
As 27
|
Di & Mo 34
|
Sb 41
|
Nb 48
|
Bi 55
|
|
O 7
|
S 14
|
Fe 21
|
Se 28
|
Ro & Ru 35
|
Te 43
|
Au 49
|
Th 56
|
- Seeing the word octave applied to this table may lead one to think that Newlands recognised periods of eight elements with repeating properties, as we do with the modern periodic table, for example: Li Be B C N O F Ne.
- However, each sequence of Newlands' octaves contain only seven elements. Count the elements in the columns! In Newlands' day the group 8 (18) rare gas elements, He, Ne, Ar, Kr & Xe, had not yet been discovered.
- To Newlands, H to F & F to Cl are octaves of eight elements, the eighth element repeating the properties of the first.
There are seven notes in a musical octave: A B C D E F G, after which you start again with A'; similarly for Newlands, seven elements H Li G Bo C N O, then the 8th is F and you start again. [Note that Newlands treated H as a halogen.] More here.
A B C D E F G A
Philip Stewart's musical representation:
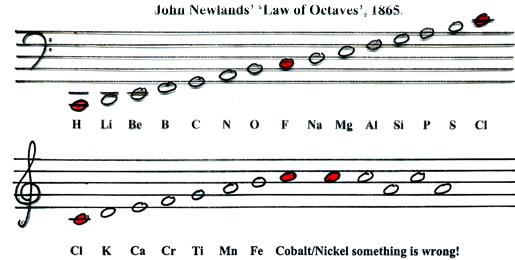
- To Newlands, H to F is an octave of eight elements.
- Today we say Li to Ne & Na to Ar are periods of eight elements, and that that Li and Na are in different periods. Indeed, the Li to Na series consists of nine elements.
- In Newlands' day the group 8 (18) rare gas elements, He, Ne, Ar, Kr & Xe, had not been discovered.
Read more about Newland's Octaves, including a commentary on the original papers in Carmen Giunta's Elements and Atoms: Case Studies in the Development of Chemistry.
| Year: 1864 | PT id = 1354, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1864
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1864 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8
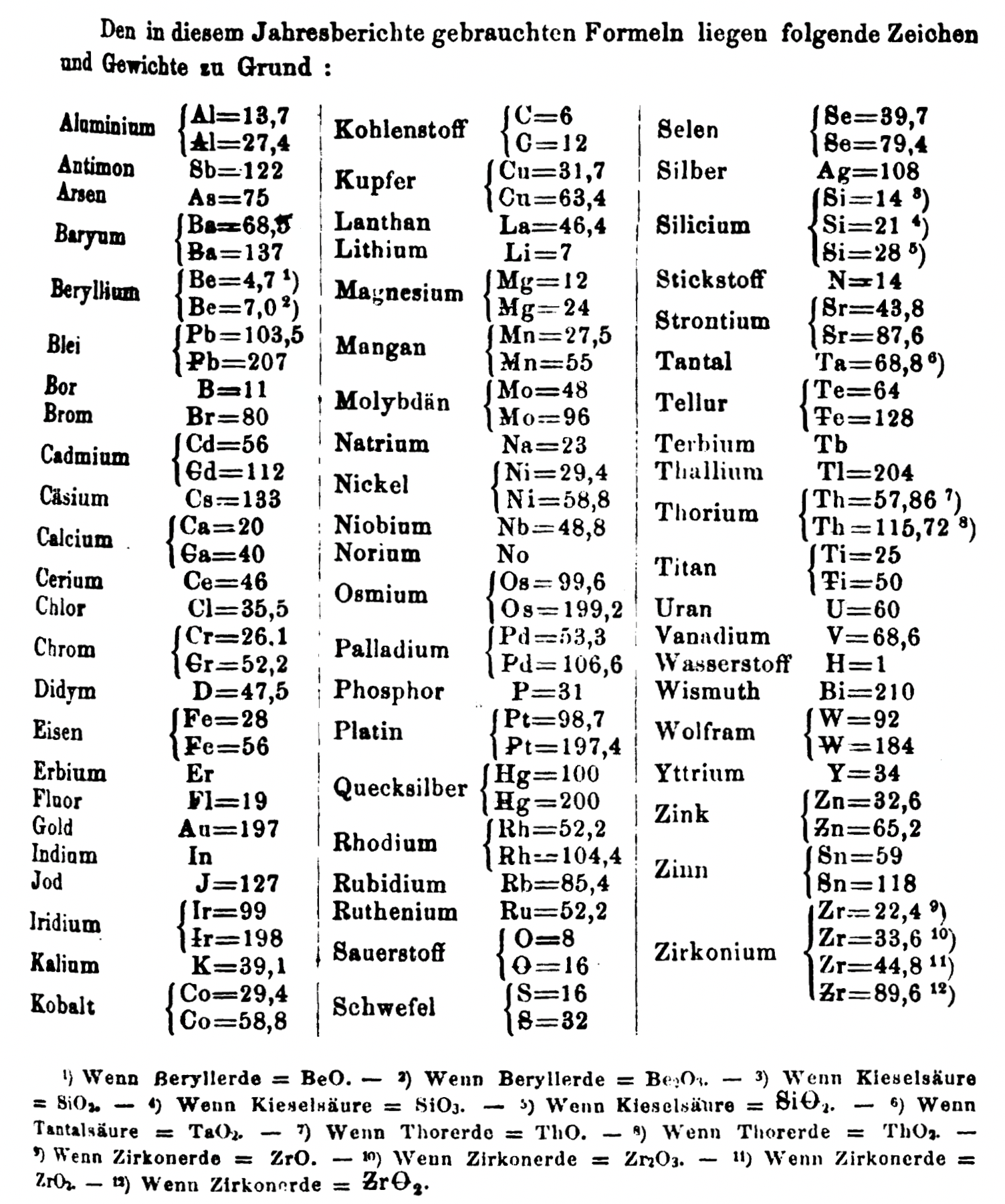
Thanks to René and Mario Rodriguez for the tip!
| Year: 1865 | PT id = 1355, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1865
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1865 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
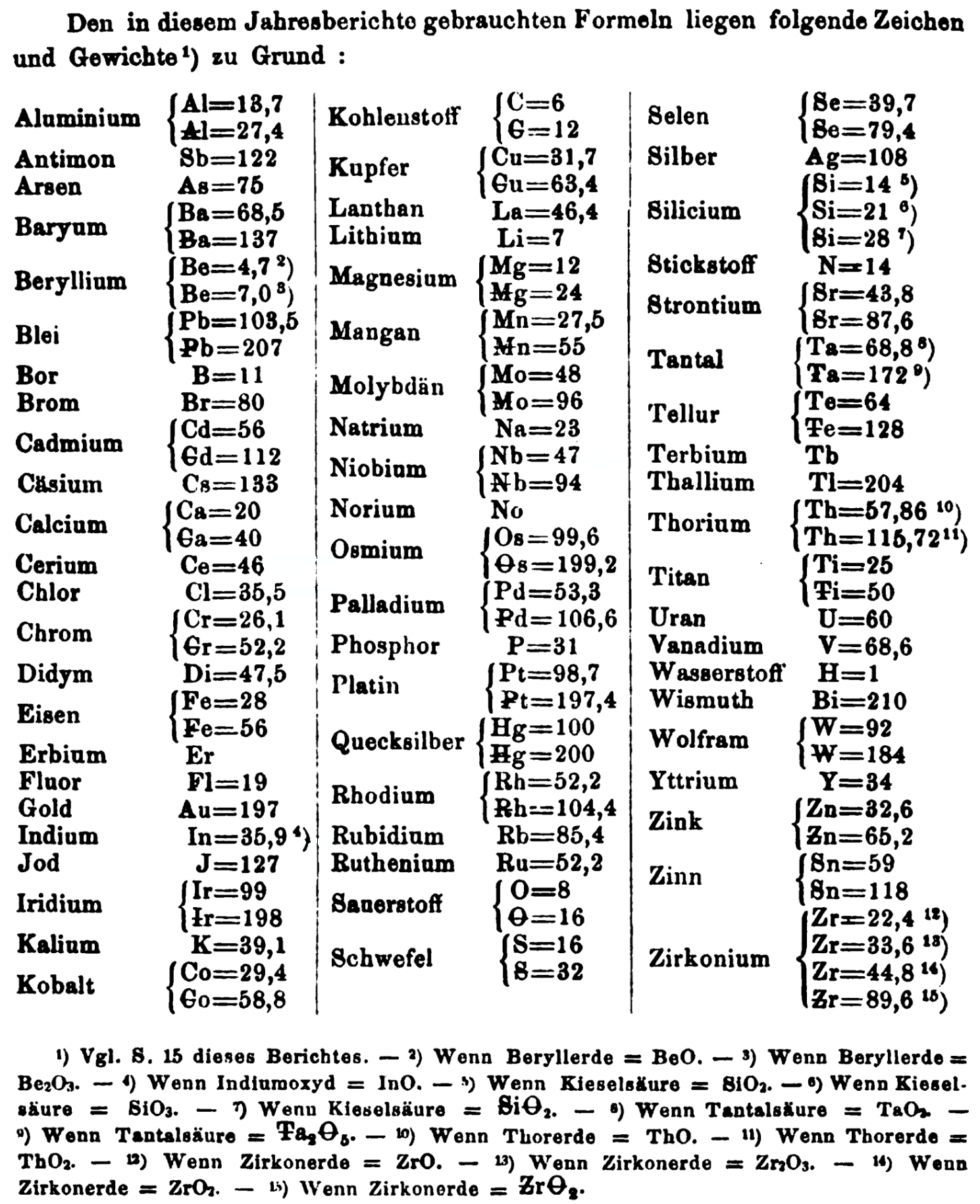
Thanks to René and Mario Rodriguez for the tip!
| Year: 1866 | PT id = 1356, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1866
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1866 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th = 57.86 and 115.72
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
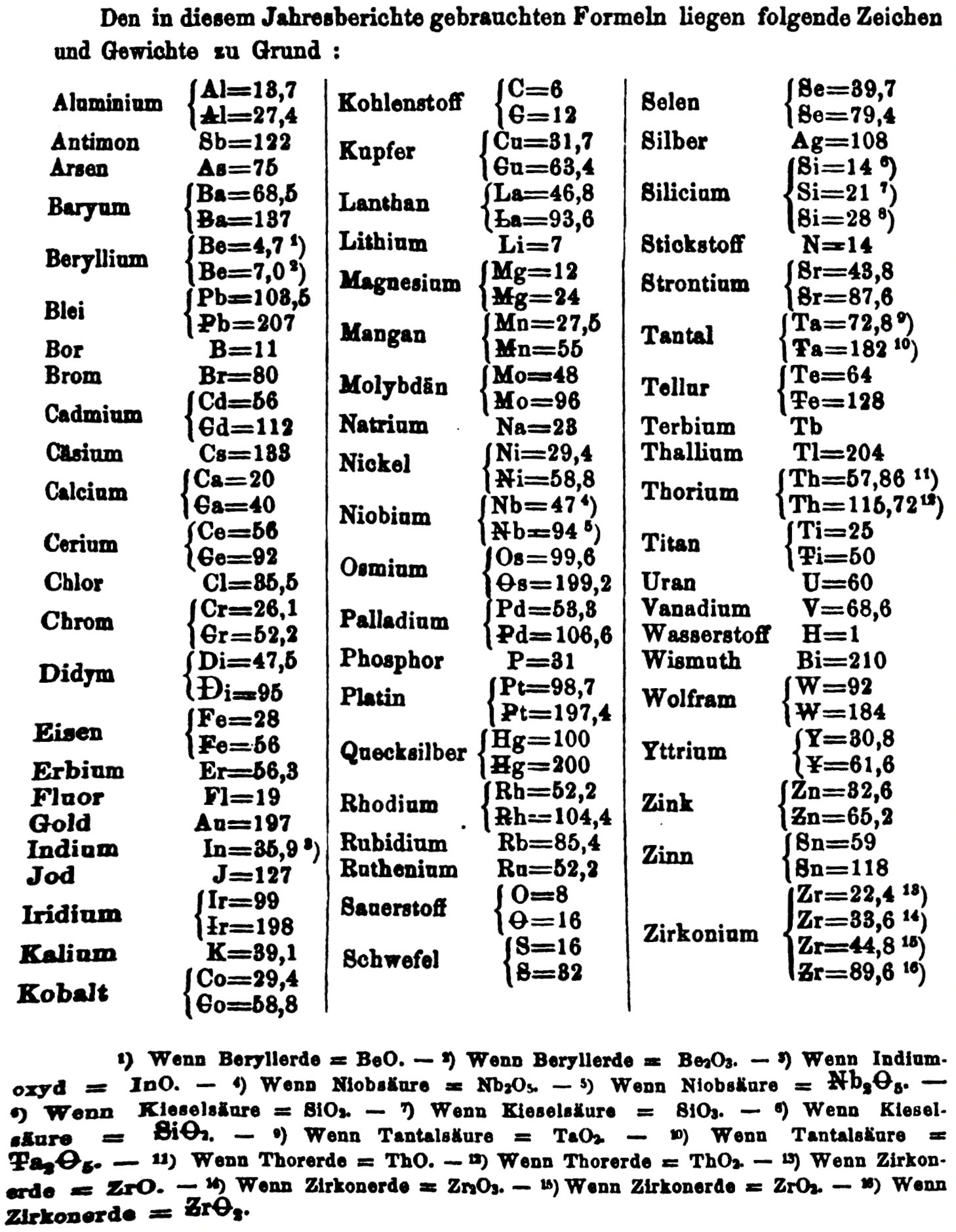
Thanks to René and Mario Rodriguez for the tip!
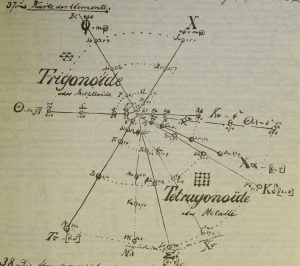
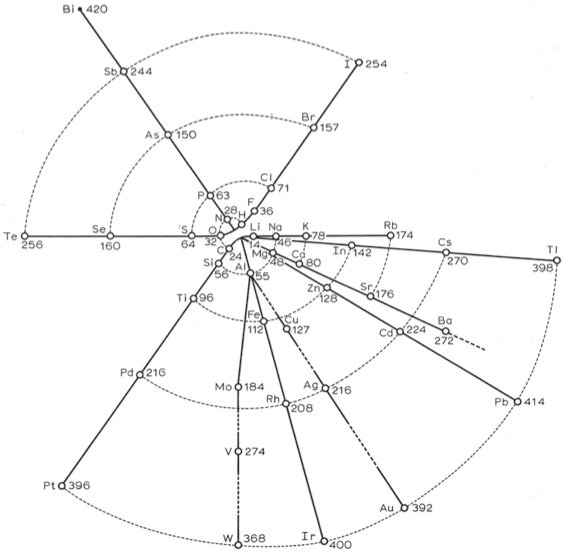
| Year: 1867 | PT id = 270, Type = formulation spiral |
Hinrichs' Programme of Atomechanics
Gustavus Detlef Hinrichs' spiral "Programme of Atomechanics". Programm der Atomechanik oder die Chemie eine Mechanik de Pantome, Augustus Hageboek, Iowa City, IA (1867).
Hinrichs' system is based on the relationship of what he called: "pantogens, with its atoms called panatoms, which explains the numerical relations of atomic weights and gives a simple classification of the elements."
This classification system culminated in 1867 in his spiral periodic table, which better clarified the groupings of elements. Hinrichs' classification, while distinctly different from the other periodic tables of this period, "seems to capture many of the primary periodicity relationships seen in the modern periodic table... it is not cluttered by attempts to show secondary kinship relationships." (Scerri)
| Year: 1867 | PT id = 1357, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1867
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1867 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th =57.86 and 115.72
- W = 92 and 184
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
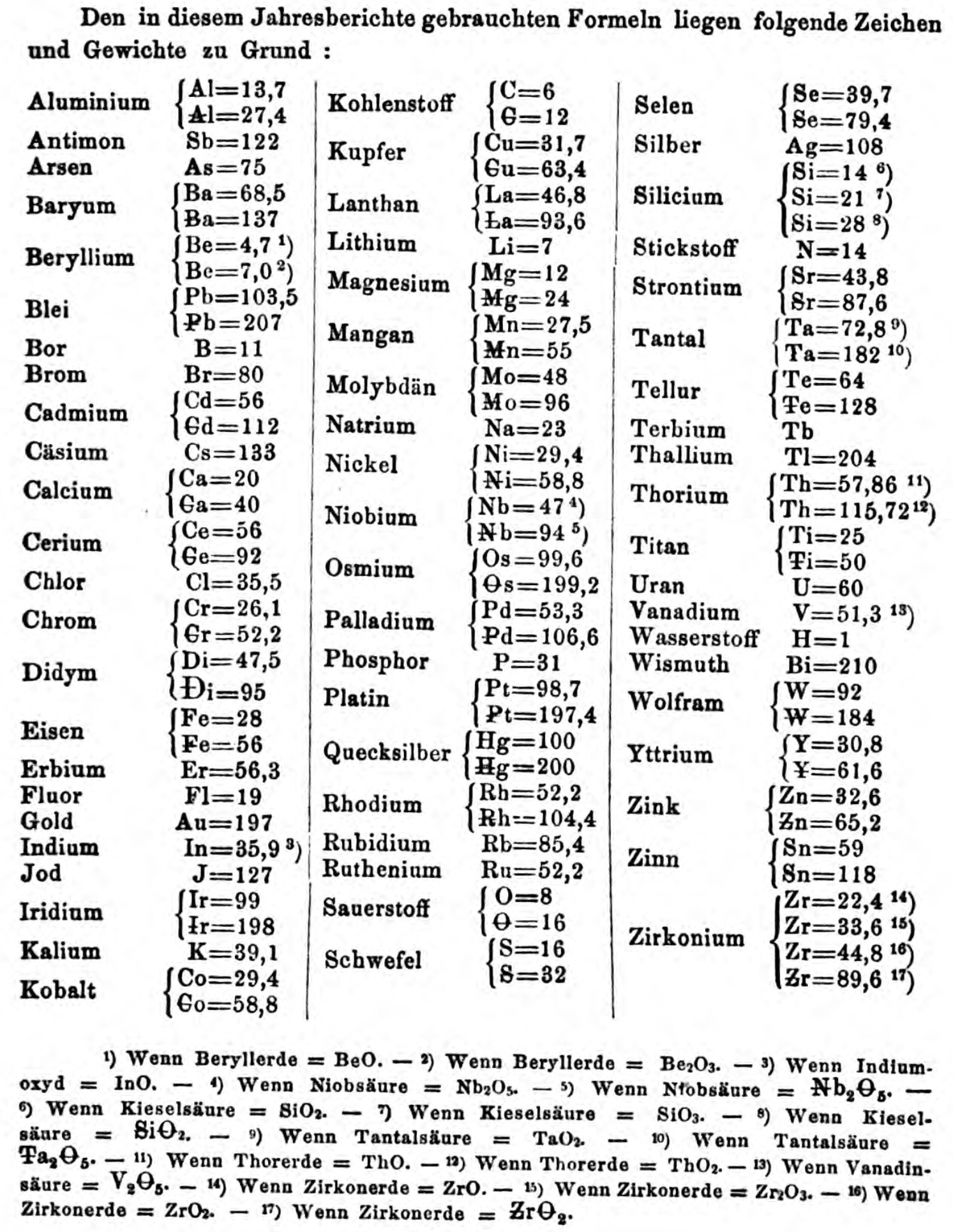
Thanks to René and Mario Rodriguez for the tip!
| Year: 1868 | PT id = 1358, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1868
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1868 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53.3 and 106.6
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 44.8 and 89.6
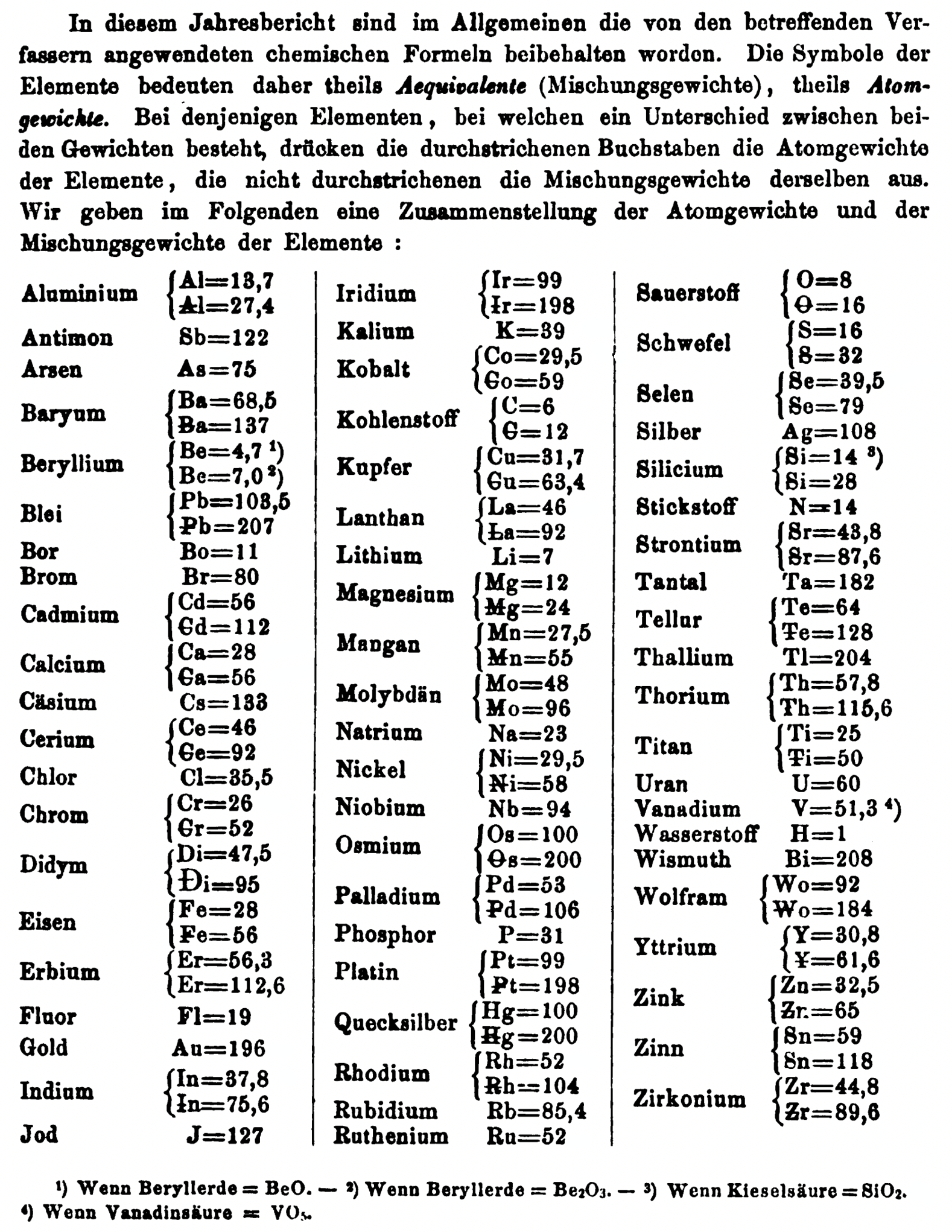
Thanks to René and Mario Rodriguez for the tip!
| Year: 1869 | PT id = 1359, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1869
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1869 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 44.8 and 89.6
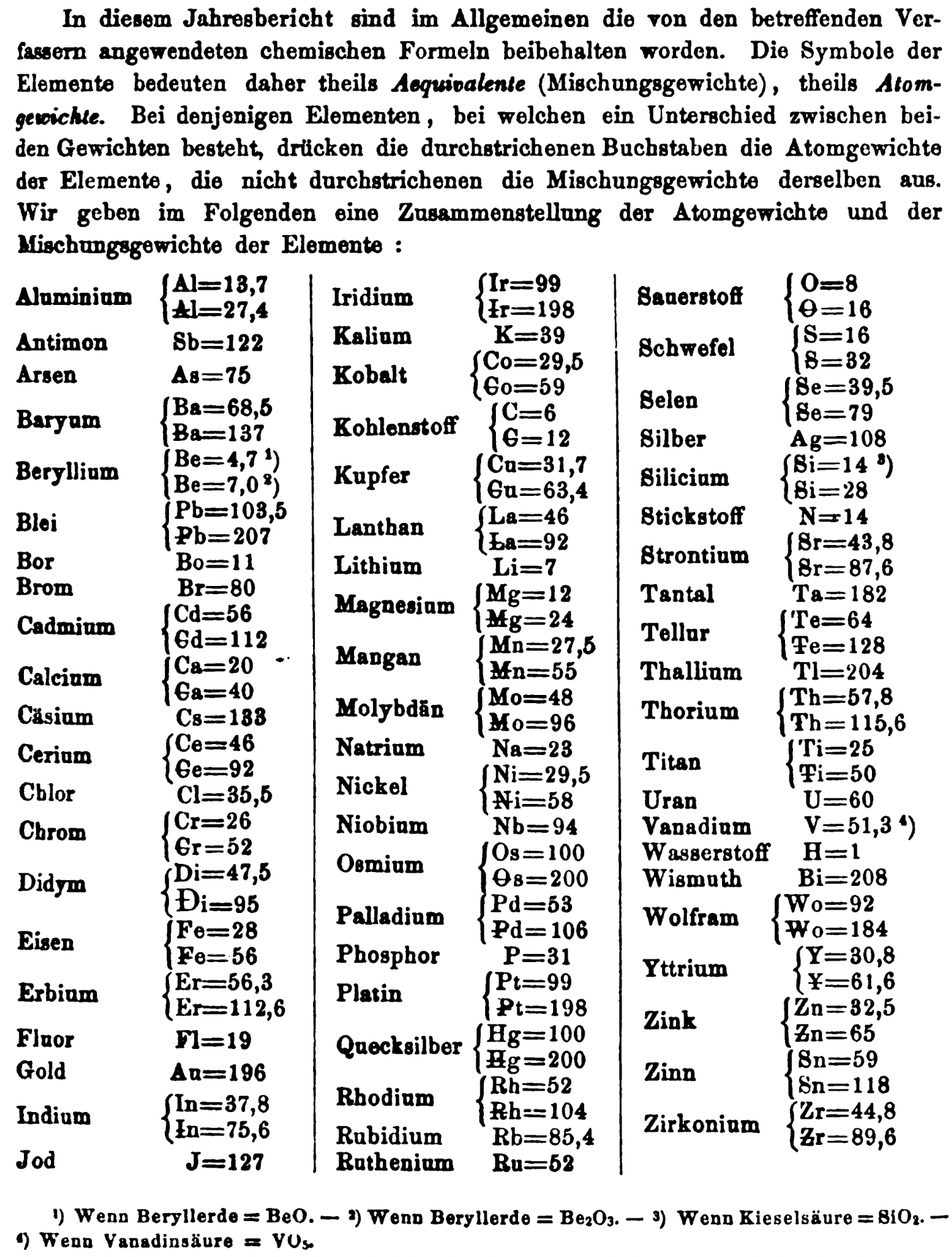
Thanks to René and Mario Rodriguez for the tip!
| Year: 1870 | PT id = 1360, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1870
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1870 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
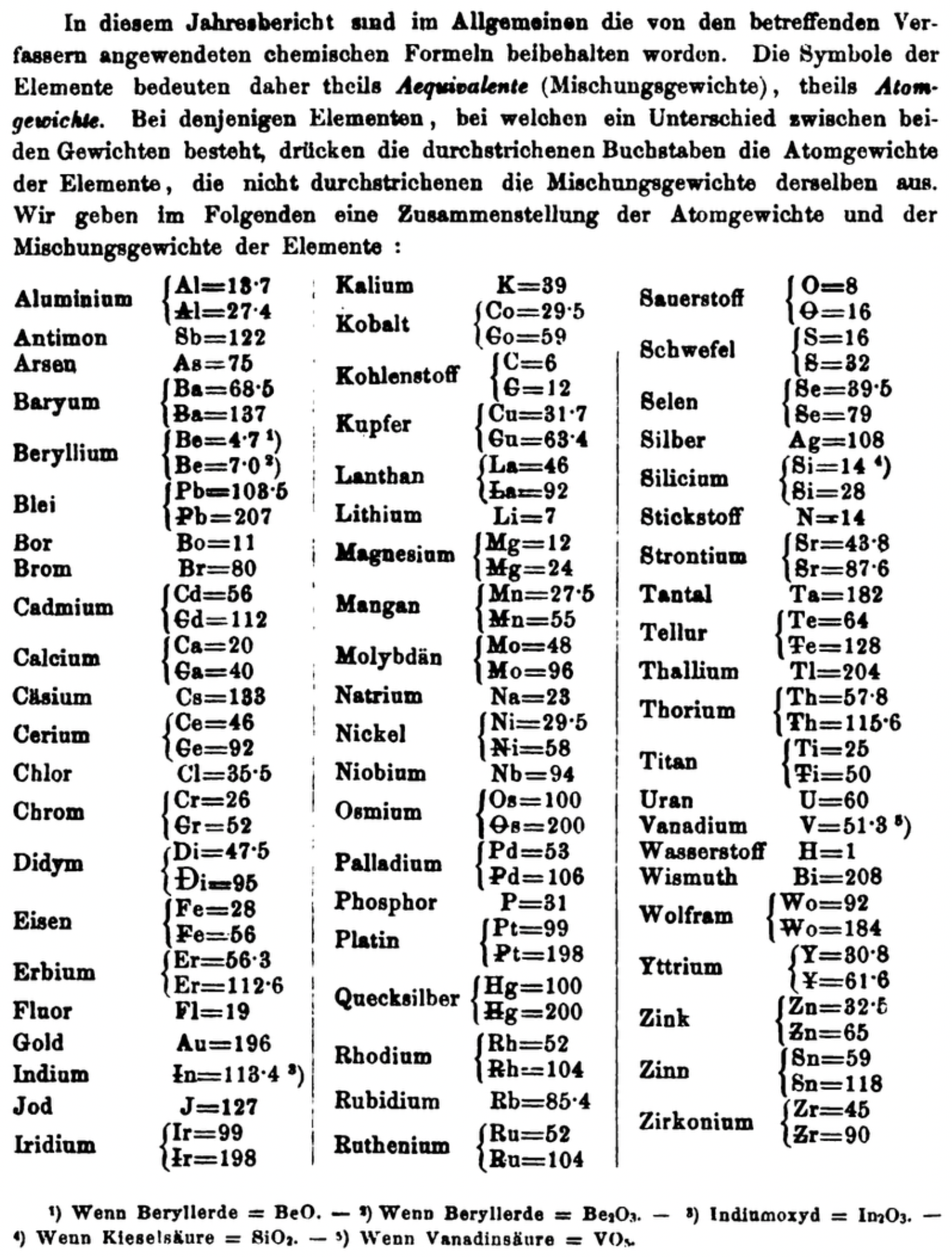
Thanks to René and Mario Rodriguez for the tip!
| Year: 1871 | PT id = 1361, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1871
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1871 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
Thanks to René and Mario Rodriguez for the tip!
| Year: 1872 | PT id = 1362, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1872
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1872 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.5 and 59
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.8 and 115.6
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 29.8 and 59.7
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
Thanks to René and Mario Rodriguez for the tip!
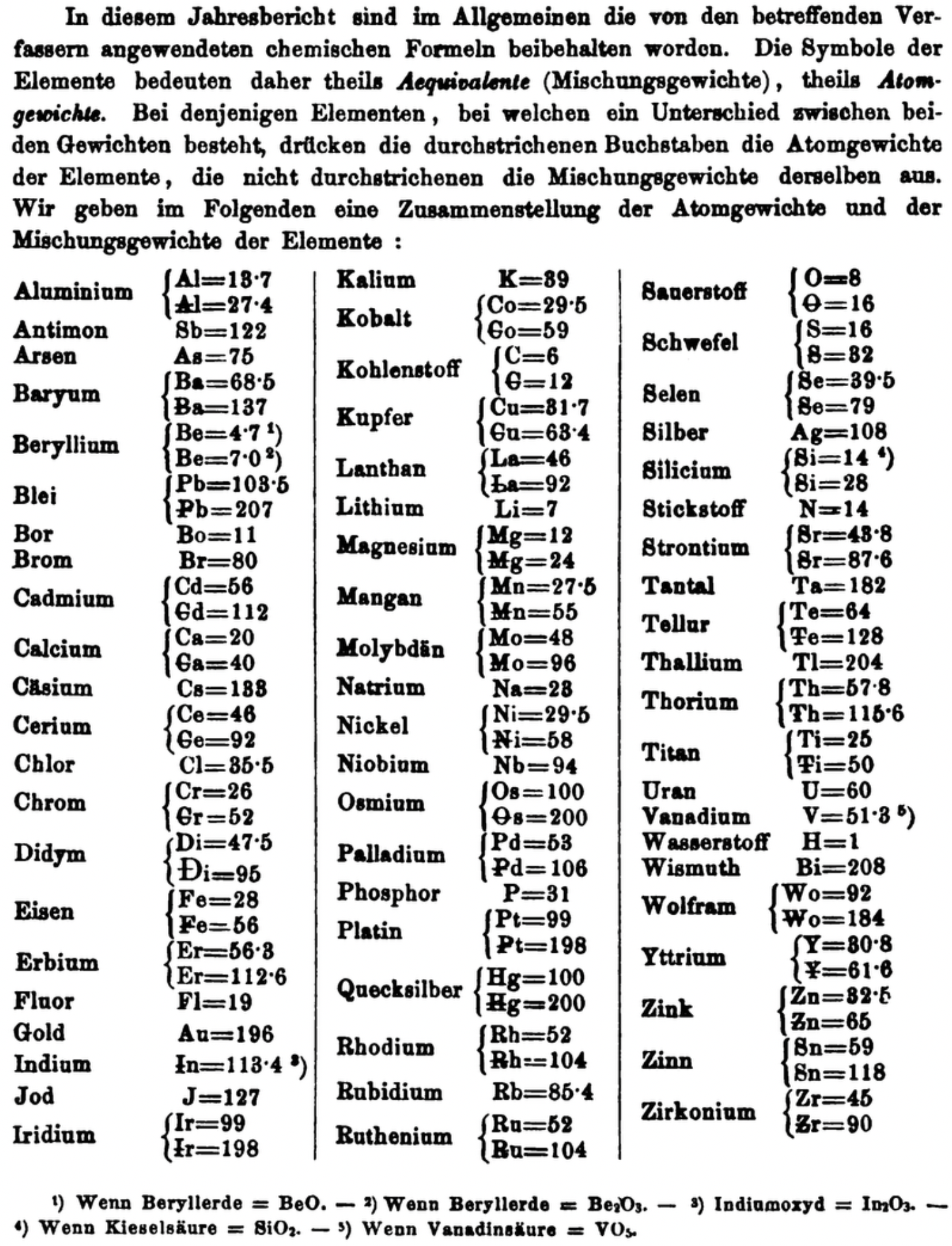
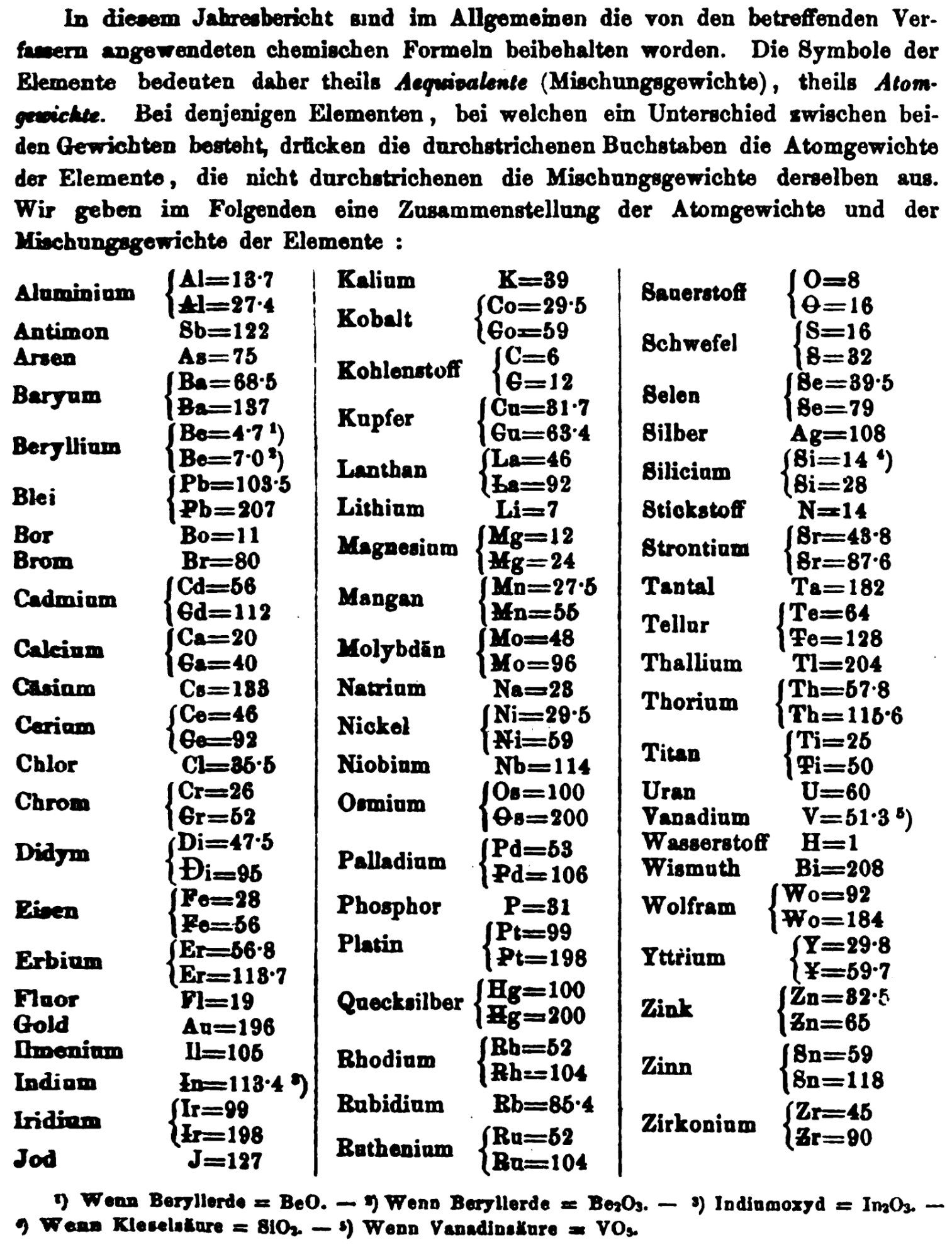
| Year: 1873 | PT id = 1363, Type = formulation review element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1873
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1873 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systematic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
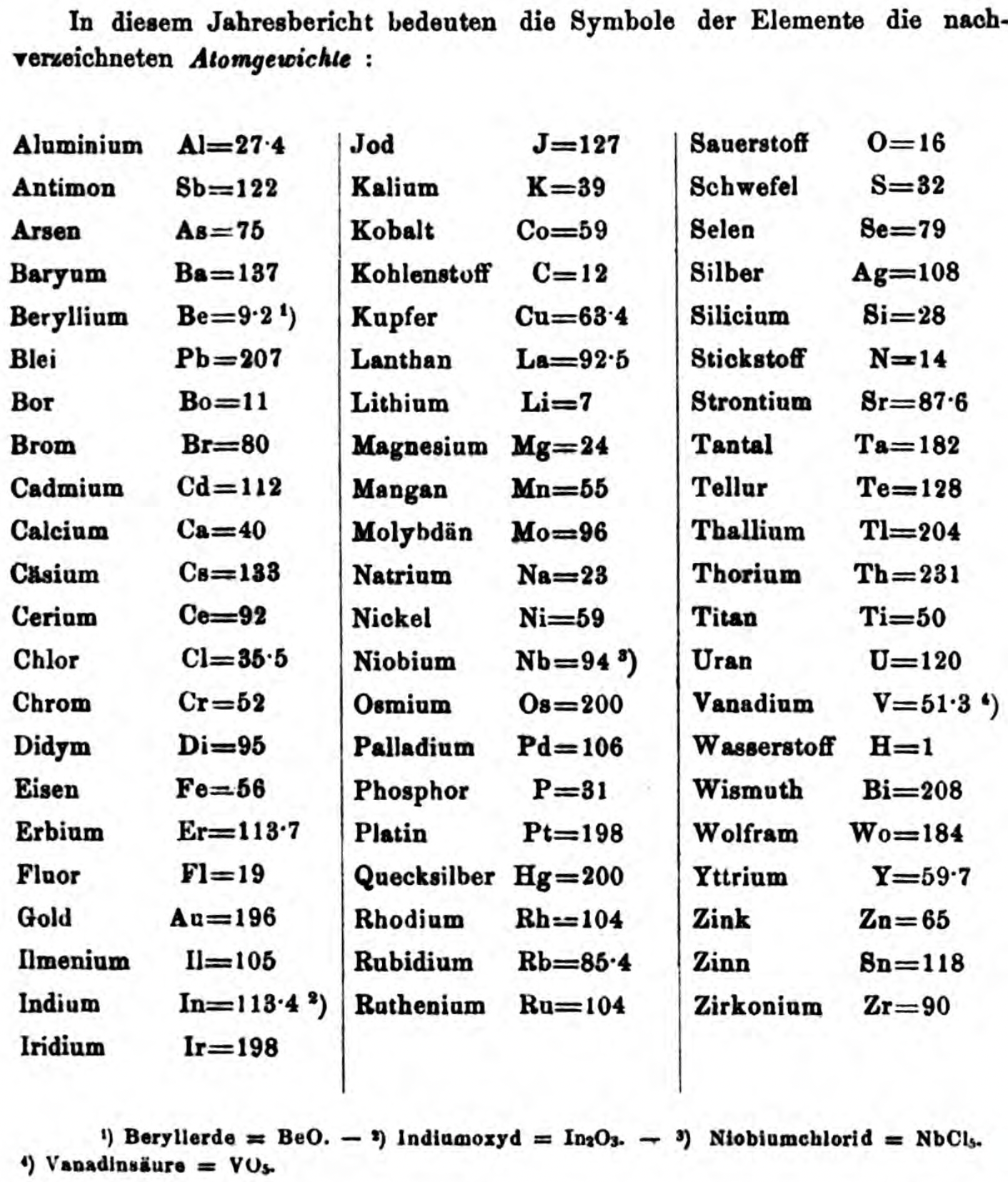
Notes:
- Didym D = 48 was actually a mixture of rare earth elements.
- Ilmenium, Il, was later found to be a mixture of niobium and tantalum.
- Generally, the elements missing had yet to be discovered (dates given below).
- The table below shows the progress from 1858 to 1873.
- By 1873 the only elements with incorrect atomic weights were the (at the time) somewhat obscure strontium, lanthanium, cerium and urananium.
- Previously, many elements were shown with two entries. Clearly, the stoichiometric and mass problems had largely been resolved (and the data agreed upon) by 1873.
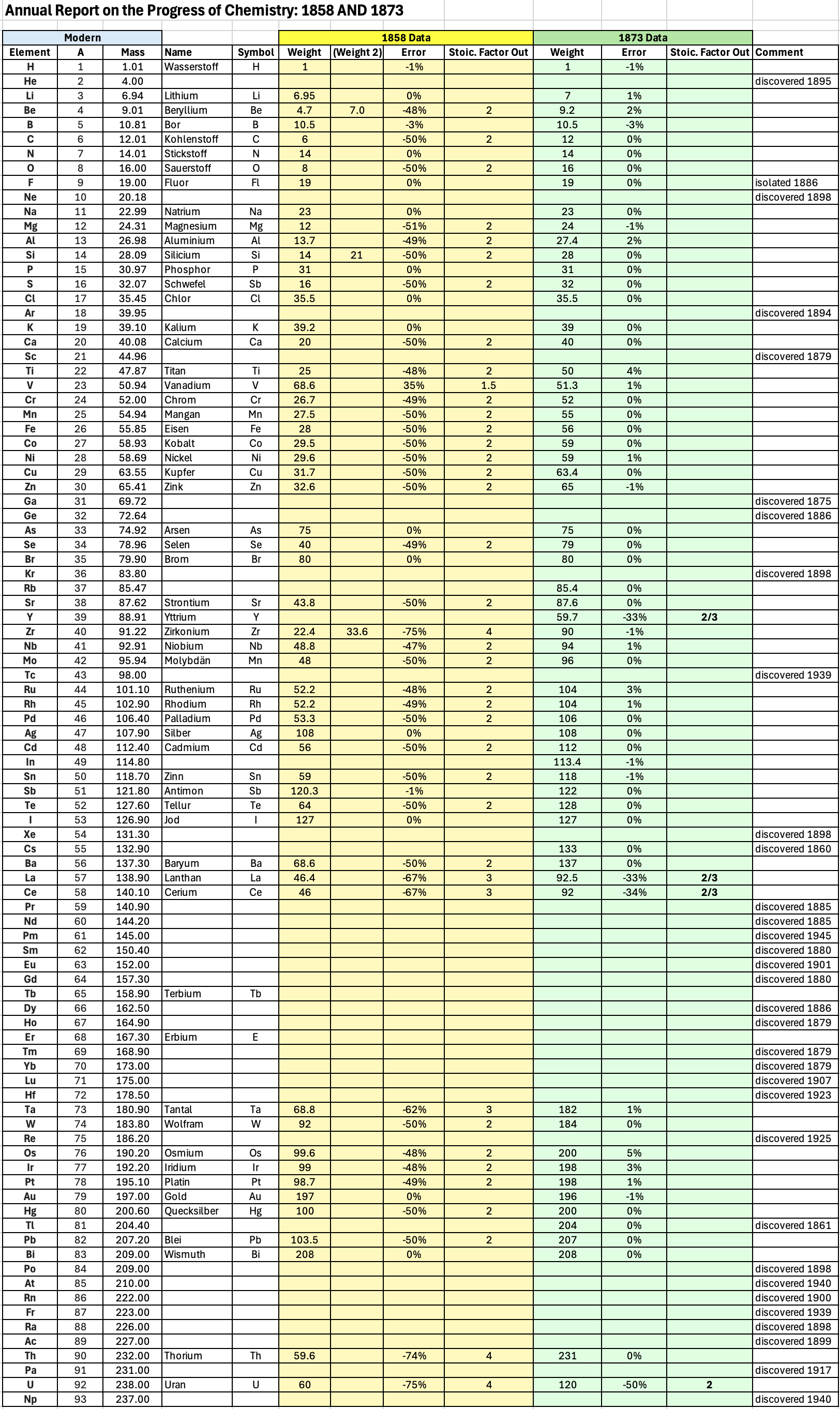
Thanks to René and Mario Rodriguez for the tip!
| Year: 1875 | PT id = 1135, Type = formulation |
Gibbes' Synoptical Periodic Table
From page 127 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:

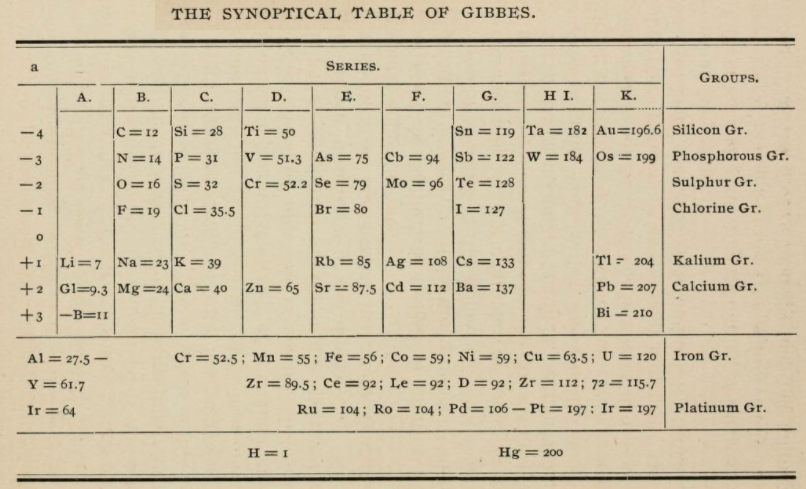
Thanks to René for the tip!
| Year: 1875 | PT id = 1136, Type = formulation spiral |
Concentric Ring Arrangement of Wiik
From page 133 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:

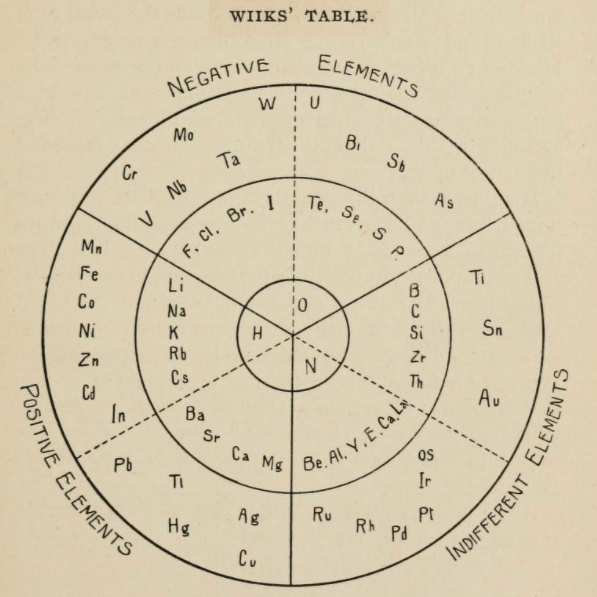
Thanks to René for the tip!
| Year: 1878 | PT id = 1137, Type = formulation |
Waechter's Numerical Regularities
From page 136 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:
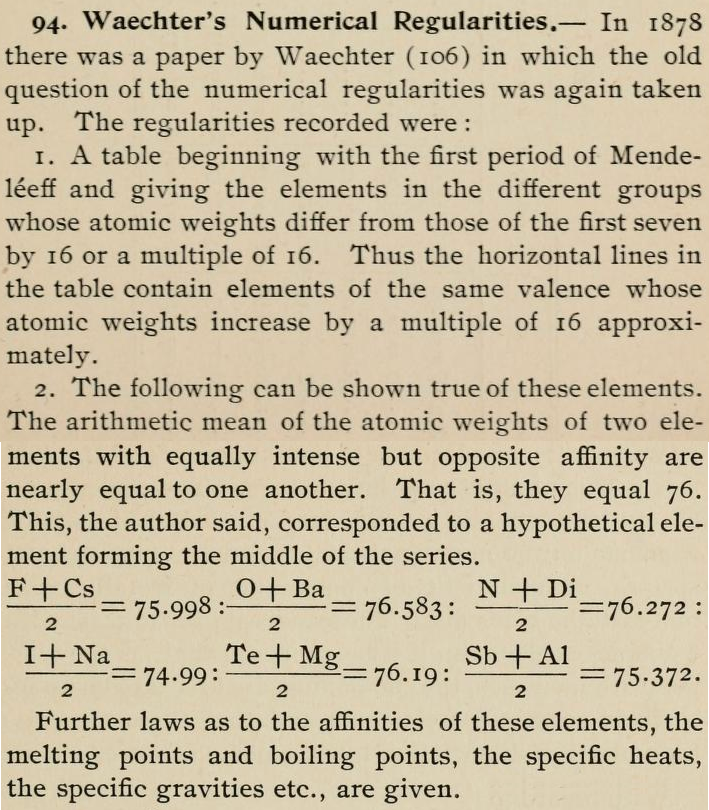
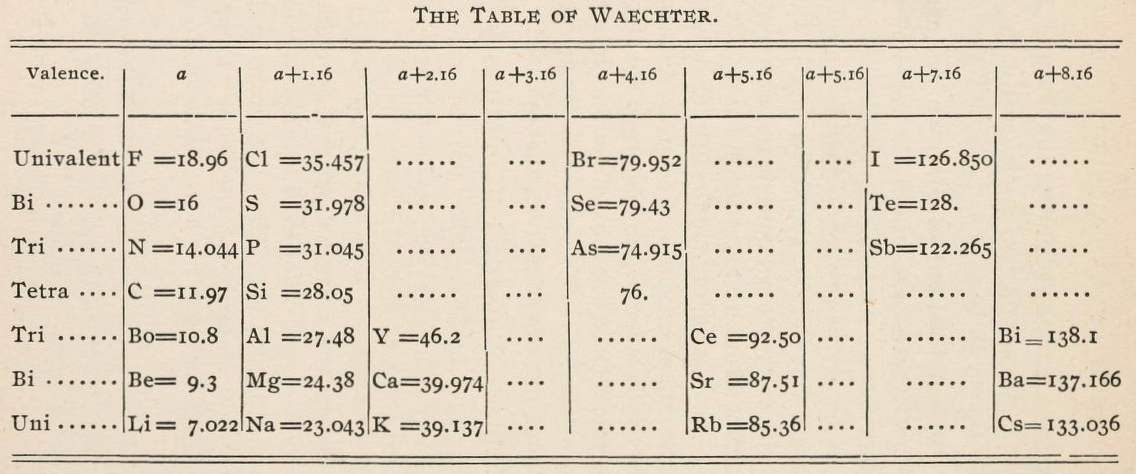
Thanks to René for the tip!
| Year: 1882 | PT id = 1138, Type = formulation |
Bayley's Attempt
From page 158 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes about Bayley:

Thanks to René for the tip!
| Year: 1885 | PT id = 1139, Type = formulation |
Carnelley & The Periodic Law
From page 172 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:

Thanks to René for the tip!
| Year: 1885 | PT id = 1145, Type = formulation review |
von Richter's Periodic System of the Elements
From page 244 of A Text-book of Inorganic Chemistry by Victor von Richter, Published by Blakiston (US ed. in English, 1885). The full text (scanned) is available from archive.org. The first edition was published in 1874 in German. von Richter was was from the Baltic region, in the the Russian empire at the time.
von Richter's work is almost certainly the first chemistry textbook based on the periodic system. Many (indeed most) modern Inorganic Chemistry texts follow this format, but NOT the Chemogenesis web book!
von Richter, writes:

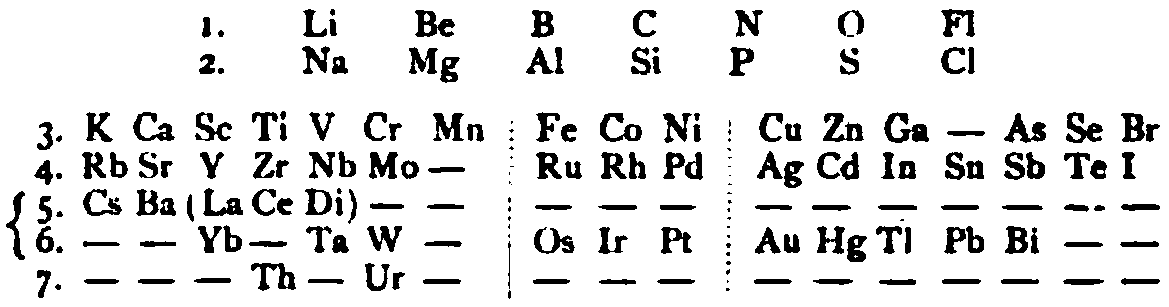

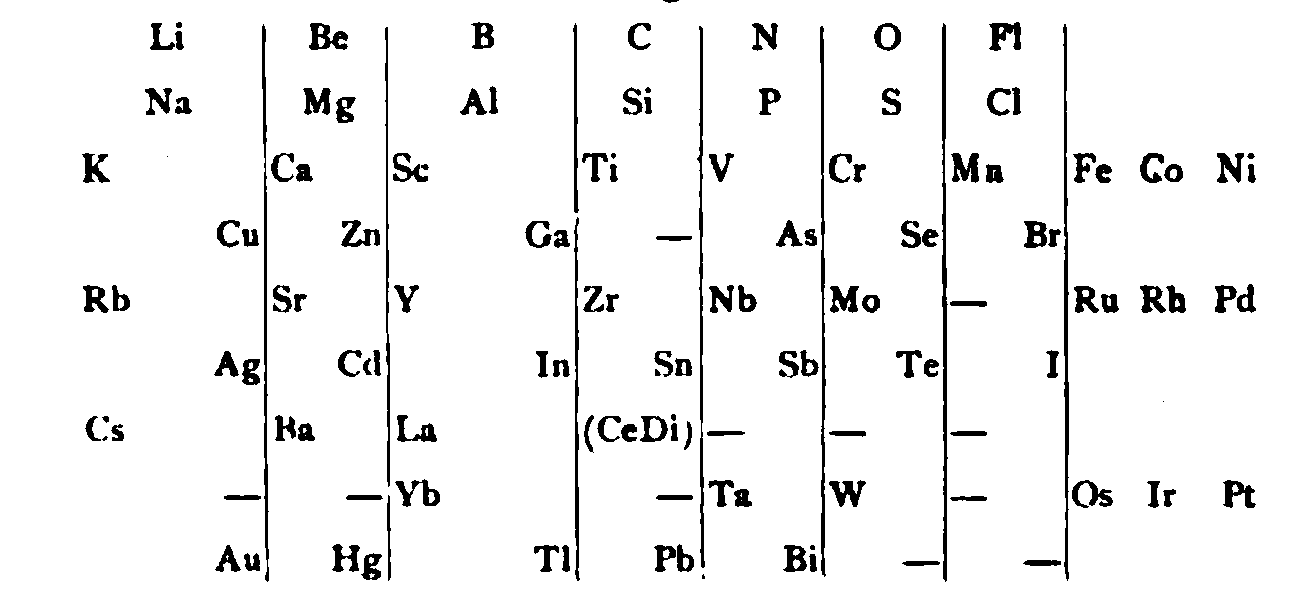
Thanks to René for the tip!
| Year: 1886 | PT id = 1107, Type = formulation spiral 3D |
Shepard's Natural Classification
Shepard's Natural Classification of the Elements, a spiral formulation with instructions for turning it into a three-dimensional table. From: Elements of Inorganic Chemistry, Descriptive and Qualitative (pp221), by J. H. Shepard, (1886), Boston MA, pub. D. C. Heath
René Vernon writes:
Note the instructions along the side, to turn the table into a tube (spiral form) and the 19 spaces from La to eka-Ce. Here, Yb needs to be moved back one column into group II, so as to leave room for Lu under La. Then eka-Ce becomes Hf. This results in La + 15 lanthanoids.
The accompanying text says:
"Elements of most distinct basic character are found towards the left; non-metals predominate in the upper and middle parts of Groups V., VI., and VII. ; while the lower part of the table is marked by the more indifferent elements.
"A double spiral will be traced beyond Si (beginning with P and V respectively) and distinguished by heavy-face and light-face type.
"The harmony of nature here exhibited is most impressive. Is it possible that the so-called elements are really compounds? Did the various 'elements' of the earth and sun once exist as hydrogen, when our solar system was a nebula? And will modern chemists ever revive the famed problem of the alchemists, and seek to turn the base metals into gold? Far more precious than gold is the search for truth; and the more we learn of science, the broader becomes our conception of what we know in part, and the deeper should be our reverence for the infinite thought of the Creator."
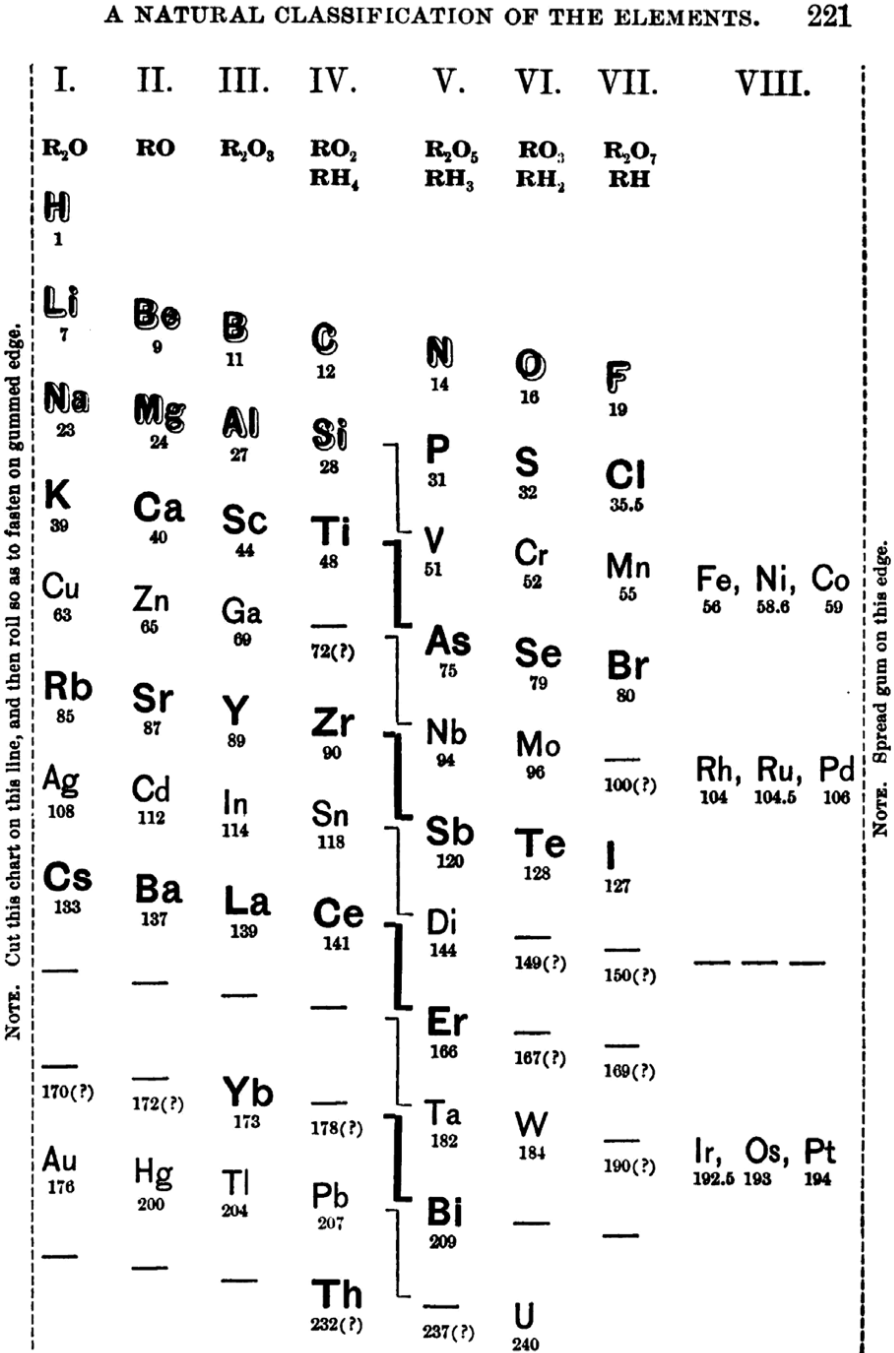
| Year: 1888 | PT id = 997, Type = formulation spiral |
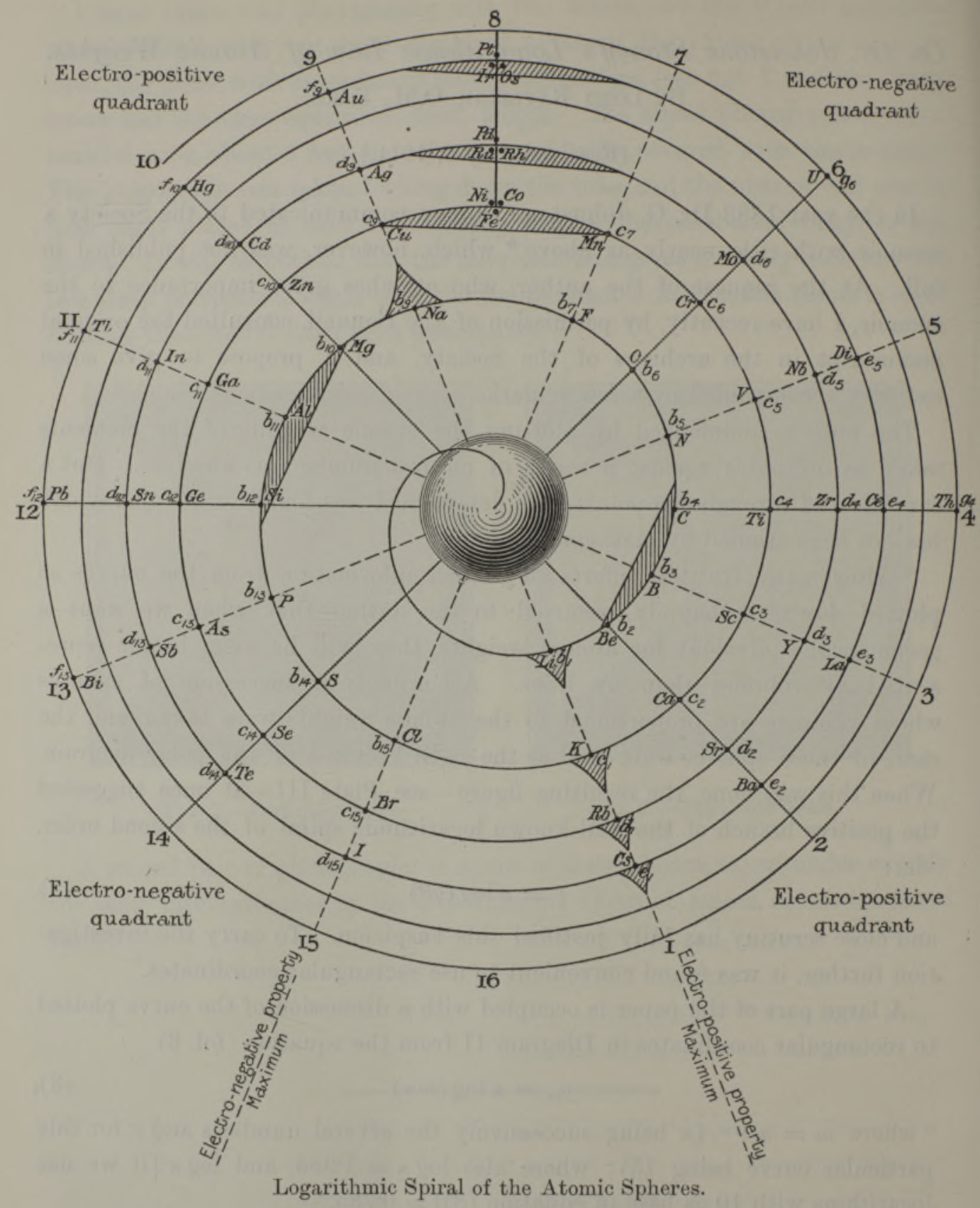
Stoney's Spiral
Johnstone Stoney's Spiral, taken from A. E. Garrett's The Periodic Law (page 167, 1909 pub. D. Appleton And Company). The reference is given – page 167 – is: Phil. Mag. [6], 4, pp 411 et seq.; Proc. Roy. Soc., 1888, p115.
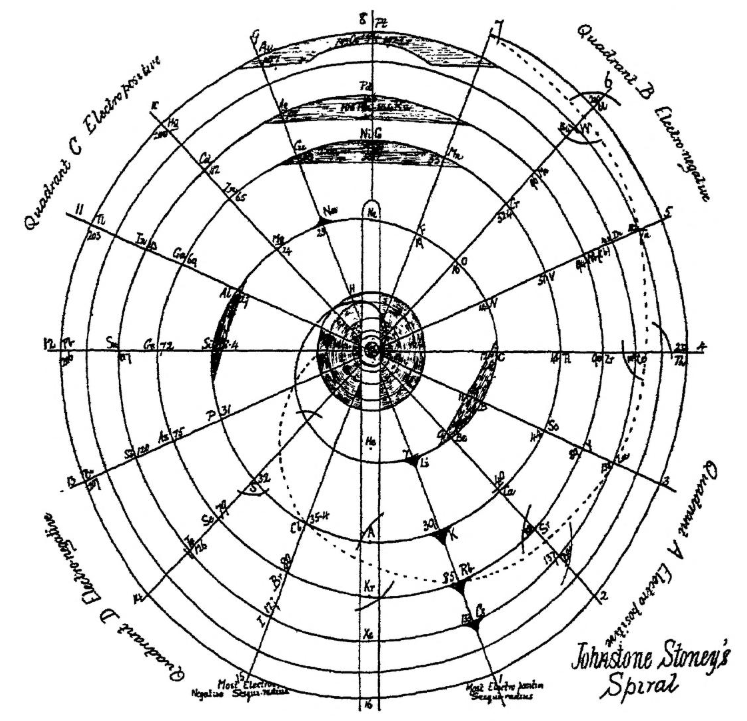
Thanks to Roy Alexander for the tip!
| Year: 1888 | PT id = 1267, Type = formulation spiral |
Stoney's Spiral Periodic Table
In the Proceedings of the Royal Society of London, Series A, Containing Papers of a Mathematical and Physical Character, Volume 85, Issue 580, Aug 1911, p. 472, there is an article On Dr. Johnstone Stoney's Logarithmic Law of Atomic Weights, by Lord Rayleigh (who co-discovered argon in 1894), who writes :
"In the year 1888, Dr. G. Johnstone Stoney communicated to the Society a memoir with title nearly as above, which, however, was not published in full. At the request of the author, who attaches great importance to the memoir, I have recently, by permission of the Council, consulted the original manuscript in the archives of the Society, and I propose to give some extracts, accompanied by a few remarks. The author commenced by plotting the atomic weights of the elements taken as ordinates against a series of natural numbers as abscissæ. But a curve traced through the points thus determined was found to be 'one which has not been studied by mathematicians.
"This sudden transition may have some connection with the fact that no elements have been found on sesqui-radius 16, although the investigation in § 3 shows that the values of m corresponding to the stations on sesqui-radius 16 cannot be dispensed with.
"The vacant places here pointed out are now occupied by the since discovered inert gases. The anticipation is certainly a remarkable one, and it goes far to justify the high claims made for the diagram, as representing in a telling form many of the leading facts of chemistry."
Comment from Mark Leach:
"Notice how the electronegative elements are positioned top right & bottom right and the electropositive elements top left & bottom right."
René Vernon writes:
"Stoney has another article in the September 1902 edition of the The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science, called Law of Atomic Weights, pp. 411–415. At the back of the journal is an updated fold-out version of Stoney’s table, image attached.
- Ar, Kr and Xe fit on the spiral, and on spoke 16.
- Neon fits on the spiral but is instead on spoke 8.
- Helium is on spoke 18 but is not on the spiral.
- The circle in the middle represents H (p. 414).
"On the page after the updated spiral, there looks to be some printed content, but it is hidden by what looks to be a folded over page."
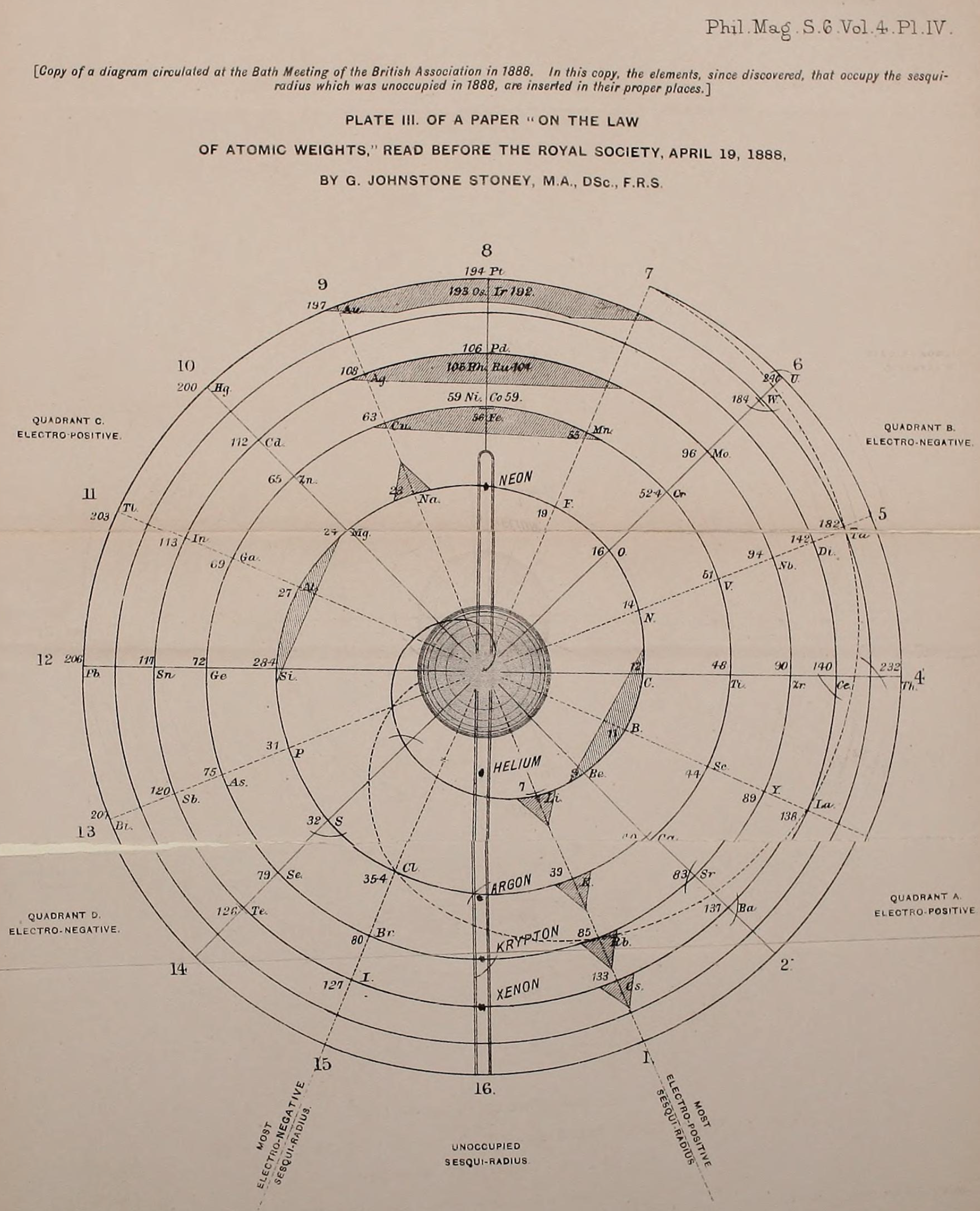
Thanks to René for the tip!
| Year: 1891 | PT id = 1140, Type = formulation |
Wendt's Generation-Tree of the Elements
From page 244 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:
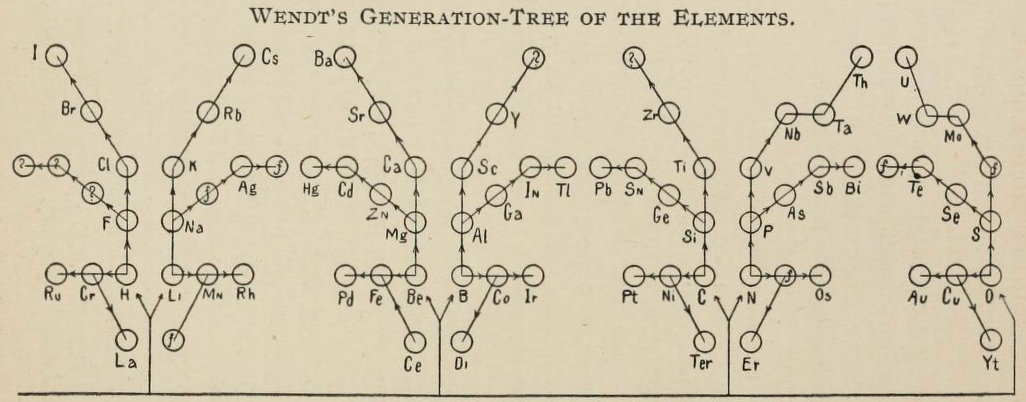
Thanks to René for the tip!
| Year: 1892 | PT id = 62, Type = formulation 3D |
Bassett's Vertical Arrangement
Bassett's Vertical Arrangement is actually designed to be a three dimensional formulation. Quam & Quam's review paper states:
"This table resembles Mendeléeff's vertical arrangement. The Cs period, however, starts far above the horizontal line of K and Rb, thereby giving space to the known and predicted elements of that period. The alkali metals appear in three horizontal lines. Co and Ni are arranged in order of their atomic weights.
"Bassett suggested cutting out the table and rolling it onto a cylinder of such circumference that similar elements would fall in line in Groups. For instance, Li, Na, K, Rb, and Cs would then fall on a line parallel to the axis of the cylinder."
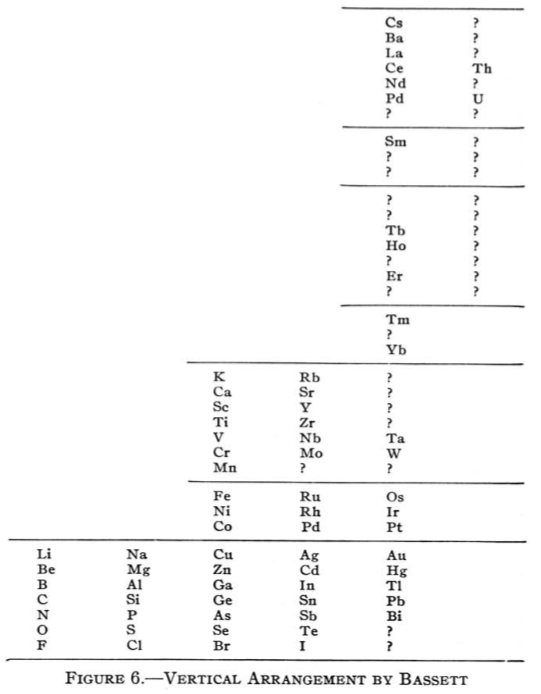
From Quam & Quam's 1934 review paper.pdf
| Year: 1893 | PT id = 63, Type = formulation |
Rang's Periodic Arrangement of The Elements
P.J.F. Rang's The Periodic Arrangement of the Elements, Chemical News, vol. 67, p. 178 (1893)
Observing that that Rang's table has four 'groups': A, B, C & D, René Vernon writes:
- Group A contains the strongest positive elements; group D the strongest negative elements. At such an early date, it's odd to see groups 1 to 3 categorised together.
- Group B are the elements with high melting points; "they are all remarkable for their molecular combinations" (presuamably, a reference to multiple oxidation states). At one side of group B are the "anhydro-combinations", probably referring to the simple chemistry of Ti, Zr, [Hf] Nb and Ta being dominated by insoluble oxides. At the other side are the "amin, carbonyl, and cyanogen combination", probably a reference to the group VIII carbonyls, as metal carbonyls had only just been discovered. Ni is shown after Fe, rather than Co.
- Group C includes the "heavy metals that have low melting points"; an early reference to frontier or post-transition metals, as a category.
- Rang says: ...if groups A and D be split up vertically in respectively three and two parts, the table presents seven vertical groups, and horizontally seven more or less complete series. Each group in each of the series 2 and 3 are represent by one element... The octave appears both horizontally and vertically in the table.
- Rang's reference to Di as representing all the triads between Ba and Ta kind of works since Hf would go under Zr, and that would leave 15 Ln or five sets of three. Thus, something like this:

Gd occupies the central position among the Ln. This arrangement won't fit however unless Rang envisaged all 15 Ln occupying the position under Y. - The location of H over | Ga | In | Tl, appears strange... but the electronegativity of H (2.2) is closer to B (2.04) than it is to C (2.55).
From Quam & Quam's 1934 review paper.pdf
| Year: 1893 | PT id = 1151, Type = formulation |
Nechaev's Truncated Cones
René Vernon (who found this formulation) writes:
This weird and wonderful table appears in Teleshov & Teleshova (2019, p. 230). It is attributed by them to Nechaev (1893) and is apparently discussed by Ipatiev (1904):
- The caption accompanying the table is: "Scanning of the projection of rotational bodies in the form of truncated cones as used in Nechaev's spatial construction of the periodic system, 1893."
- Looking at the table it seems to anticipate, after a fashion, the double periodicity noticed by later authors.
- Alternatively, if turned on its side, it would be just five columns wide.
- Between Ce (ignoring Di) and Yb, there are spaces for 12 missing elements, which is one too many.
- Pulling Yb back by one position would have done the trick.
"... We would also like to mention one more version of the periodic table, namely the one offered by V. Ipatiev. Ipatiev's version was one of the first to have been applied in a school textbook, and is also concise and accompanied by a detailed methodological commentary. More specifically, Ipatiev is important in directing our attention to the fact that an essential feature common to all elements should be chosen if the elements are to be systematized. Furthermore, Ipatiev also offered another crucial insight in arguing that this selected feature must satisfy certain conditions, namely: 1) it must be measurable, 2) it must be common to all elements and 3) it must be paramount, i.e. that all the remaining properties of the elements must depend on it [Ipatiev]."
References:
Ipat'ev, V. & Sapozhnikov, A. (1904). Kratkij kurs himii po programme voennyh uchilishh [A concise course in chemistry for military academies]. Sankt-Peterburg: tip. V. Demakova.
Nechaev N. P. (1893). Graficheskoe postroenie periodicheskoj sistemy jelementov Mendeleeva. Sposob Nechaeva [Graphic construction of Mendeleev's periodic system of elements. Nechaev's way]. Moskva: tip. Je. Lissnera i Ju. Romana
Teleshov S, Teleshova E.: The international year of the periodic table: An overview of events before and after the creation of the periodic table. In V Lamanauskas (ed.).: Science and technology Education: Challenges and possible solutions. Proceedings of the 3rd International Baltic Symposium on Science and Technology Education, BalticSTE2019, Šiauliai, 17-20 June, 2019. pp. 227-232, (2019)
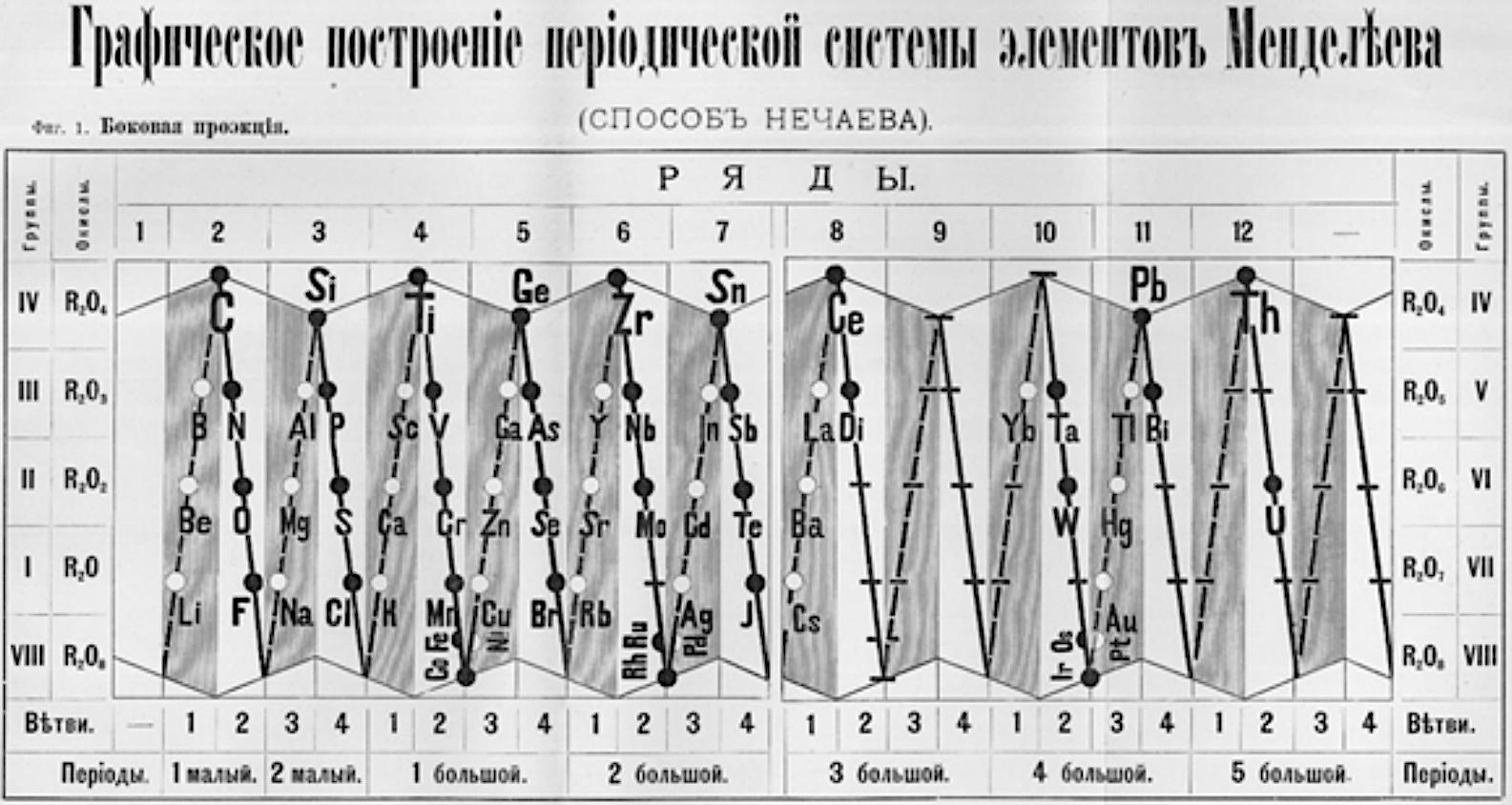
| Year: 1896 | PT id = 1087, Type = formulation |
Ramsay's Elements Arranged in the Periodic System
From The Gases of the Atmosphere, The History of Their Discovery by William Ramsay (and from the Gutenberg Project.)
The author writes pp 220-221:
"In 1863 Mr. John Newlands pointed out in a letter to the Chemical News that if the elements be arranged in the order of their atomic weights in a tabular form, they fall naturally into such groups that elements similar to each other in chemical behaviour occur in the same columns. This idea was elaborated farther in 1869 by Professor Mendeléeff of St. Petersburg and by the late Professor Lothar Meyer, and the table may be made to assume the subjoined form (the atomic weights are given with only approximate accuracy):—"
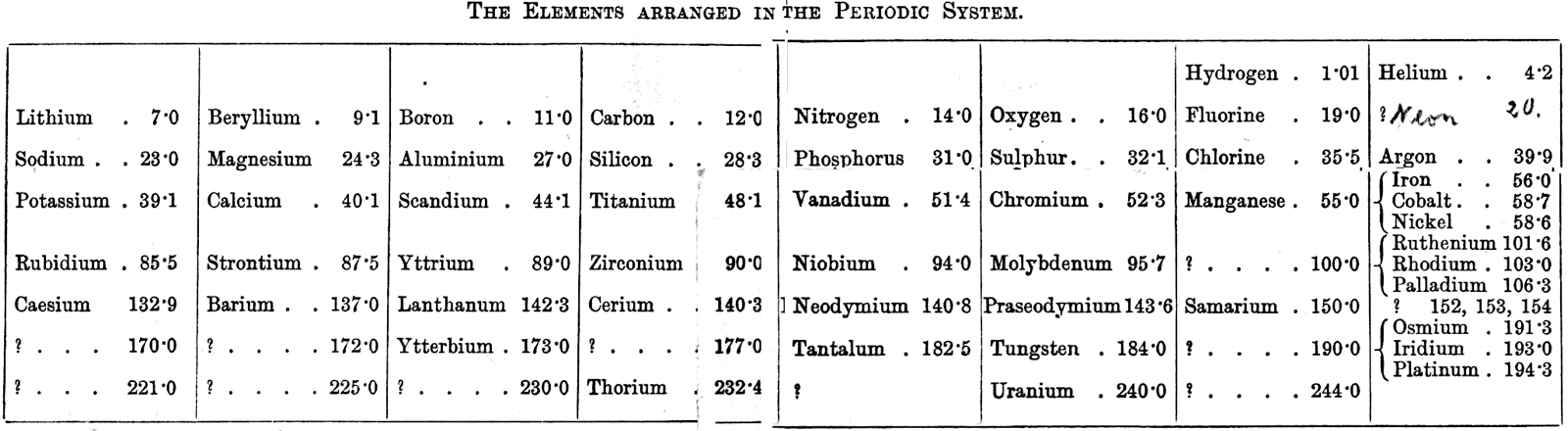
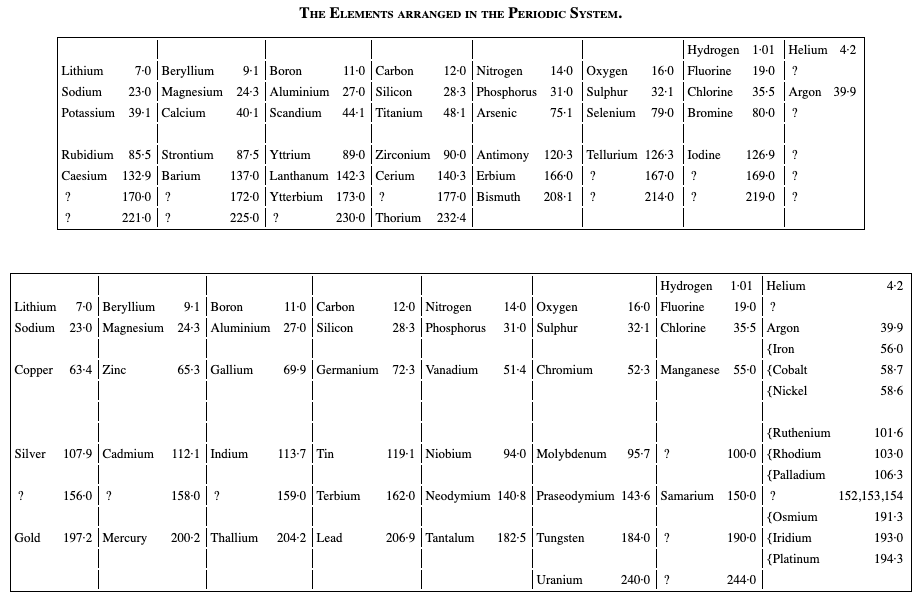
Thanks to René for the tip!
| Year: 1896 | PT id = 1383, Type = structure |
Discovery of Radioactivity
From The Nuclear Wallchart:
In 1896 Henri Becquerel was using naturally fluorescent minerals to study the properties of x-rays, which had been discovered in 1895 by Wilhelm Roentgen. He exposed potassium uranyl sulfate to sunlight and then placed it on photographic plates wrapped in black paper, believing that the uranium absorbed the sun’s energy and then emitted it as x-rays.
This hypothesis was disproved on the 26th-27th of February, when his experiment "failed" because it was overcast in Paris. For some reason, Becquerel decided to develop his photographic plates anyway. To his surprise, the images were strong and clear, proving that the uranium emitted radiation without an external source of energy such as the sun. Becquerel had discovered radioactivity.
Becquerel showed that the radiation he discovered could not be x-rays. X-rays are neutral and cannot be bent in a magnetic field. The new radiation was bent by the magnetic field so that the radiation must be charged and different than x-rays. When different radioactive substances were put in the magnetic field, they deflected in different directions or not at all, showing that there were three classes of radioactivity: negative, positive, and electrically neutral.
The term radioactivity was actually coined by Marie Curie, who together with her husband Pierre, began investigating the phenomenon recently discovered by Becquerel. The Curies extracted uranium from ore and to their surprise, found that the leftover ore showed more activity than the pure uranium. They concluded that the ore contained other radioactive elements. This led to the discoveries of the elements polonium and radium. It took four more years of processing tons of ore to isolate enough of each element to determine their chemical properties.
Ernest Rutherford, who did many experiments studying the properties of radioactive decay, named these alpha, beta, and gamma (α, β and γ) particles, and classified them by their ability to penetrate matter. Rutherford used an apparatus similar to Becquerel's. When the air from the chamber was removed, the alpha source made a spot on the photographic plate. When air was added, the spot disappeared. Thus, only a few centimeters of air were enough to stop the alpha radiation.
- α-particles carry more electric charge, are more massive, and move slowly compared to β and γ particles, they interact much more easily with matter.
- β-particles are much less massive and move faster, but are still electrically charged. A sheet of aluminum one millimeter thick or several meters of air will stop these electrons [and positrons].
- γ-rays carry no electric charge, they can penetrate large distances through materials before interacting–several centimeters of lead or a meter of concrete is needed to stop most γ-rays.
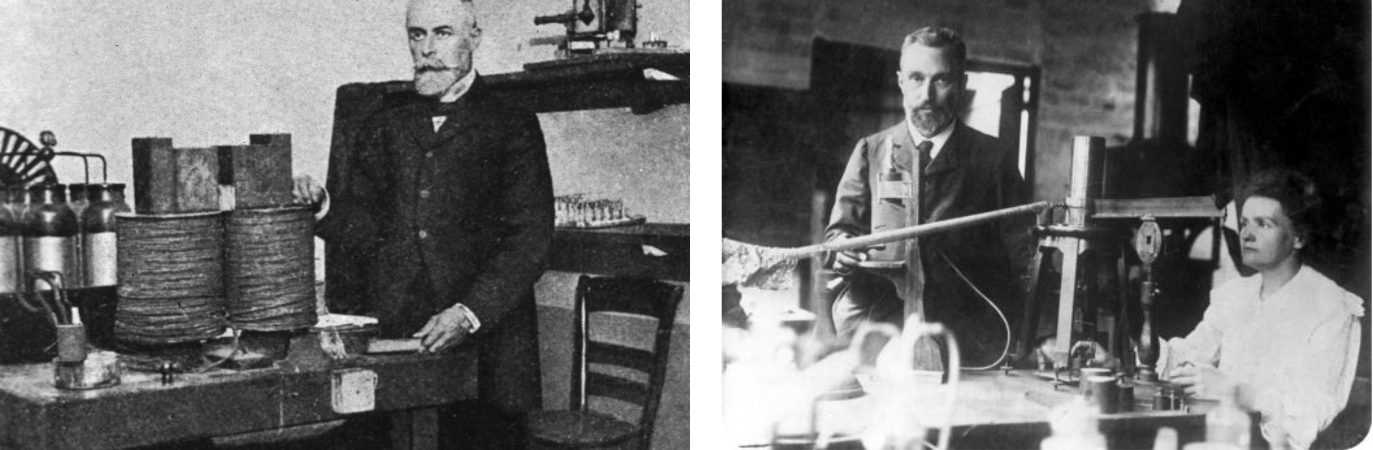
Henri Becquerel and Marie & Pierre Curie in their labs:
| Year: 1896 | PT id = 1134, Type = review |
Venable's The Development of The Periodic Law
The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896).
The full text (scanned) is available from archive.org.


Thanks to René for the tip!
| Year: 1905 | PT id = 64, Type = formulation |
Werner's Arrangement
Werner's Arrangement is the first modern looking PT formulation. It appeared before the structure of the atom was known, before the importance of atomic number was recognised and before quantum mechanics had been developed.
Berichte der Deutschen Chemischen Gesellschaft (1905), 38, 914-21 and J. Chem. Soc., Abstr. 88, II, 308-9 1905:
From Quam & Quam's 1934 review paper.pdf
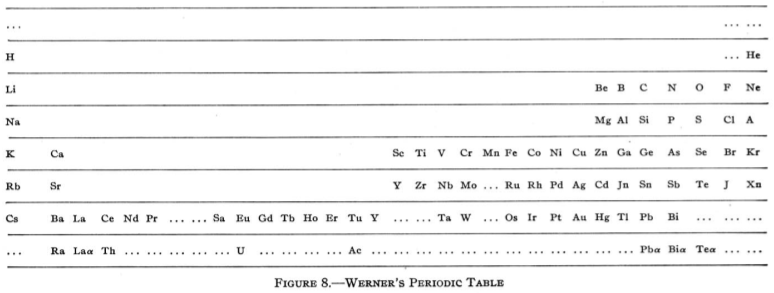
Eric Scerri comments that the interesting features are:
- A remarkably modern looking formulation in that it separates not only the transition metals but also the rare earths into separate blocks to give what we would now call a "long-form 32 column table". Except Werner guessed wrong as to how many rare earths exist, with the result that he shows 33 groups.
- This formulation is also interesting for showing an element between H and He and two elements before H.
- Werner computed the average gaps between atomic weights for the second through the fifth periods as 1.85, 2.4, 2.47 and 2.5, respectively.
- From this he extrapolated the gap for the first period as 1.5, which coincidentally was also half the difference between the atomic weights of H and He. Werner thus predicted a new element with atomic weight 2.5.
- Moseley's work of 1913 showed there were no elements before H and none between H and He.
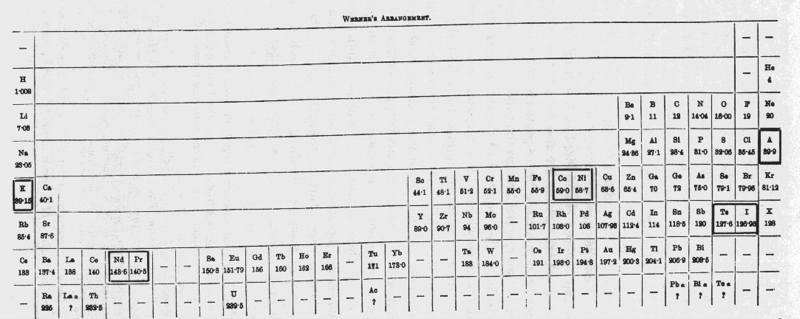
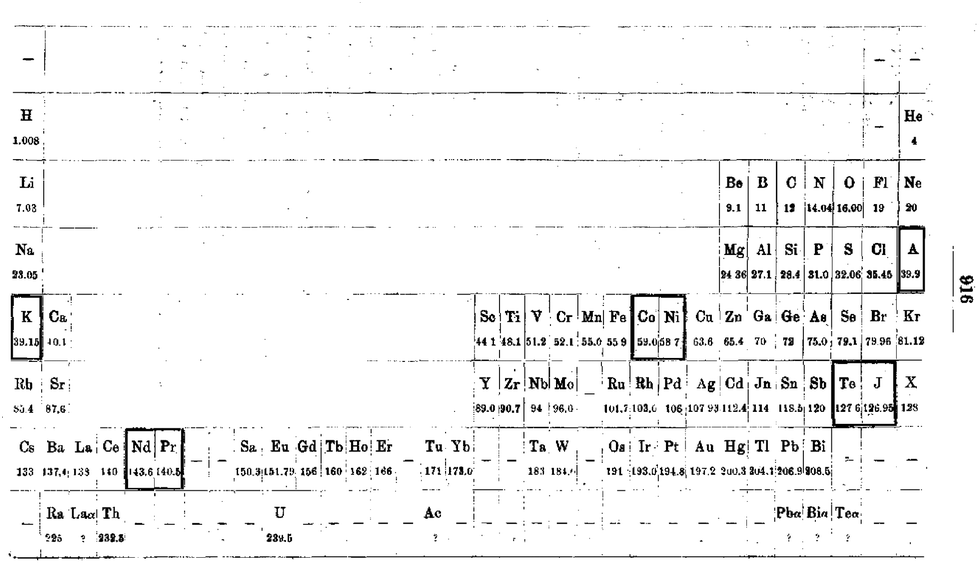
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1907 | PT id = 1105, Type = formulation |
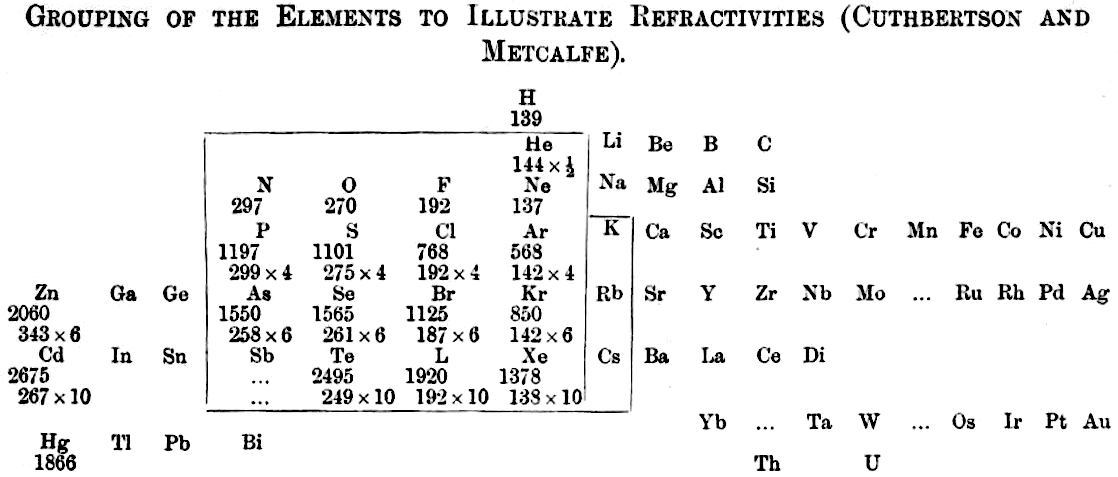
Grouping of The Elements to Illustrate Refractivity
From C. Cuthbertson & E. Parr Metcalfe, Part III On The Refractive Indices of Gaseous Potassium, Zinc, Mercury, Arsenic Selenium and Tellurium, Phil. Trans. A: Mathematical & Physical Sciences, vol 207, pp135–148, 1907.
René Vernon writes:
"A curious periodic table which runs from group 12 on the left to group 13 on the right (see below). It seems to have done that way to bring out the pattern in multiples of refractivities i.e. x½ x 4 x 6 x10. The border around the elements in groups 15 to K-Rb-Cs in group 1 denotes this relatively strong regularity among the refractivity values. The L for iodine is a printer's error."
| Year: 1909 | PT id = 1106, Type = review |
Garrett's The Periodic Law
A book reviewing The Periodic Law by A.E. Garrett, pub. D. Appelton & Co (1909). This work shows the state of knowledge in the first decade of the 20th century.
René Vernon writes:
"On page 43 Garrett notes that, '[Thomas] Carnelley was the first English chemist to work out in detail the manner in which the properties of the elements are periodic functions of their atomic weights. His papers on this subject appeared in the Philosophical Magazine between the years 1879 and 1885.' "

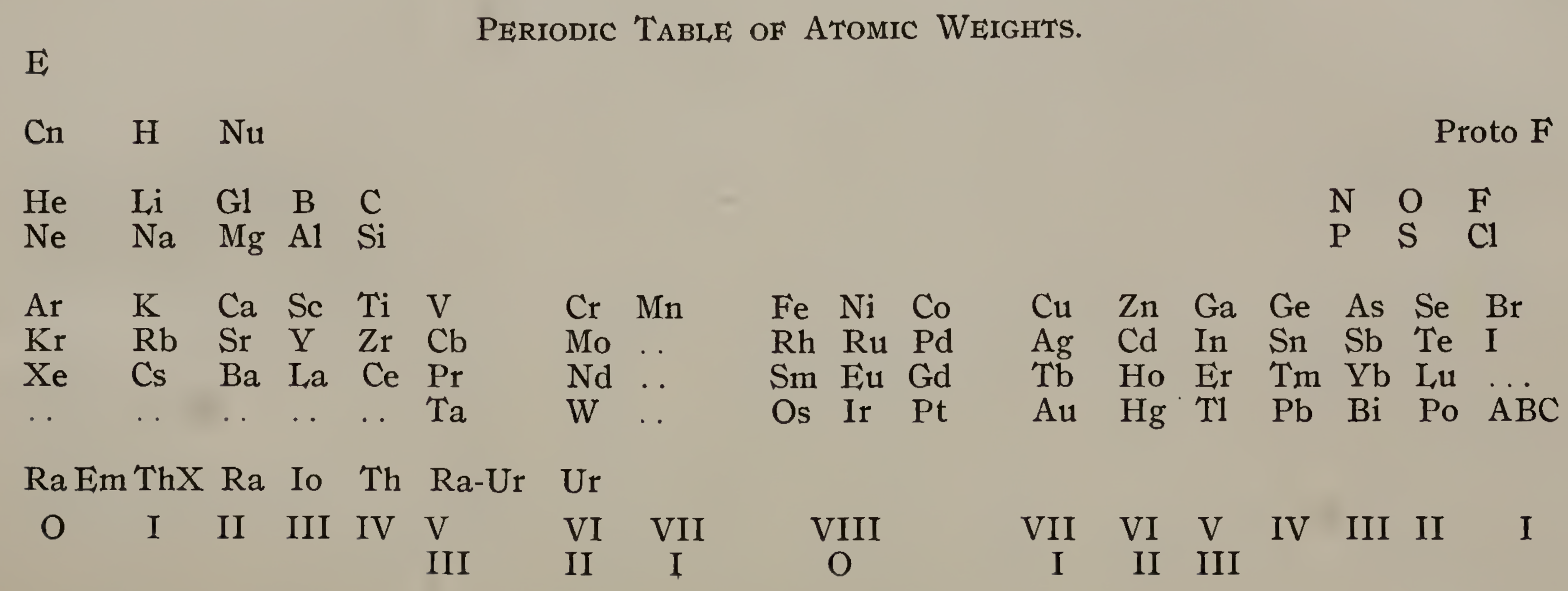
| Year: 1911 | PT id = 1296, Type = formulation |
Emerson's Periodic Table of Atomic Weights
Emerson BK, Helix chemica: A study of the periodic relations of the elements and their graphic representation, American Chemical Journal, vol. 45, pp. 160–210 (1911). The formulation below appears on page 173; a scanned pdf version of the paper can be viewed here.
René Vernon writes:
Emerson includes two elements before hydrogen: "E" (either the luminiferous ether or the electron) and "Coronium". There are also two elements between hydrogen and helium: "Nebulium" and "Protofluorine".
This is the first time I have seen a PT showing four extra elements and where they are supposed to fit.
After La, Emerson incorporates 13 lanthanides (Ce to Lu) as transition elements into his 7th period.
Emerson missed dysprosium, between Tb and Ho.
"A, B and C" at the bottom right are supposed to be 'halogen emanations'.
Mark Leach adds that Emerson's very odd Periodic Table of Atomic Weights does not actually show any atomic weights.
| Year: 1913 | PT id = 59, Type = formulation |
Rydberg's Table
René Vernon writes:
My source is the 1914 French translation of Rydberg’s 1913 German article.
- Rydberg 1913, Untersuchungen über das System der Grundstoffe, Lunds Univ. Årsskrift, (Acta Univers, Lundensis), vol. 9, no. 18, pp. 1-41
- — 1914, Recherches sur le système des éléments, Journal de Chimie Physique, vol. 12, pp. 585–639, https://doi.org/10.1051/jcp/1914120585

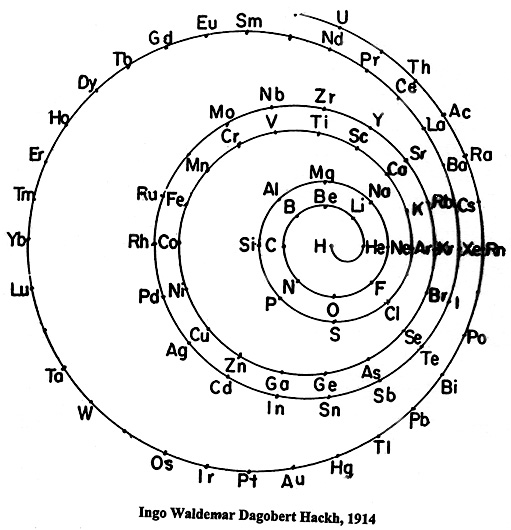
| Year: 1914 | PT id = 23, Type = formulation spiral |
Hackh's Spiral Periodic Table
Ingo Hackh's spiral periodic table of 1914, from Das Synthetisches System der Atome, Hamburg, Hephaestos.
Philip Stewart says:
"I believe that Hackh's 1914 spiral is of special interest it is the first spiral to take account of Mosley's atomic numbers, and the first to show successively larger pairs of coils. It is also interesting because H stands alone in the centre. I have only seen Mazurs' redrawn (as usual!) version, but Mazurs gives SciAm Supplement 1919 as one reference."
This is the Mazurs version:
| Year: 1917 | PT id = 1155, Type = formulation |
Friend's Periodic Table (1917)
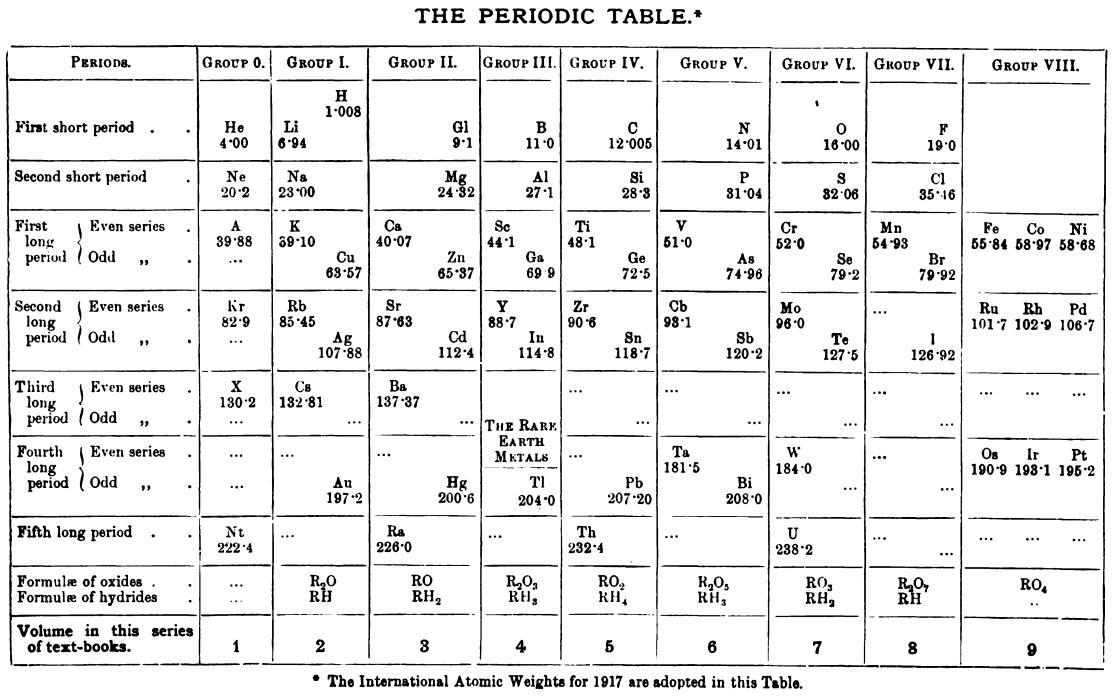
Thanks to René Vernon for the tip.
| Year: 1918 | PT id = 1300, Type = formulation |
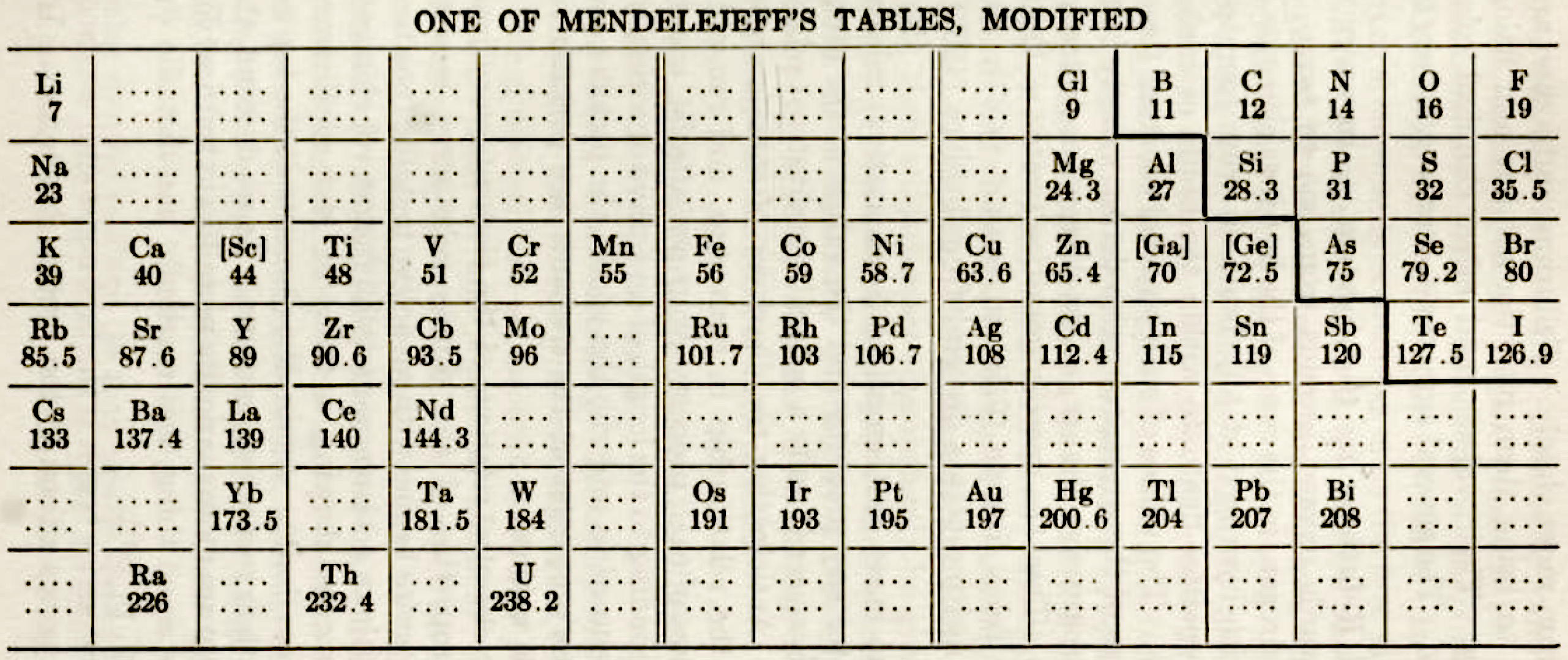
One of Mendelejeff's Tables, Modified
From Smith A 1918, General Chemistry for Colleges, 2nd ed., The Century Co., New York, p. 299
René Vernon writes:
- H is missing, as are the noble gases.
- Consequently, the period numbers are out by one apiece.
- Seven groups are on the left and seven are on the right (the ever present allure of symmetry).
- After La, Ce is placed under Zr, and Nd is placed under columbium/technetium.
- According to Smith the rest of the lanthanide elements do not fit into any series, because their valences and other chemical properties do not permit most of them to be distributed over so many different groups.
- Po is expected to be a metal which is what it turned out to be Smith has anticipated that astatine will be a metal. Nine decades later, Hermann, Hoffmann & Ashcroft (2013) predicted the same thing: Hermann, A.; Hoffmann, R.; Ashcroft, N. W. (2013). Condensed astatine: Monatomic and metallic. Physical Review Letters, 111 (11), 116404-1–116404-5
- While he does not discuss it, Smith appears to have allowed for missing elements between Li and Gl and between Na and Mg.
- The three elements inside square brackets are those predicted by Mendeleev.
| Year: 1918 | PT id = 1260, Type = formulation |
Cherkesov: Two Periodic Tables
von Bichowsky FR, The place of manganese in the periodic system, J. Am. Chem. Soc. 1918, 40, 7, 1040–1046 Publication Date: July 1, 1918 https://doi.org/10.1021/ja02240a008
René Vernon writes:
"In this curious article, von Bichowsky, a physical chemist (1889-1951), mounted an argument for regarding Mn as belonging to group 8 (see table 1 below) rather than group 7 (table 2). His article has effectively been assigned to the dustbin of history, having apparently gathered zero citations over the past 103 years.
"Items of note in his 24-column table:
- While Mn, 43 and 75 are assigned to group 8 they remain in alignment with group 7. Se is shown as Sc
- 14 lanthanides, from Ce to Yb, make up group 3a; If La and Lu are included, there are 16 Ln
- Gd is shown as Cd
- Positions of Dy and Ho have been reversed
- Tm and Tm2
- Po shown as "RaF"
- Ra shown as "RaEm"
- Pa shown as Ux2
von Bichowsky made his argument for Mn in group 8, on the following grounds:
- by removing the Ln from the main body of the table all of the gaps denoted by the dashes (in table 2) were removed
- the eighth group links Cr with Cu; Mo with Ag; and W with Au
- the symmetry of the table is greatly increased
- the triads are replaced by tetrads and a group of 16 Ln which accords better with "the preference of the periodic system for powers of two"
- about eight chemistry-based differences between Ti-V-Cr and Mn, including where Mn shows more similarities to Fe-Co-Ni, for example:
- divalent Ti, V, Cr cations are all powerful reducing agents, Cr being one of the most powerful known; divalent Mn, Fe, Co, Ni are either very mild reducing agents as divalent Mn or Fe, or have almost no reducing power in the case of divalent Co or Ni;
- metal titanates, vanadates and chromates are stable in alkaline solution and are unstable in the presence of acid whereas permanganates are more stable in acid than alkali; their oxidizing power is also widely different.
I can further add:
- Mn, Fe, and Co, and to some extent Ni, occupy the "hydrogen gap" among the 3d metals, having no or little proclivity for binary hydride formation
- the +2 and +3 oxidation states predominate among the Mn-Fe-Co-Ni tetrad (+3 not so much for Mn)
- in old chemistry, Mn, Fe, Co, and Ni represented the "iron group" whereas Cr, Mo, W, and U belonged to the "chromium group": Struthers J 1893, Chemistry and physics: A manual for students and practitioners, Lea Brothers & Co., Philadelphia, pp. 79, 123
- Tc forms a continuous series of solid solutions with Re, Ru, and Os
Moving forward precisely 100 years, Rayner-Canham (2018) made the following observations:
- Conventional classification systems for the transition metals each have one flaw: "They organise the TM largely according to one strategy and they define the trends according to that organisation. Thus, linkages, relationships, patterns, or similarities outside of that framework are ignored."
- There are two oxide series of the form MnO and Mn3O4 which encompass Mn through Ni. Here the division is not clear cut since there are also the series Mn2O3 for Ti-Cr and Fe; and MnO2 for Ti to Cr.
- Under normal condition of aqueous chemistry, Mn favours the +2 state and its species match well with those of the following 3d member, Fe.
Rayner-Canham G 2018, "Organizing the transition metals" [a chapter in] in E Scerri & G Restrepo, Mendeleev to Oganesson: A multidisciplinary perspective on the periodic table, Oxford University Press, Oxford, pp. 195–205
I've also attached a modern interpretation of von Bichowsky’s table. It's curious how there are eight metals (Fe aside) capable of, or thought to be capable of, achieving +8. I am not sure that a table of this kind with Lu in group 3 is possible, without upsetting its symmetry."
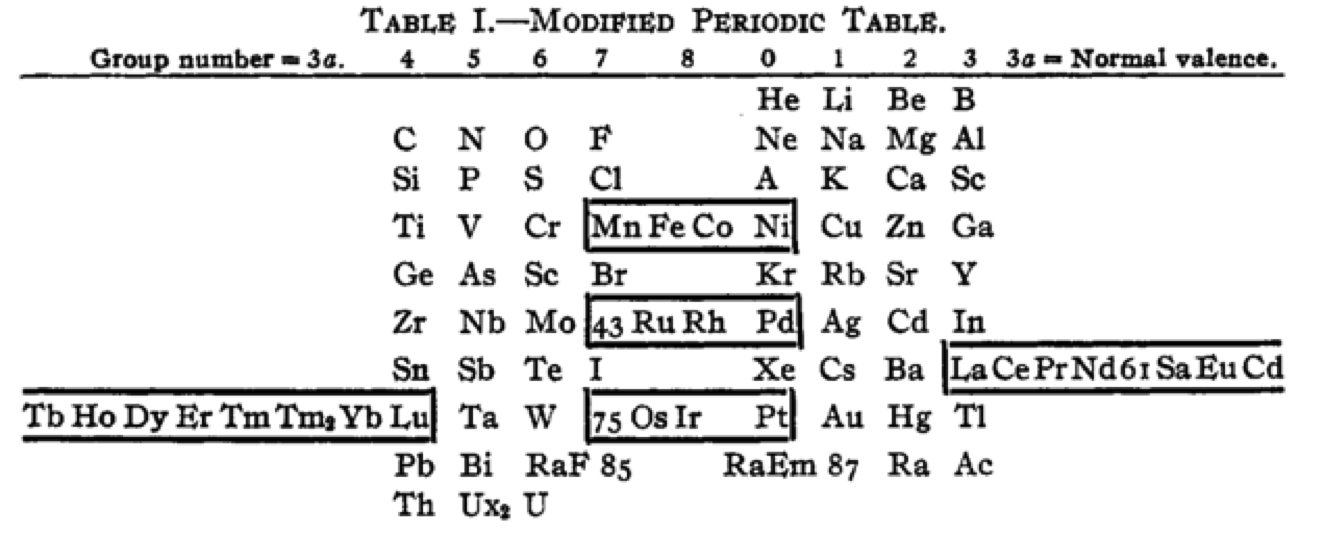
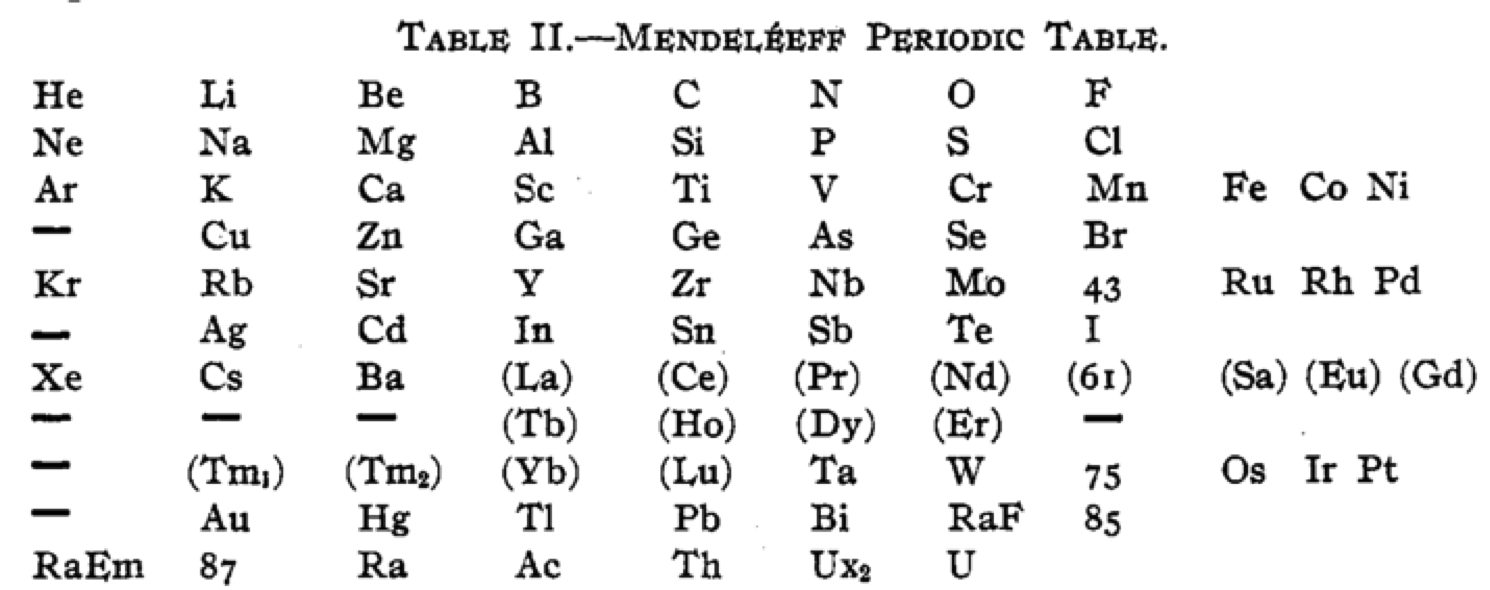
| Year: 1919 | PT id = 1293, Type = formulation |
Snyder's Fundamental Periodic Table of The Elements
Snyder MB 1919, The Fundamental Periodic Table of the Chemical Elements, filed in Congressional Library, Washington.
René Vernon writes:
"Notable for:
- Its attempted integration of the Ln and An into the short form of the periodic table
- Placement of H over He, Li and F
- Elements 108 = Pleon; 126 = Akron; 143 = Ultine"
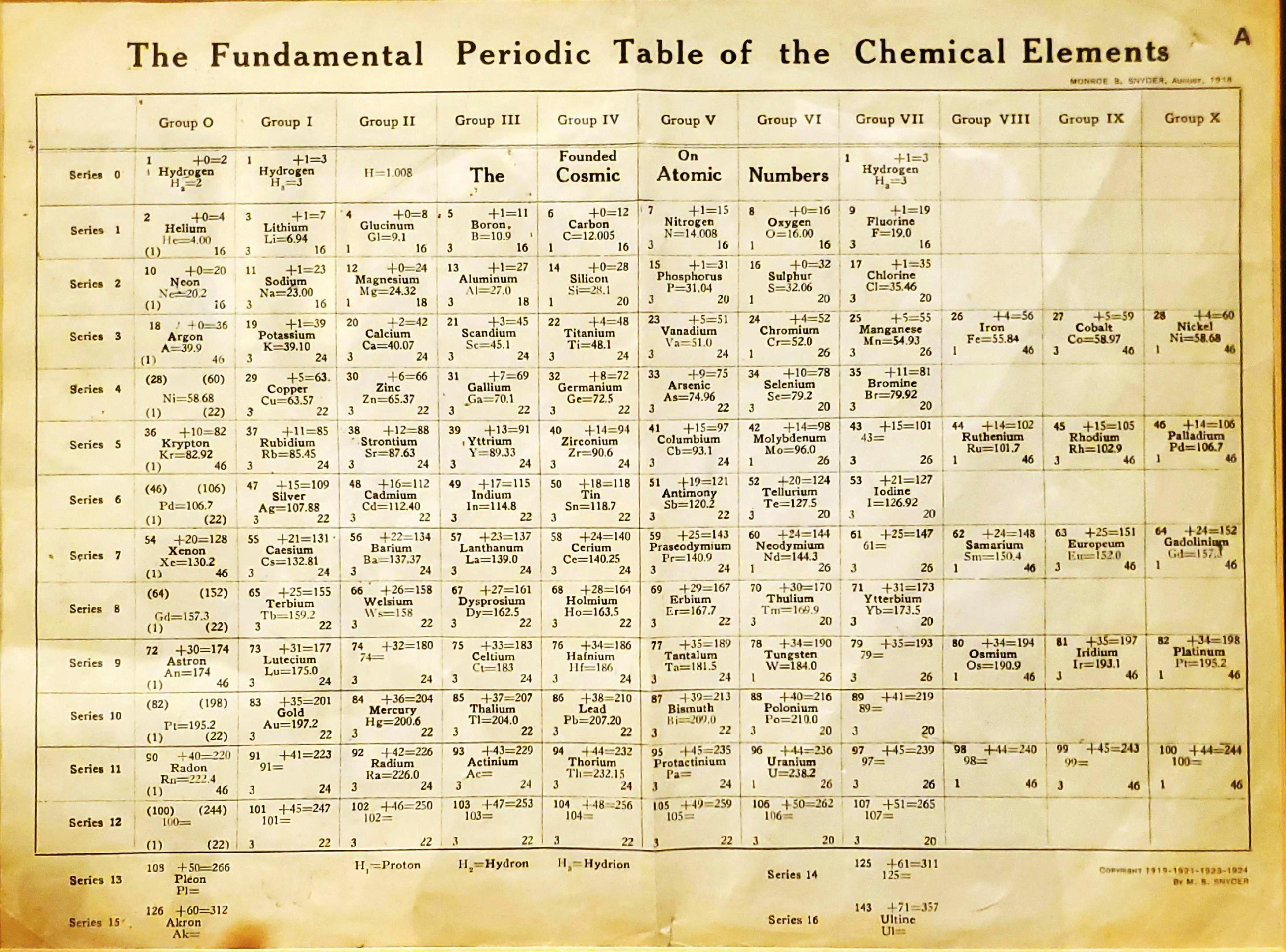
| Year: 1919 | PT id = 547, Type = formulation |
Hackh's Classification of the Elements, Updated
From a Scientific American in March 1919, an article by Ingo W. D. Hackh discussing the classification of the elements.
Shown is a periodic table slightly updated from a version from two years before, and referenced by Quam & Quam:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1919 | PT id = 1386, Type = formulation |
Hackh's Classification of The Chemical Elements
Hackh, I. W. D. (1919). The classification of the chemical elements: The fundament of chemistry, Scientific American, 87 (supp. no. 2253), pp. 146–149 (148). https://zenodo.org/records/2454321
René Vernon writes:
Note that Group 4 (including Lu) appears twice, on the left and right.
Hackh does not get it quite right when he refers to a vertical similarity prevailing in the upper half of the table and a horizontal similarity in the lower half. A horizontal similarity prevails along the first row of the transition metals; vertical similarities tend to prevail among the second and third row dyads of the transition metals. That said, a horizontal similarity does prevail among the lanthanides.
On the noble gases, Hackh (p. 146) wrote: "...they combined the two extreme ends of a period, they formed the bridge from a non-metallic halogen (electro-negative element) to a metallic alkali (electro-positive element). For this reason we may speak of these elements, the rare or inert gases, as the terminals of the periods, which are either positive nor negative... The first three elements following an inert gas are always strong positive, while the last three before an inert gas are always strong negative and thus a kind of a transition is formed by the fourth element, or the elements of the carbon group."
For chemical properties he wrote: "The chemical characteristics of the elements can equally well be studied, for there are the acid- and base-forming elements on the chart, whose zones gradually infiltrate from strong basic to weak basic to atmospheric to weak acid to strong acid or vice versa."
Read more in the paper.
| Year: 1920 | PT id = 1070, Type = formulation |
Black & Conant's Periodic Classification Of The Elements
From N.H. Black NH & J.B. Conant's Practical Chemistry: Fundamental Facts and Applications to Modern Life, MacMillan, New York (1920)
Eric Scerri, who provided this formulation writes (personal communication):
"Notice conspicuous absence of H. And, Conant was the person who gave Kuhn his first start in the history of science at Harvard."
René Vernon tells us that Conant and his coauthor write:
"The position of H in the system has been a matter of some discussion, but it is not of much consequence. It seems to be rather an odd element. Perhaps the best place for it is in group IA as it forms a positive ion." (p. 350)
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1920 | PT id = 1075, Type = formulation |
Stewart's Arrangement of The Elements
From A.W. Stewart, Recent Advances in Physical and Inorganic Chemistry, 3rd ed., Longmans, Green and Co., London (1920)
René Vernon writes:
"Stewart discusses the 'forced symmetry' of Mendeleev's table, and the distinction between 'facetious symmetry' (as he calls it) and the actual correlation of facts (as he saw them at that time)."
Extracts:
237. Mendeleev... objected strongly to the employment of graphic methods of expressing the Periodic Law, on the ground that such methods did not indicate the existence of a limited and definite number of elements in each period.
239. The Periodic Table, as laid down by Mendeleeff in his writings, exhibits a symmetry which was one of its greatest assets. For some psychological reason, symmetry has an attraction for the human mind; and we are always apt to prefer a regular arrangement to one in which irregularities pre- dominate. Psychological peculiarities are, however, undesirable guides in the search for truth; and a careful examination of the Table in the light of our present knowledge will suffice to show that it can boast of no such symmetry as we are led to expect from the text-books of our student days.
For example, owing to the omission of some of the rare earth elements and by the insertion of blanks, the Table in its original form attained a very high degree of regularity; but since there are, as we know from the X-ray spectra results, only sixteen elements to fill the eighteen vacant spaces in the Table, it is evident that the symmetry of Mendeleeff s system is purely factitious.
Further, in order to produce the appearance of symmetry, Mendeleeff was forced to place copper, silver, and gold in the first group, although there is no known oxide Au2O and the stable chloride of gold is AuCl3.
These examples are well-known, and are mentioned here only for the purpose of enforcing the statement that the symmetry of Mendeleef's system cannot be sustained at the present day. Fascinating though its cut-and-dried regularity may be, we cannot afford to let symmetry dominate our minds when in actual fact there is no symmetry to be found.
240. The most superficial examination shows that, instead of being a symmetrical whole, the Table is really pieced together from a series of discrete sections.
250. The first attempt to arrange all the elements in a periodic grouping took the form of a three-dimensional model the Telluric Helix of de Chancourtois and it is not surprising that from time to time attempts have been made to utilize the third dimension as an aid to classification. It cannot be said that much light has been thrown on the matter by these essays; but some account of them must be given here for the sake of completeness.
251. The main drawback to the spiral representation appears to be that in it no new facts are brought to light, and there is no fresh collocation of the allied elements which might give it an advantage over the ordinary forms of classification. Also, in most cases it is more difficult to grasp as a whole.
253 ...if we have to choose between factitious symmetry and actual correlation of facts, we must decide in favour of the latter, discomforting though the choice may be.
255. The following new grouping seems worth considering. Although it has many good points, it is not to be regarded as a final solution, but is put forward mainly in the hope that an examination of it may suggest some more perfect system.
| Year: 1920 | PT id = 78, Type = formulation 3D spiral |
Schaltenbrand's Helical Periodic Table
G. Schaltenbrand, Darstellung des periodischen Systems der Elemente durch eine räumliche Spirale, Z. anorg. allgem. Chem., 112, 221-4 (Sept. 1920)
From Quam & Quam's 1934 review:
"The elements are arranged in order of atomic weights on an eccentric spiral. The four sets of curves include positions of similar elements. The first small turn carries H and He; the remainder of the inert elements and the halogens are on successive small turns in analogous positions.
"On the next larger turn are found the alkali, alkaline-earth, and aluminum family elements.
"The long periods require larger turns and the period containing the rare-earth elements requires the longest turn of all. Elements of the same group are found in the same plane passing through the axis of the spiral."
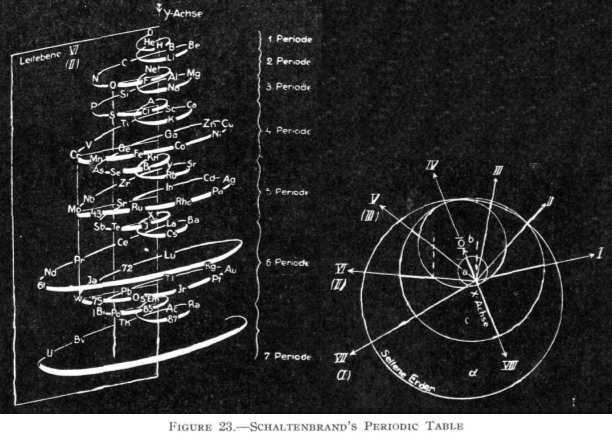
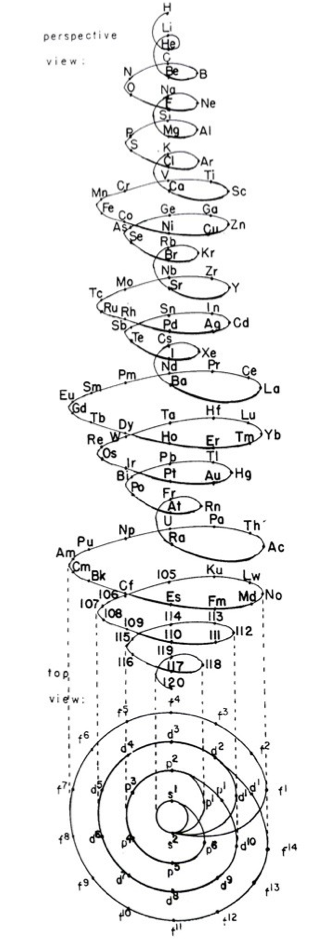
Commissioned in 2019 to match George Schaltenbrand's 1920 design for a helical gathering of the elements – albeit extended to all 118 current elements – and signed by Yuri Oganessian, it is almost certainly the most expensive periodic table in the world."


| Year: 1921 | PT id = 1192, Type = formulation |
Formánek's Periodic Table
Formánek J. 1921, Short Outline of Inorganic Chemistry (in Czech), 2nd ed., Ministerstvo zemedelstvi CSR, Praha. p. 281
René Vernon writes:
Here is an eight column table with some interesting features.
Main groups 0, Ia, IIa, Vb, VIb, and VIIb, correspond to what we have today:
- 0 Noble gases
- Ia Alkali metals
- IIa Alkaline earths
- Vb Pnictogens
- VIb Chalcogens
- VIIb Halogens
Main group IIIa is B-Al-Sc-Y... Ac whereas these days B-Al have been moved over Ga on electronic grounds. This happened despite the fact that the average trend line for chemical and physical properties v Z going down B-Al-Sc-Y... Ac is more regular.
In main group IV, notice how C and SI are positioned in the middle of the cell, unlike their neighbours to either side. The group thus bifurcates after Si into a Ti branch and a Ge branch. This is quite reasonable since there is not much difference in the average trendlines going down either option. In any case, C-Si came to be moved over Ge again on electronic grounds.
He survived the electronic revolution, staying over Ne.
| Year: 1921 | PT id = 1237, Type = formulation |
Margary's Modified Table
Ivan D. Margary B.A. (1921) XXXVI. A modification more in accord with atomic structure, The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 42:248, 287-288.
An old school table showing group 3 as B-Al-Sc-Yt-Rare earths.
Thanks to René for the tip!
| Year: 1921 | PT id = 1020, Type = formulation |
Bury's Periodic Arrangement based on Langmuir's Theory
Using Langmuir's theory of the arrangement of electrons in atoms, J.Am.Chem.Soc., 41, 868 (1919), Charles R. Bury formulated a Periodic Arrangement: C.R. Bury, Langmuir's theory of the arrangement of electrons in atoms and molecules, J. Am. Chem. Soc., 43, 1602-1609 (1921).
This formulation seems to be the basis of Seaborg's formulations of 1939, 1942 & 1945.
Ricardo R Contreras, Avances en Química, 14(1), 41-60 (2019), has re-drawn the Bury PT and writes [Google Translate]:
"This version emphasizes periods and electronic configurations.
"There is a long period in which the metals of titanium to copper are found, which he calls transition elements. [This formulation] leaves spaces for the element atomic number 43, technetium, discovered by Perrier Segre in 1937; for the element 72, hafnium, discovered in 1932 by D. Coster and G. von Hevesey; for the element 87, the eka-cesium, which corresponds to francium (Fr), discovered in 1939 by the French physicist Marguerite C. Perey (1909-1975) and, at the end of the group of halogens, for the element 85, the astatine (At), synthesized for the first time in 1940 by American physicists Dale R. Corson (1914-2012), Kenneth R. MacKenzie (1912-2002) and the Italian-American physicist Emilio G. Segrè (1905-1989) at the University of Berkeley (California), bombarding bismuth with particles.
"Bury uses 'A' as the symbol argon, 'Nt' (niton) for radon (Rn) and, the symbol 'Bv' (brevium) for proctactinium (Pa)."
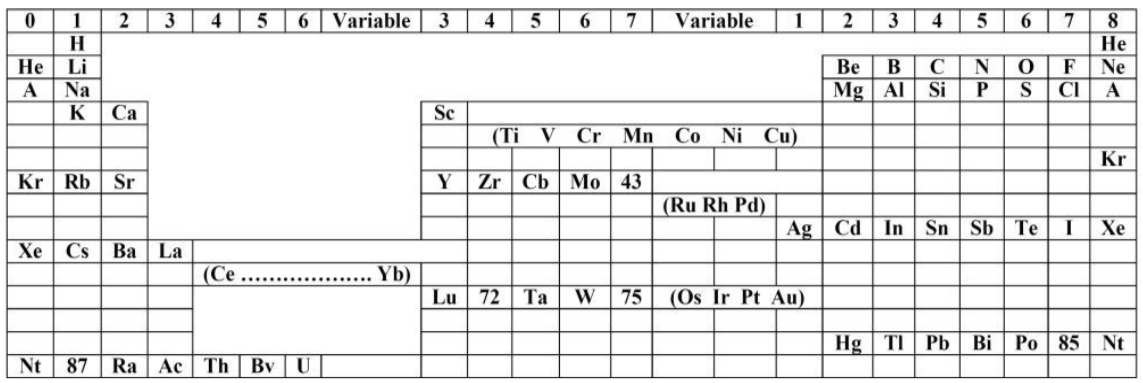
| Year: 1923 | PT id = 1198, Type = formulation |
Fajans' Periodic Table
Fajans K., Radioactivity and the latest developments in the study of the chemical elements, trans. TS Wheeler, WG King, 4th German edition, Methuen & Co., London, pp. 116-117, 1923.
René Vernon writes: "An addition to the long list of tables with B-Al over Sc."
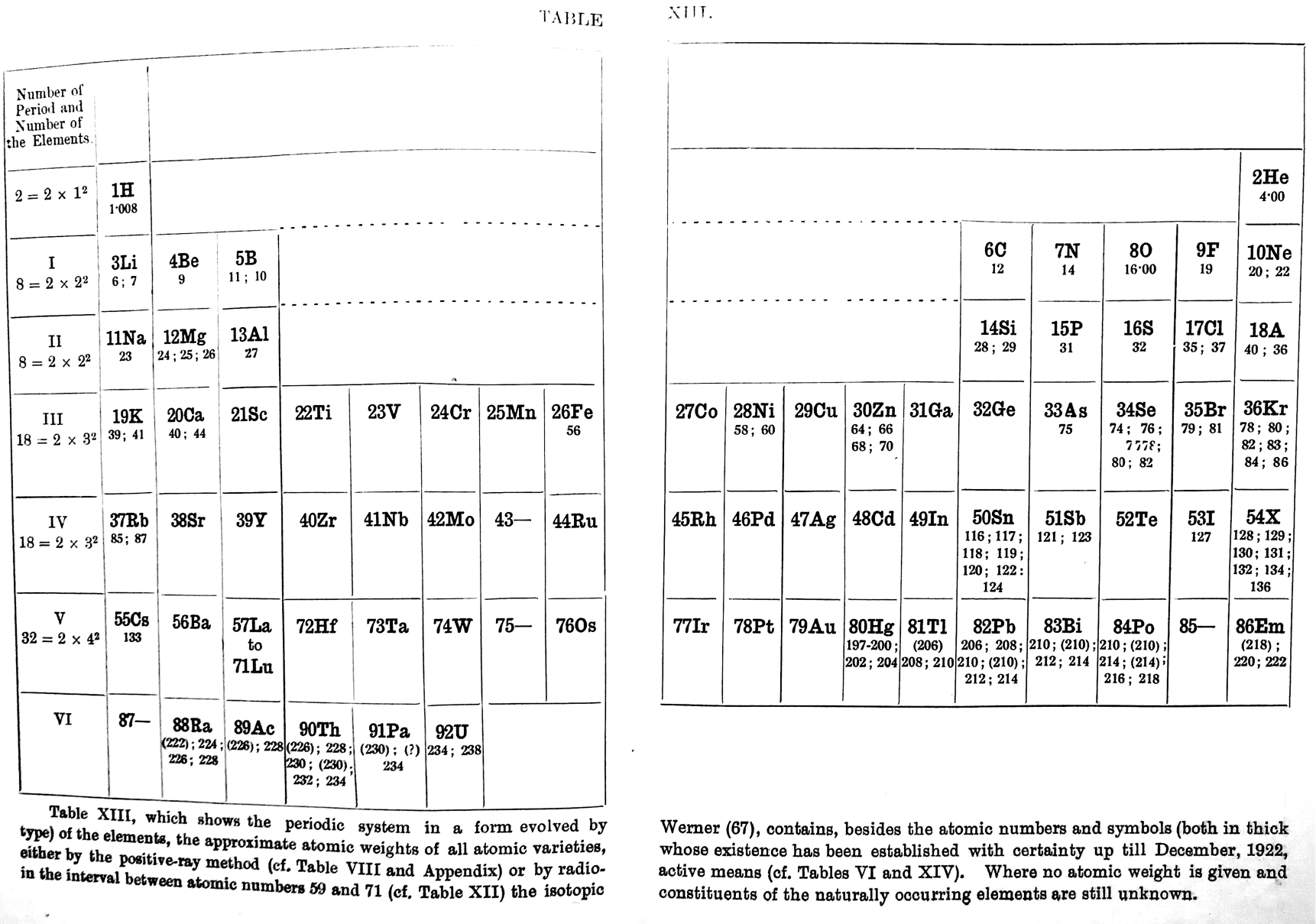
| Year: 1923 | PT id = 1256, Type = formulation review |
Deming's Periodic Table With Commentry by Vernon
René Vernon writes:
Deming's 1923 periodic table is credited with popularizing the 18-column form.
I now see Deming used different thickness sloping lines to represent the different degrees of similarity between the main groups and their corresponding transition metal groups.
- The line between Li-Na and group 11 is dashed, denoting the weakest relationship.
- Be-Mg are in group 2 The line between Be-Mg and group 12 is not dashed, denoting a stronger relationship.
- B-Al are in group 3
- The line between B-Al and Ga-In-Tl is thicker yet.
When I plot up to 20 chemical properties v Z going down these options I get the following values for the average smoothness of the trendlines:
- 73.5% for Li-Na-Cu(+2)-Ag(+1)-Au(+3) versus 84% for Li-Na-K-Rb-Cs
- 70% Be-Mg over Zn versus 85% for Be-Mg-Ca-Sr-Ba
- 81% for B-Al-Ga-In-Tl versus 88% B-Al-Sc-Y-La
I would have thought the smoothness for the line between Li-Na and Cu would be < 70%, consistent with Deming’s dashed line. But the thickness of the line would depend on what Deming took into account when he drew it. The common wisdom about groups 1 and 11 is that their similarities are: "confined almost entirely to the stoichiometries (as distinct from the chemical properties) of the compounds in the +1 oxidation state." (Greenwood & Earnshaw 2002, p. 1177). Kneen et al. (1972, p. 521) say that, "the differences between the properties of the group IA and IB elements are those between a strongly and weakly electropositive metal." On this basis I follow Deming’s dashed line. I’ve appended some notes about Group 1 and Group 11.
- Main group 4 is C-Si-Ge-Sn-Pb
- The line between Si and Ti-Zr-Hf is thick
- The line between N-P and V is less thick
- The line between O-S and Cr is less thick again
- The line between F-Cl and Mn is dashed
I have [calculated] a smoothness for C-Si-Ti-Zr-Hf of 86% versus 70% for C-Si-Ge-Sn-Pb. Since Ti shows some transition metal chemistry but not C-Si, it is perhaps plausible to keep C-Si-Ge-Sn-Pb together (as Deming did ).
Deming was a smart author. Nigh on a century later and the metrics check out.
More about group 1 and group 11
There may be a little more to the relationship between Li-Na & Cu-Ag-Au, than is ordinarily appreciated. For example:
- The resulting composite "group" has two electropositive metals and three more electronegative metals so its overall nature is more nuanced then purely group 1 or purely group 11
- The ionic radii of Li+ and Cu+ are 0.76 and 0.77 Å, and there is at least some discussion in the literature about substitution phenomena (Vasilev et al. 2019, p. 2-15; Udaya et al. 2020, p. 98; Kubenova 2021 et al.)
- Group 1 and 11 metal atoms form clusters relatively easily including Au_42+, Ag_64+, Rb_75+, Na_43+ (Mile et al. 1991, p. 134; Wulfsberg 2000, p. 631).
- In an organometallic context, Schade & Scheyler (1988, p. 196) wrote that, "There is much evidence that differences between group 1 and group 11 metals are not of principal but rather gradual manner."
- Although most nonmagnetic metals exhibit superconductivity it is significant that the Group 1 and 11 metals do not become superconducting at very low temperatures (Rao & Gopalakrishnan 1997, p. 398).
- Gold forms intermetallic compounds with all alkali metals (Schwerdtfeger et al. 1989. p. 1769)
References
- Greenwood NN & Earnshaw A 2002, Chemistry of the Elements, 2nd ed., Butterworth Heinemann, Oxford
- Kubenova et al. 2021, "Some thermoelectric phenomena in copper chalcogenides replaced by lithium and sodium alkaline metals", Nanomaterials 2021, vol. 11, no. 9. article 2238, https://doi.org/10.3390/nano11092238
- Mile et al. 1991, "Matrix-isolation studies of the structures and reactions of small metal particles", Farady Discussions, vol. 92, pp. 129–145 (134), https://doi.org/10.1039/FD9919200129
- Rao CNR & Gopalakrishnan J 1997, New Directions on Solid State Chemistry, 2nd ed., Cambridge University Press, Cambridge
- Schade C & Schleyer PVR 1988, "Sodium, potassium, rubidium, and cesium: X-Ray structural analysis of their organic compounds", Advances in Organometallic Chemistry, vol. 27, Stone FGA & West R (eds), Academic Press, San Diego, pp. 169–278
- Schwerdtfeger et al. 1989, "Relativistic effects in gold chemistry. I. Diatomic gold compounds.", The Journal of Chemical Physics, vol. 91, no. 3, pp. 1762–1774. https://doi.org/10.1063/1.457082
- Udaya et al. 2020, Metal sulphides for lithium-ion batteries, in Inamuddin, Ahmer & Asiri (eds), Lithium-ion batteries: Materials and applications, Materials Research Forum, Millersville PA, pp. 91–122
- Vasiliev AN et al. 2019, Low-dimensional Magnetism, CRC Press, Boca Raton
- Wulfsberg 2000, Inorganic chemistry, University Science Books, Sausalito, CA
| Year: 1924 | PT id = 31, Type = formulation |
Hubbard Periodic Chart Of The Atoms
The American classic Henry Hubbard Periodic Chart Of The Atoms went through 12 editions.
A 1924 original on a dining room wall:

The current Sargent Welch version of the Henry Hubbard Periodic Table:
| Year: 1924 | PT id = 1372, Type = structure |
de Broglie and Wave–Particle Duality
de Broglie, L. Recherches sur la théorie des quanta. Annales de Physique, 3, 22–128 (1925). (Doctoral thesis, submitted 1924)
"Louis Victor Pierre Raymond, 7th Duc de Broglie was a French theoretical physicist and aristocrat known for his contributions to quantum theory. In his 1924 Ph.D. thesis, de Broglie postulated the wave nature of electrons and suggested that all matter has wave properties.
"This concept, now known as the de Broglie hypothesis, an example of wave–particle duality, and forms a central part of the theory of quantum mechanics. In 1929, de Broglie won the Nobel Prize in Physics, after the wave-like behaviour of matter was experimentally confirmed in 1927."

| Year: 1925 | PT id = 1035, Type = formulation 3D |
Model of the Periodic System of de Chancourtois
From the Science Museum in the UK collection, a model of the Periodic System of de Chancourtois from 1862:
"Model demonstrating the telluric screw periodic system of Alexander-Emile Beguyer de Chancourtois proposed in a paper published in 1862.
"This model, made by the Science Museum in 1925, provides a rare physical realisation of arguably the earliest periodic system of for the elements. It was devised by the French geologist, Alexander-Emile Beguyer de Chancourtois in 1862, 7 years prior to Dmitri Mendeleev's periodic table.
"De Chancourtois arranged the elements in the order of their atomic weights along a helix which was traced on the surface of a vertical cylinder, with an angle of 45 degrees to its axis. The base of the cylinder was divided into 16 equal parts (the atomic weight of oxygen), and the lengths of the spiral corresponding to the weights of the elements were found by taking the one-sixteenth part of a complete turn as a unit":

| Year: 1925 | PT id = 1373, Type = structure |
Pauli and The Exclusion Principle
Pauli, W. Über den Zusammenhang des Abschlusses der Elektronengruppen im Atom mit der Komplexstruktur der Spektren. Zeitschrift für Physik, 31, 765–783 (1925).
"Wolfgang Pauli was an Austrian–Swiss theoretical physicist and a pioneer of quantum mechanics. In 1945, after having been nominated by Albert Einstein, Pauli received the Nobel Prize in Physics 'for the discovery of the Exclusion Principle, also called the Pauli Principle'. The discovery involved spin theory.
"The Pauli exclusion principle (German: Pauli-Ausschlussprinzip) states that two or more identical particles with half-integer spins (i.e. fermions) cannot simultaneously occupy the same quantum state within a system that obeys the laws of quantum mechanics. This principle was formulated by Austrian physicist Wolfgang Pauli in 1925 for electrons, and later extended to all fermions with his spin–statistics theorem of 1940.
"In the case of electrons in atoms, the exclusion principle can be stated as follows: in a poly-electron atom it is impossible for any two electrons to have the same two values of all four of their quantum numbers, which are: n, the principal quantum number; ℓ, the azimuthal quantum number; mℓ, the magnetic quantum number; and ms, the spin quantum number.
"If two electrons reside in the same orbital, then their values of n, ℓ, and mℓ are equal. In that case, the two values of ms (spin) pair must be different. Since the only two possible values for the spin projection ms are +1/2 and –1/2, it follows that one electron must have ms = +1/2 and one ms = –1/2.
"To preserve the conservation of energy in beta decay, Pauli proposed the existence of a small neutral particle, dubbed the neutrino by Enrico Fermi, in 1930. Neutrinos were first detected in 1956. "
| Year: 1925 | PT id = 926, Type = formulation |
Sommerfeld's Electon Filling Diagram
Arnold Sommerfeld diagram appears in an issue of Memoirs and Proceedings of the manchester Literary and Philosophical Society for 1925-26. volume 70, p. 141-151.
Eric Scerri writes:
"The electron groupings are not exactly the same as what is believed to exist today but it amounts to the same order of filling. For example p orbitals were thought to consist of two groups of 2 and 4 electrons, rather than 2, 2, 2 as believed today. Similarly d orbitals were thought to be formed of two groups of 4 and 6 electrons. With that in mind you will see that Sommerfeld was the first to propose an aufbau filling system: The occupation of 4s before 3d or as represented here the 2 electrons in orbit 11 followed by the 4 and 6 from orbits 3,s and 3,3.
"Sommerfeld does indicate sub-shells. They are just not the same groupings as the current ones. For example 2,1 and 2,2 indicates subshells within the 2nd main shell. Similarly the 3rd shell is presented as 3,2 and 3,3. The totals are of course the same, namely 6 for what we now call p orbitals and 10 for what we call d orbitals. All this came before the discovery of the 4th or spin quantum number. This is in keeping with Bohr's original assignment of shells and sub-shells.
"The discovery of sub-structure to electron shells was not an 'all or nothing' development, but a gradual and almost organic evolution."
Eric has a new book out – A Tale of Seven Scientists and a New Philosophy of Science – in which the gradual evolution of electronic structure involving Bohr, Sommerfeld, Bury, Main Smith, Pauli and others is traced out.
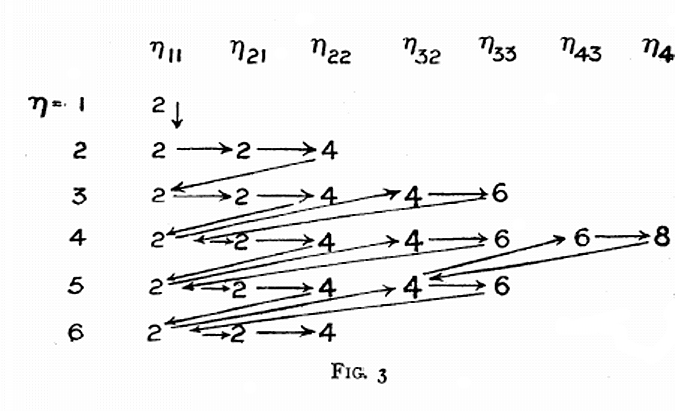
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1925 | PT id = 735, Type = formulation 3D spiral |
Courtines' Model of the Periodic Table or Periodic Classification
Published in J. Chem. Ed., 2, 2, 107-109 in 1925 by M. Courtines of the Laboratory of Experimental Physics, College of France, Paris.
Q&Q write:
"The unfolded tower arrangement appears much like a modernised Chauvierre chart cut on a line between Ni and Cu, Cu, with the right part fitted to the left in order of increasing atomic numbers. The rare-earth elements, however, are placed on a novel accordion-like folded strip with ends made secure just below Xt and between Ba and Hf. The author describes in detail the method of folding the chart into a tower-like cylindrical model. H is folded back to show its lack of relationship other groups of elements. In the space for each symbol, electron arrangements and isotopes are also enumerated."
And, in what appears to be a 'top down' view of the above 3D formulation, Courtine M 1926, Oùen est la physique, Gauthier-Villars et Cie, Paris:
Thanks to Eric Scerri for the tip & René Vernon's additions!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1926 | PT id = 1375, Type = structure |
Schrödinger Wave Equation
Schrödinger, E. Quantisierung als Eigenwertproblem (Quantization as an eigenvalue problem) (Parts I–IV). Annalen der Physik, 79, 361–376; 489–527; 734–756; 80, 437–490 (1926).
"Erwin Schrödinger was an Austrian–Irish theoretical physicist who developed fundamental results in quantum theory. In particular, he is recognised for devising the Schrödinger equation, an equation that provides a way to calculate the wave function of a system and how it changes dynamically in time."
"A special case of the Schrödinger equation is the position-space Schrödinger equation for a single nonrelativistic particle in one dimension:
"The ψ is a wave function, a function that assigns a complex number to each point x at each time t. The parameter m is the mass of the particle, and V(x,t) is the potential energy function that represents the environment in which the particle exists. The constant i is the imaginary unit, and ħ is the reduced Planck constant, which has units of action (energy multiplied by time).
"Schrödinger coined the term 'quantum entanglement' in 1935. Schrödinger shared the 1933 Nobel Prize in Physics with Paul Dirac 'for the discovery of new productive forms of atomic theory.''
"The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. Conceptually, the Schrödinger equation is the quantum counterpart of Newton's second law in classical mechanics. Given a set of known initial conditions, Newton's second law makes a mathematical prediction as to what path a given physical system will take over time. The Schrödinger equation gives the evolution over time of the wave function, the quantum-mechanical characterisation of an isolated physical system. The equation was postulated by Schrödinger based on a postulate of Louis de Broglie that all matter has an associated matter wave. The equation predicted bound states of the atom in agreement with experimental observations."
Three videos on the Schrödinger Wave Equation. (The middle one is very detailed.):

| Year: 1926 | PT id = 1156, Type = formulation |
Friend's Periodic Table (1926)
Vallance RH & Eldridge AA, A Text-Book of Inorganic Chemistry, Vol. VII, Part III, Chromium and its Congeners, JN Friend (ed.) Charles Griffin & Company, London (1926), front paper.
René Vernon (who found this formulation) writes:
"I can't recall seeing a table in which the lanthanoids were allocated in quite such a manner: across seven groups. And, 16 such lanthanoids shown. Even curiouser, Argon = A; xenon = X; are shown in group 0. Wonderful nomenclature from nearly a century ago."
| Year: 1927 | PT id = 1377, Type = structure |
Fifth Solvay Conference on Physics
"The most famous conference was the fifth Solvay Conference on Physics, which was held from 24 to 29 October 1927. The subject was Electrons and Photons and the world's most notable physicists met to discuss the newly formulated quantum theory. The leading figures were Albert Einstein and Niels Bohr. Seventeen of the 29 attendees were or became Nobel Prize winners, including Marie Skłodowska-Curie who, alone among them, had won Nobel Prizes in two separate scientific disciplines. The anti-German prejudice that had prevented Einstein and others from attending the Solvay conferences held after the First World War had melted away. Essentially all of those names who had contributed to the recent development of the quantum theory were at this Solvay Conference, including Bohr, Born, de Broglie, Dirac, Heisenberg, Pauli, Planck, Lorentz, Compton, Ehrenfest, and Schrödinger. Heisenberg commented:
"Through the possibility of exchange between the representatives of different lines of research, this conference has contributed extraordinarily to the clarification of the physical foundations of the quantum theory. It forms, so to speak, the outward completion of the quantum theory."
"The photo taken of this conference's participants is sometimes entitled 'The Most Intelligent Photo Ever Taken', for its depiction of the world's leading physicists gathered together in one shot."
A. Piccard, E. Henriot, P. Ehrenfest, E. Herzen, Th. De Donder, E. Schrödinger, J.E. Verschaffelt, W. Pauli, W. Heisenberg, R.H. Fowler, L. Brillouin;
P. Debye, M. Knudsen, W.L. Bragg, H.A. Kramers, P.A.M. Dirac, A.H. Compton, L. de Broglie, M. Born, N. Bohr;
I. Langmuir, M. Planck, M. Skłodowska-Curie, H.A. Lorentz, A. Einstein, P. Langevin, Ch. E. Guye, C.T.R. Wilson, O.W. Richardson
Fifth conference participants, 1927. Institut International de Physique Solvay in Leopold Park.
| Year: 1927 | PT id = 1015, Type = formulation |
LeRoy's Periodic Table
R.H. LeRoy, Teaching the Periodic Classification of Elements, School Science and Mathematics 1927, 27: 793-799. This formulation thulium in group IC and has the actinides in the C groups, analogous to the lanthanides, two decades before Seaborg.
René adds:
"This 1927 formulation has several remarkable features.
"The lighter and heavier lanthanides and actinides are shown in numbered C groups i.e. C4, C5, C6, C7 and C1, C2, and C3. The 14 remaining elements between C7 and C1 are labelled as transition elements, analogous to the old chemistry notion of the ferromagnetic and platinum metals in IUPAC groups 9 to 11 being labelled as transition elements. There is no known Tm(I) although this would not be inconceivable. Nd is in group C6, which doesn't quite work since there is no Nd(VI) although such an oxidation state is not inconceivable given the existence of Pr(V). in group C7, Pm(VII) is not known. For the actinides, Md(I) has been reported but not confirmed.
"B-Al-Sc-Y-La-Ac are shown as main group metals; that would be consistent with their chemistry. While Sc-Y-La-Ac are routinely classified as transition metals their chemistry is largely that which would be expected of main group metals following the alkaline earths in IUPAC group 2.
"The author refers to the noble gases as 'transitional'. The noble gases bridge the most reactive groups of elements in the periodic table – the alkali metals in group I and the halogens in group VII. That's a concept that's rarely referred to these days even though it's still quite valid.
"Ga-In-Tl are shown as B3 metals, falling just after Zn-Cd-Hg in group B2, and Cu-Ag-Au in group B1. That doesn't work for Ga etc, which are nowadays regarded as main group metals.
"H is shown floating above the A elements, and in the transitional zone, with links to F and to Li."
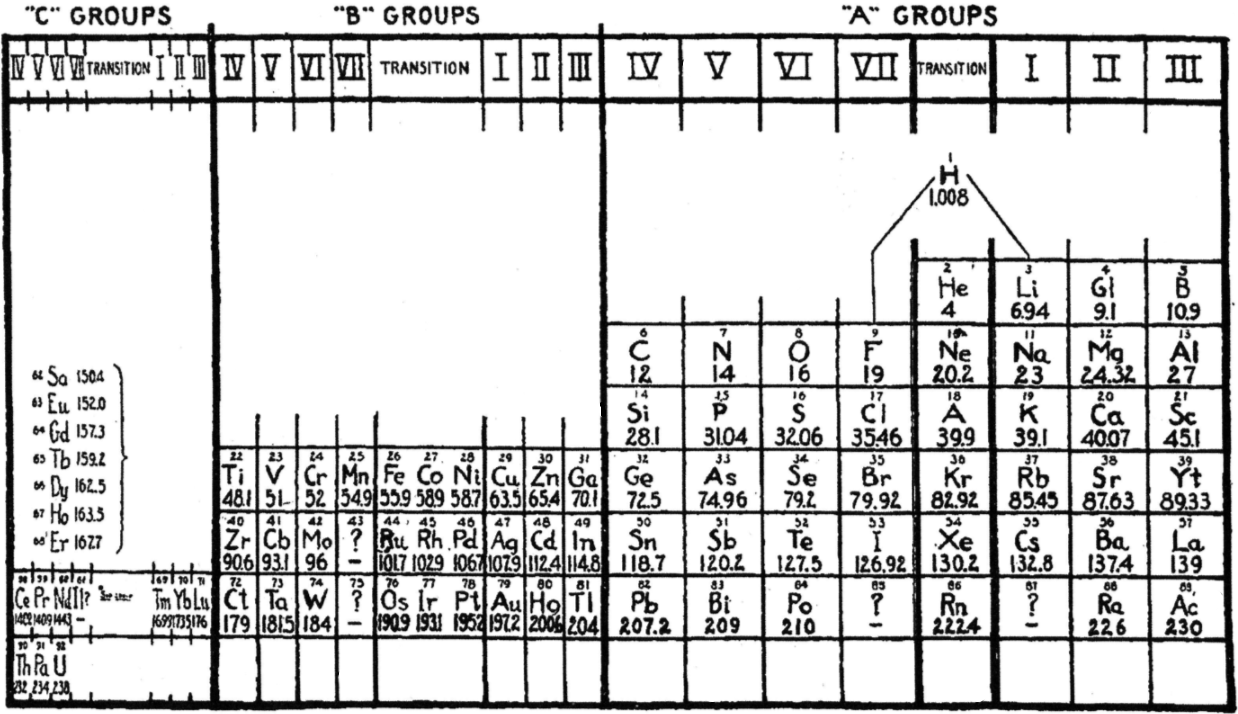
Thanks to John Marks for the tip, and to René for the comments/analysis!
| Year: 1930 | PT id = 154, Type = formulation spiral |
Janet's Shell Filling Diagram
Janet produced six papers, in French, which are almost unobtainable as he had them privately printed and didn't distribute them properly. The shell-filling diagram dated from November 1930, six years before Madelung. Note that Janet uses Bohr's radial quantum number, k, which is l+1. In the text he formulates the n+k-1 rule. Information supplied by Philip Stewart.
| Year: 1930 | PT id = 1264, Type = formulation |
Gardner's Table of Electronic Configurations of the Elements
A table of electronic configurations of the elements. Nature 125, 146 (1930). https://doi.org/10.1038/125146a0
Abstract:
"MR. ROY GARDNER gave an interesting paper on A Method of Setting out the Classification of the Elements at a recent meeting of the New Zealand Institute. The paper included the accompanying Table, which shows the distribution of electrons into groups corresponding to the principal quantum numbers for all the elements and at the same time preserves the most essential features of the two-dimensional arrangement of Mendeleef. Elements having the same complete groups (that is, all stable groups of 8 or 18) are placed in the same horizontal row, and the vertical columns include elements with the same number of electrons in the incomplete outer groups. The electronic configurations are those given by Sidgwick ("Electronic Theory of Valency", 1927). An asterisk marks elements for which the 'normal' atom is thought to have only one electron in the outermost group, but as practically all these give divalent ions, the point is of minor interest chemically. Distribution of electrons into k-subgroups is unnecessary; these have at present little significance for chemical purposes, and in any case the subgroups are considered to be filled in order to the maxima 2, 6, and 10."
René Vernon writes:
In this table Gardner emphasises the existence of four types of elements:
- those with all "groups" complete
- those with one incomplete group
- those with two incomplete groups (transition elements)
- those with three incomplete groups (rare earth elements)
The upper limits of existence of covalencies of 8, 6, and 4 are marked by heavy horizontal lines.
Note:
- there are nine groups of d-block elements [as we would now call them], and but 13 f-block elements
- La and Lu are treated as d-block elements
- while Yb is counted as an f-block element it was later realised (1937) that the 4f shell is full at Yb, hence it is not clear where Gardner would have placed it (Yb)—seemingly in the 0 column
| Year: 1931 | PT id = 1017, Type = formulation |
LeRoy's Periodic Table
R.H. LeRoy, Teaching the Periodic Classification of Elements, School Science and Mathematics 1927, 27: 793-799. This formulation thulium in group IC and has the actinides in the C groups, analogous to the lanthanides, two decades before Seaborg.
René adds:
"This 1927 formulation has several remarkable features.
"The lighter and heavier lanthanides and actinides are shown in numbered C groups i.e. C4, C5, C6, C7 and C1, C2, and C3. The 14 remaining elements between C7 and C1 are labelled as transition elements, analogous to the old chemistry notion of the ferromagnetic and platinum metals in IUPAC groups 9 to 11 being labelled as transition elements. There is no known Tm(I) although this would not be inconceivable. Nd is in group C6, which doesn't quite work since there is no Nd(VI) although such an oxidation state is not inconceivable given the existence of Pr(V). in group C7, Pm(VII) is not known. For the actinides, Md(I) has been reported but not confirmed.
"B-Al-Sc-Y-La-Ac are shown as main group metals; that would be consistent with their chemistry. While Sc-Y-La-Ac are routinely classified as transition metals their chemistry is largely that which would be expected of main group metals following the alkaline earths in IUPAC group 2.
"The author refers to the noble gases as 'transitional'. The noble gases bridge the most reactive groups of elements in the periodic table – the alkali metals in group I and the halogens in group VII. That's a concept that's rarely referred to these days even though it's still quite valid.
Ga-In-Tl are shown as B3 metals, falling just after Zn-Cd-Hg in group B2, and Cu-Ag-Au in group B1. That doesn't work for Ga etc, which are nowadays regarded as main group metals.
"H is shown floating above the A elements, and in the transitional zone, with links to F and to Li."

Thanks to John Marks for the tip, and to René for the comments/analysis!
| Year: 1932 | PT id = 1051, Type = formulation |
Bacher & Goudsmith's Periodic System and Index
R.F. Bacher RF and S.A. Goudsmith, Atomic Energy States, McGraw-Hill, New York, p. xiii. 1932:
Thanks to René for the tip!
| Year: 1932 | PT id = 1211, Type = formulation |
Bejerrum's Periodic Table
Bjerrum N, Inorganic chemistry, trans. (1936) from the 3rd Danish edition (1932) by N Bjerrum and RP Bell, William Heinemann, London
René Vernon observes:
- There are split blocks everywhere in Bjerrum's periodic system: s once; f once; d twice; p twice.
- As per old chemistry: B and Al are over Sc
- The group numbering is interesting: eight groups and eight sub-groups
- Bjerrum says the metals fall naturally into two groups: the light metals with a density below 4 gm/cm^3; the heavy metals with a density above 7 gm/cm^3, many of which form coloured salts
- Bjerrum refers to the transition metals as being those in subgroups 8a, 8b and 8c
| Year: 1934 | PT id = 435, Type = formulation |
Brazilian Version of The Hubbard Periodic Chart Of The Atoms
A Brazilian Version of the American classic Henry Hubbard Periodic Chart Of The Atoms from a lecture theater in Rio, rediscovered by Martyn Poliakoff of PeriodicVideos.com and The University of Nottingham. From the early 1930s:

- Ma – Masurium (43) Disputed claim to discovery of technetium.
- Cb – Columbium (41) Former name of niobium
- Ab – Alabamine (85) Discredited claim to discovery of astatine.
- Il – Illinium (61) Discredited also
- Sa – Samarium (62) Current symbol is Sm
- Sp – Spectrium (70) Suggested name for ytterbium
- Cb – Columbium (41) Former name of niobium (also called Pelopium)
The current Sargent Welch version of the Henry Hubbard Periodic Table:
| Year: 1935 | PT id = 1011, Type = formulation |
Rysselberghe's Periodic Table
Pierre Van Rysselberghe J. Chem. Educ. vol. 12, no. 10, pp. 474—475 1935.
The author writes:
"The usual relationships between analogous elements are preserved and are in fact emphasized by this new arrangement. The only missing regularity is the natural succession of atomic, numbers, but all periodic classifications have to sacrifice it on account of the rare earths. Moreover, it can easily be restored by reading the horizontal lines n the order indicated by the numbers written on the left of the heavy frame line. Each horizontal line is limited by the frame of the table. For instance, K and Ca on the one hand, Cu and Zn on the other hand, form two distinct horizontal lines, as shown by the different numbers given to these groups. They are at the same level because the valence electrons have the same quantum numbers."
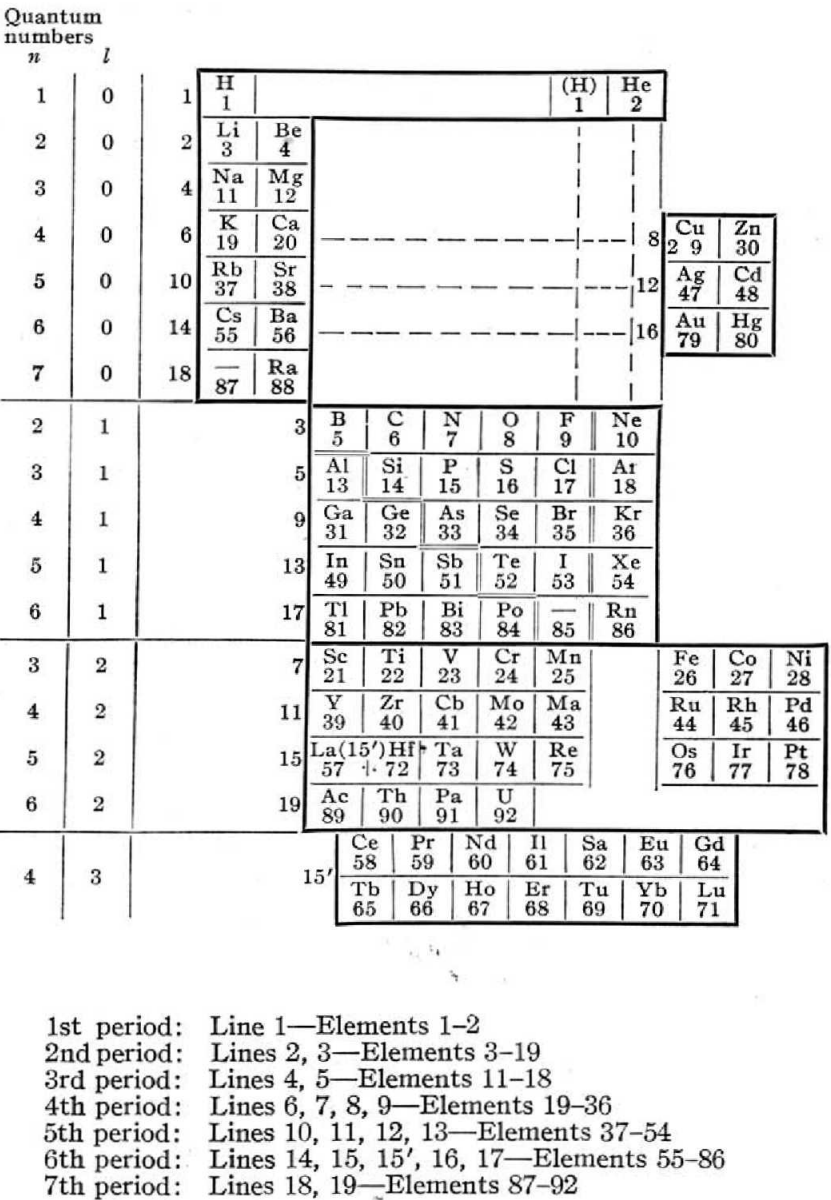
Thanks to René for the tip!
| Year: 1936 | PT id = 777, Type = formulation data misc |
Orbital Filling With Electrons
Students of chemistry are often confused why the orbitals fill with electrons: 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p6... etc., because the 3d10 seems to be 'out of sequence'.
This 'out of sequence' difficulity is nicely explained if the orbitals are arranged in a slightly different way:
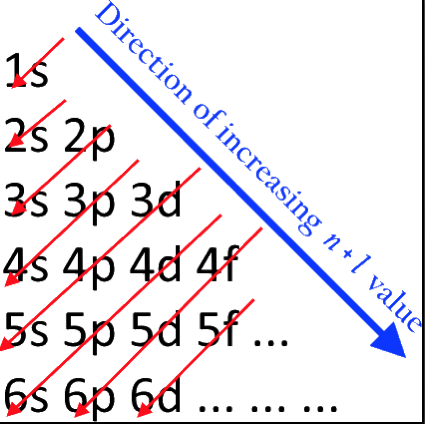
The aufbau principle states that in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy levels before occupying higher levels. For example, the 1s shell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible.
The order in which these orbitals are filled is given by the n + ![]() rule, also known as the Madelung rule (after Erwin Madelung), the Janet rule or the diagonal rule.
rule, also known as the Madelung rule (after Erwin Madelung), the Janet rule or the diagonal rule.
Orbitals with a lower n + ![]() value are filled before those with higher n +
value are filled before those with higher n + ![]() values. In this context, n represents the principal quantum number and ? the azimuthal quantum number. The values
values. In this context, n represents the principal quantum number and ? the azimuthal quantum number. The values ![]() = 0, 1, 2, 3 correspond to the s, p, d and f orbital lables.
= 0, 1, 2, 3 correspond to the s, p, d and f orbital lables.
Julio Gutiérrez Samanez writes:
"I send you the diagram below that reconciles quantum mechanics (diagram for filling the electronic cells) with the Janet table or LSPT. Explaining the duplication of periods with the duplication of the quantum number n, and the introduction of Tao (T) spin of the level or spin of the period, which explains the parity of the symmetric periods."
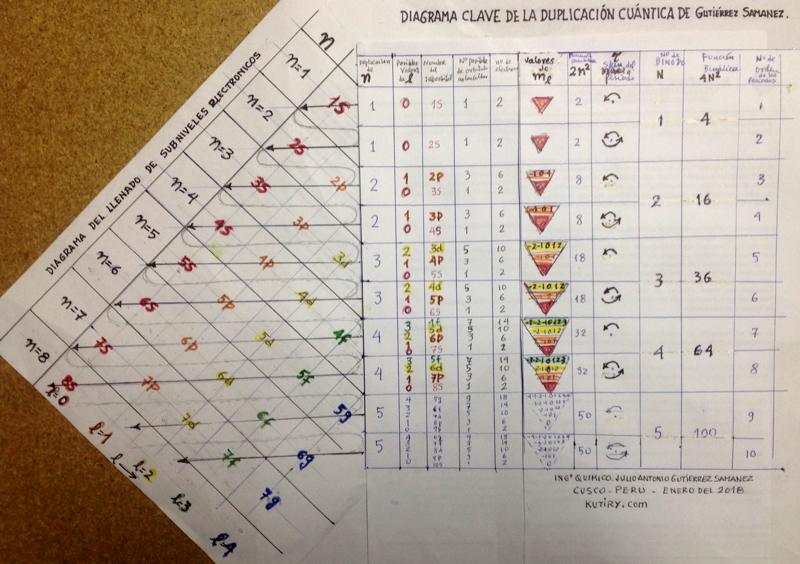
| Year: 1936 | PT id = 1290, Type = formulation |
Van Wert Periodic table (after Guertler-Leitgebel)
Van Wert LR, An Introduction to Physical Metallurgy, McGraw-Hill, New York, 1936, pp 17. Van Wert says the periodic table is after "Guertler-Leitgebel", which is presumably Guertler WM & Leitgebel M 1929, Vom Erz zum metallischen Werkstoff: Leitlinien und Rüstzeug der metallurgischen und metallkundlichen Wissensgebiete, Akademische Verlagsgesellschaft, m.b.H., Leipzig
From René Vernon who writes:
In this almost symmetrical presentation, Van Wert divides the periodic table metals into:
Strongly Electropositive: Groups 1 to 3, Ln
High-melting Heavy Metals: Transition metals
Low-melting Heavy Metals: Post-transition metalsIf the 15 Rare Earths had been shown as 14, and moved one cell to the left we would have a perfectly symmetrical table.
Elsewhere (p. 38) Van Wert refers to the noble metals as follows:
"With respect to corrosion, the noble metals — gold, the platinum metals, and to a less degree, silver — are in a class by themselves. They are comparatively chemically inert to all common corrodents; only silver is appreciably attacked by sulphur gas."
Van Wert's table also refers to non-metals and to inert gases. On page 7 mention is made of the metalloids:
"There are a few elements, also, that partake of the nature of both metals and nonmetals, under many—indeed, under most—conditions they seem metallic enough, but on occasion their behavior is decidedly nonmetallic. These metalloids, as they are sometimes called, add a further difficulty in the attempt to frame a satisfactory definition of the metallic state."
By 1936, it was known that metalloids had a predominately nonmetallic chemistry (Newth 1894, pp. 7??8; Friend 1914, p. 9). So, on the nonmetal side of house are metalloids; "nonmetals"; and noble gases. Separating out the halogens from the nonmetals yields: metalloids; "nonmetals"; halogens; noble gases.
The net result is four types of metals and four of nonmetals = more symmetry.
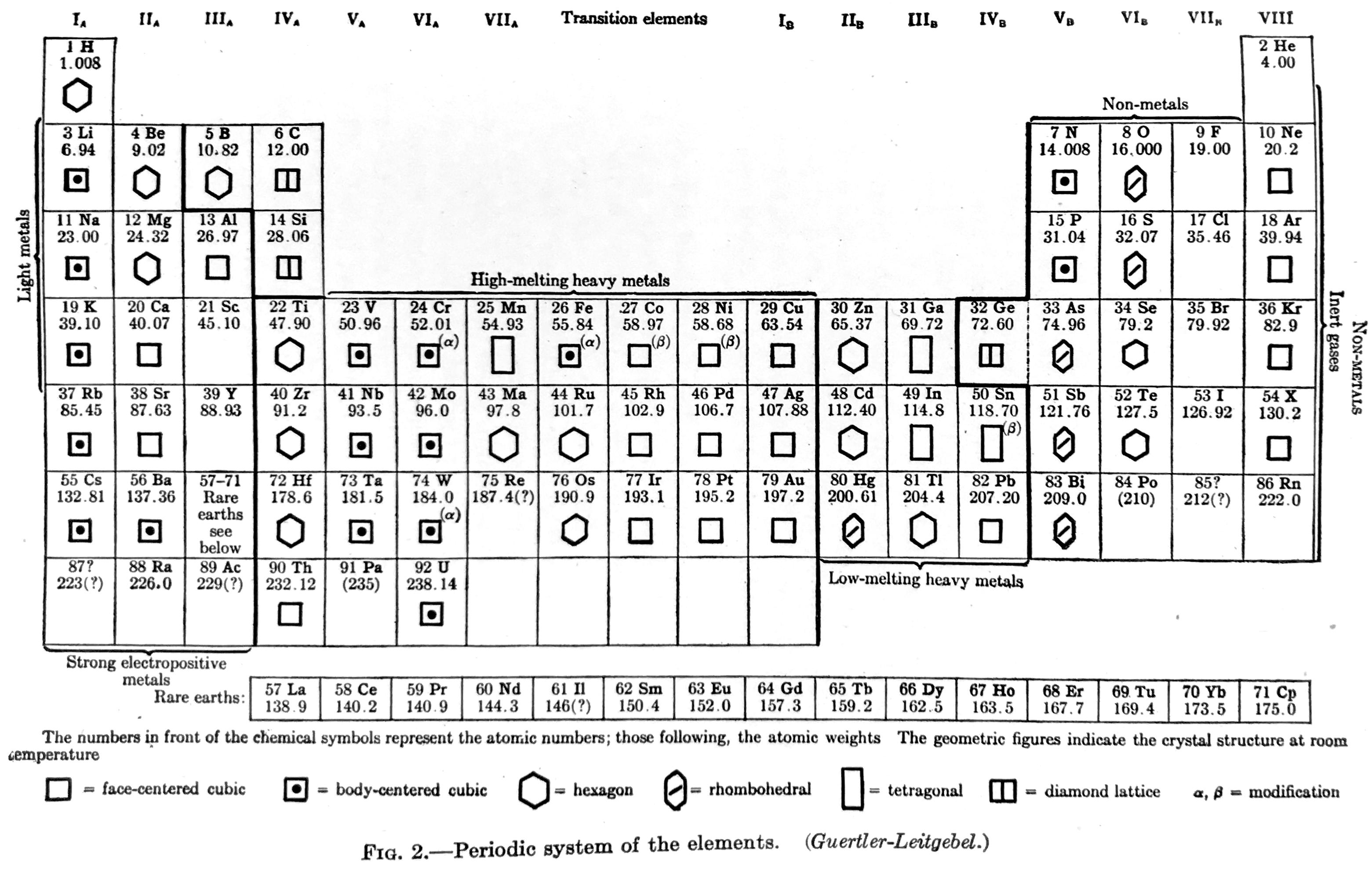
| Year: 1936 | PT id = 595, Type = formulation |
Nekrasov Periodic Table
From here, using Google Translate:
The shape of the table is presented by Bohr effect of considering the properties of the elements as simple substances and for reactions to occur with the intervention of such substances. But for the study of compounds and reactions that occur between them, the key factor is the electron configurations of atoms in states of valence to them on the given compounds.
It follows that a more complete picture of the periodic table would be when you take into account the peculiarities of atoms in both its neutral state and in all its particular valence states. This is the proposal of Boris Nekrasov, a member of the Academy of Sciences in Moscow.
Nekrasov distinguishes three types of analogies between elements Total analogs are those in which the analogy is shown in all its valence, all analogs compared to the valence valences except for the group corresponding to the number that can be called characteristic and analogous to the valence characteristic .
Thus, in the table shown here distinguish the elements entirely analogous joined by continuous lines, such as Na and K.
Those analogies in all except the characteristic valences joined by dotted lines. This is the case of Na and Cu in both cases if you lose an electron (valence feature) your setup is different. In the first case is 8 (1s2, 2P6) and the second 18 (3s2, 3p6, 3d10).
Lastly presenting exclusively analogies valence are connected with dashed lines. This is the case both S and Cr +6 elements have their valence electron configuration similar in the last layer 8 (2s2, 2p6) for the S and 8 (3s2, 3p6) for Cr.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1936 | PT id = 909, Type = formulation spiral |
Libedinski's Periodic Classification of The Elements
Simón Libedinski: PERIODIC CLASSIFICATION OF THE ELEMENTS, from his book: Dialectical Materialism, in Nature, in Society and in Medicine, Ediciones Ercilla, Santiago de Chile, 1938, pp 56-57:
"Mendeleev's Table, like that of Werner and others, are not, however, more than flat projections of the actual ordering of the elements. There is as much difference between Mendeleev's Table and the real group as there is between the planisphere and a rotating globe. A rational representation, starting from the simplest element – the negative electron –, would be a spiral line that, surrounding said central point, first gave a small turn, touching only two bodies: hydrogen and helium. From here it would jump to a much larger orbit, in which it would touch eight bodies and then another equal, also of eight. From here, another jump to a much larger orbit, comprising eighteen bodies, and then another equal; from this point one jumps to another orbit, again augmented, comprising thirty-two bodies (including rare earths); and when this round is over, the last one begins, to vanish a short distance.
"In the dialectical grouping of the elements, which I have the satisfaction of exposing, the classic arrangement of the same is respected. Only the arrangement changes, which instead of being rectilinear, is spiral. So I managed to suppress the anomaly of the double columns, and comfortably incorporate the important group of rare earths. I can not give my graphic the name of Tabla, because it is just the opposite: it aims to give the idea of ??space, and of movement in space. The double columns of the Classic Table can be found here as well, but only if you look through the whole, considered as a planetary system of conical shape, with the electron at the vertex. Effectively: column 1 coincides, through space, with column 1a; column 4 with column 4 bis, etc. The dialectical grouping also allows us to easily appreciate the remarkable dialectical character of the properties of matter: these properties are repeated periodically. These are the "returns" to qualities or previous properties, but not exactly equal to those, but only similar: and this resemblance, only to a certain extent. The difference is that that quality, those properties or some characteristic, are exalted to each dialectical return."
Contributed by Julio Antonio Gutiérrez Samanez, Cusco, Peru, March 2018 (using Google Translation)
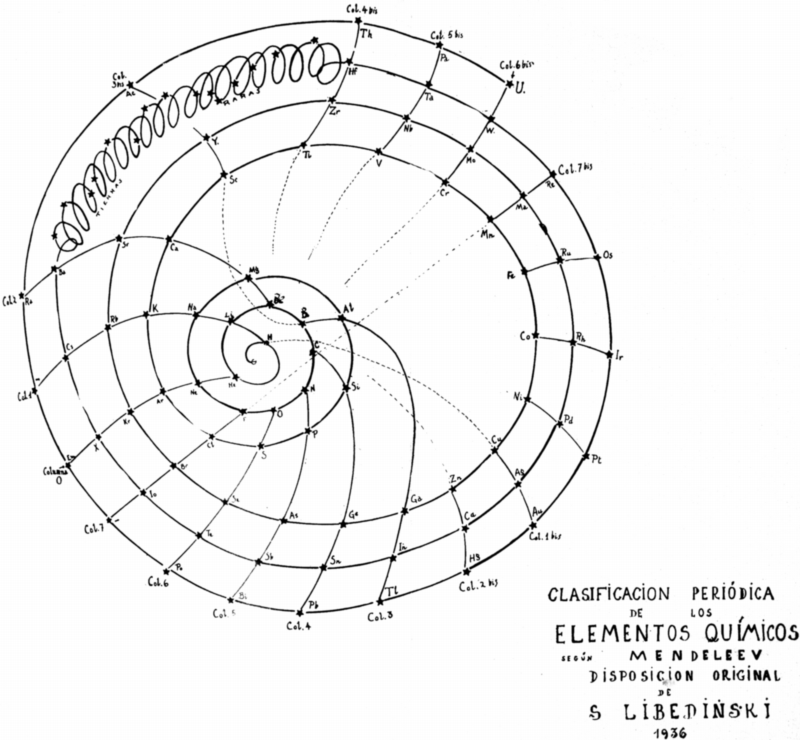
| Year: 1939 | PT id = 1056, Type = formulation |
Foster's Periodic Arrangement
L.S. Foster, "Why not modernise the textbooks also? I. The periodic table", Journal of Chemical Education, vol. 16, no. 9, pp. 409–412, https://pubs.acs.org/doi/10.1021/ed016p409
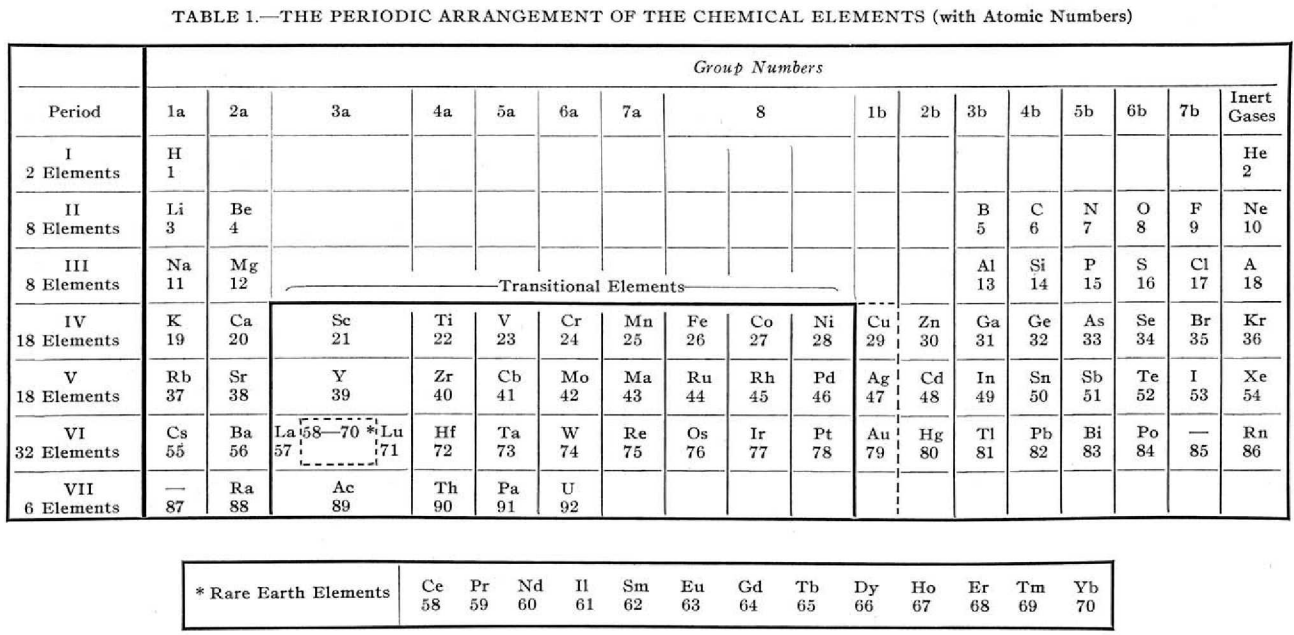
Foster writes:
"The [above] modern periodic table is simply an orderly array of the elements with all unnecessary ornamentation omitted, has been found highly satisfactory for instructional purposes.
"The transitional elements, with two unfilled electron shells, are separated from the non-metallic elements.
"The rare-earth elements, defined as those with three incomplete electron shells, are shown to be those of atomic numbers 58 to 70, while La and Lu, which have only two incomplete electron shells are classified as transitional elements.
"Copper, silver, and gold act as transitional elements except when the state of oxidation is one."
René Vernon writes:
Foster couldn't show the coinage metals – with their full d10 complements – as transitional elements, but by adding a broken line around them he was showing they had the capacity to act as if they were.
I tried to work out how he distinguished La & Lu from Ce to Yb. Foster seems to be saying that La 5d1 6s2, has incomplete 5th and 6th (ie. 6p) shells.
Same for Lu 4f14 5d1 6s2 having incomplete 5th and 6th shells. Whereas, for example, Ce 4f1 5d1 6s2 has incomplete 4th, 5th and 6th shells. Presumably this was in the years before the fact that the 4f shell became full at Yb was widely appreciated. So, strictly speaking, group 3a should have read:
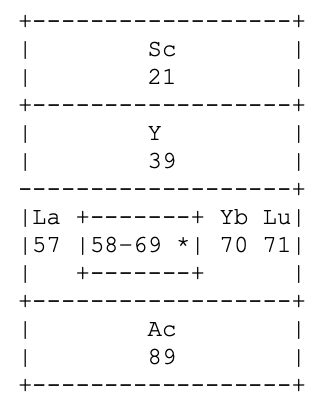
On the other hand, Yb3+ has an f13 configuration, so it does meet his three unfilled shells criterion. Had he known, he probably would've put a broken line around Yb to indicate its full f14 complement but that it normally acted as a rare earth, with an incomplete 4f shell; whereas neither La nor Lu have this capacity.
Good to see Foster put so much thought into organising his table, and his experience with using it for instructional purposes.
Van Spronsen does not mention Forster's table. Mazurs has a reference to Foster's table but lumps it in with the other medium-long tables, not appreciating its subtlety.
Mark Leach writes:
This formulation is very much like the XBL 769-10601, Periodic Table Before World War II used by Seaborg and the Manhattan project and is a precursor to the modern periodic table.
| Year: 1939 | PT id = 627, Type = formulation spiral |
Periodic Chart in Spiral Form
Periodic Chart in Spiral Form from K. Gordon Irwin, Periodicity Patterns of The Elements J.Chem.Ed. 1939 16 (7), 335 DOI: 10.1021/ed016p335
Thanks to René Vernon for the reference:

| Year: 1939 | PT id = 943, Type = formulation |
XBL 769-10601, Periodic Table Before World War II
An internal document of the Lawrence Berkeley Laboratory XBL 769-10601 shows a late 1930s (pre World War II) periodic table.
Note that this formulation erroneously predicts positions for transuranium elements:
- thorium is shown in the group below hafnium
- protactinium below tantalum
- uranium below tungsten
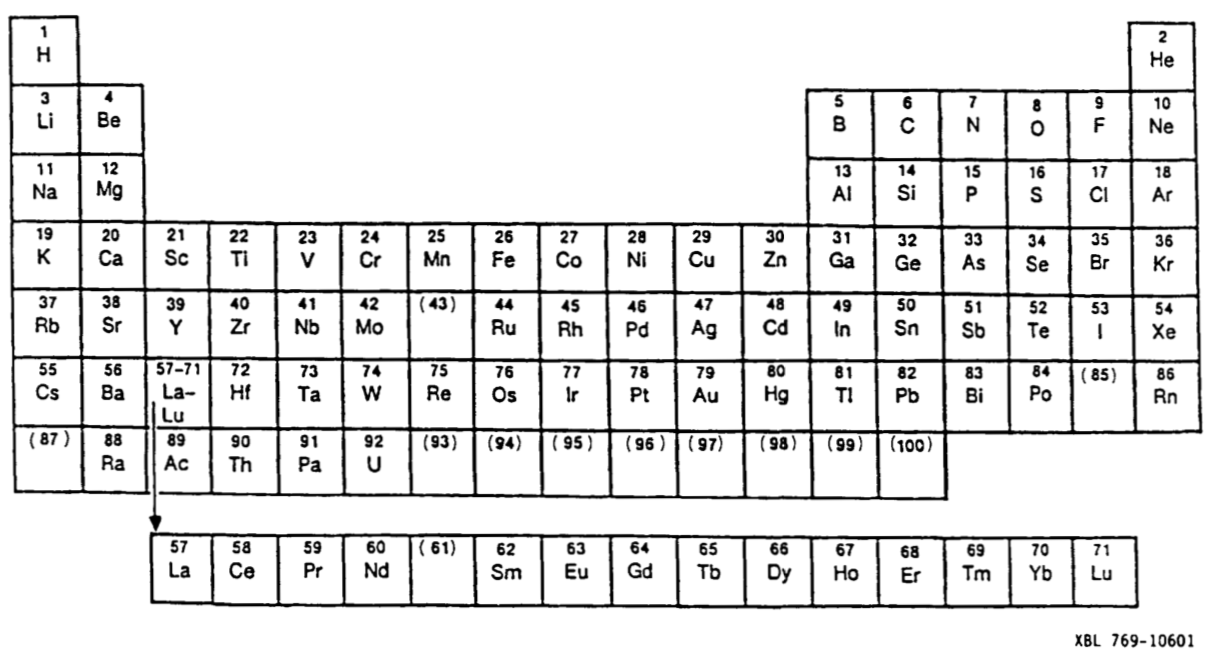
| Year: 1940 | PT id = 1262, Type = formulation review |
Hsueh & Chiang's Periodic Properties of the Elements
Hsueh & Chiang, Periodic Properties of the Elements, J. Chinese Chem. Soc., 5, 5, 253-275. See the PDF.
René Vernon writes:
"A mathematical expression of the periodic law was put forward in 1937 in an article by Chin-Fang Hsueh and Ming-Chien Chiang: J Chinese Chem Soc, 5, 263 (In English.) They derived a property equation from which the numerical magnitude of a property P is related to the atomic number Z of the element in question in terms of valence V, a function of the periodic factor y, the principal quantum number n, and two parameters a and p, which are constants for a given family of elements but different for different families."
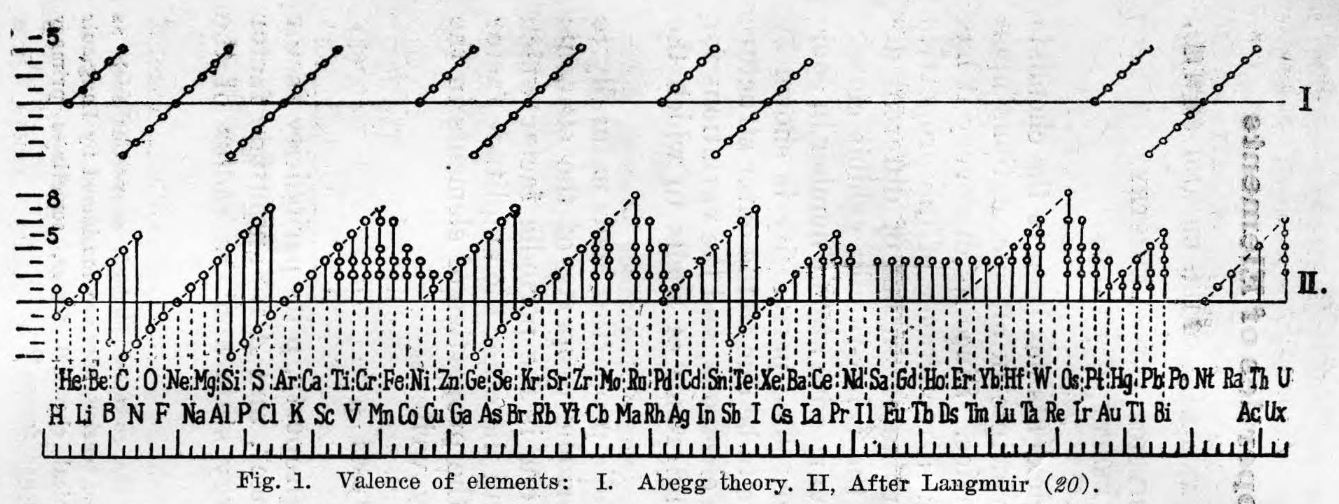
| Year: 1942 | PT id = 521, Type = formulation |
Seaborg's Periodic Table of 1942
In his Priestly Medal Address, The Periodic Table: Tortuous Path to Man-Made Elements printed in C&EN April 16, 1979 and reprinted in Modern Alchemy: Selected Papers of Glenn T. Seaborg (1994), page 181, Glenn Seaborg describes how the extension of the PT, caused the discovery of the transuranic elements, plutonium and neptunium, resulted in a new "uranide" group.
The formulation below is the working (and at the time top-secret) formulation used by the Manhatten atomic bomb project. The Lawrence Berkeley Laboratory internal reference number for this document is XBL 798-2509.
Like the 1939 formulation, XBL 769-10601, the formulation below erroneously predicts positions for the heaviest elements:
- thorium is shown in the group below hafnium
- protactinium below tantalum
- uranium below tungsten
| Year: 1944 | PT id = 1305, Type = formulation spiral |
Emerson's Spiral Formulation
Emerson EI, 1944, A new spiral form of the periodic table, JChemEd., vol. 21, no. 3, pp. 111–115
René Vernon writes:
Emerson says that the elements in the A groups are called the representative elements because, as Eble states, they "include metals, nonmetals, inert elements, liquids, and gases." Eble RL, 1938, Atomic structure and the periodic table, JChemEd., vol. 15, p. 575
Note the inclusion in Emerson’s table of the neutron as element 0. Astonishingly, Emerson writes: "Element 0, possibly neutron [sic], is considered as a noble gas. Because of its probable chemical inertness and extreme density it might not be detected in a sizeable amount until some future scientist succeeds in sampling the center of the Earth." (p. 111)
(Mark Leach adds: The date is 1944 when the Manhattan Project was in full swing and nothing was being published about nuclear physics and/or neutron interactions. This idea may have come from some type of Popular Science story?)
Other features:
- The A and B groups are diametrically opposed in their positioning. "The electron structure of H either as H+ or H– finds a counterpart in the structure of element 0 or 2." (p. 113)
- The cell spaces for Be and Mg have been stretched on account of uncertainty as to whether they belong to group 2 or group 12. "The break between the periphery of the loops of the spiral along the spaces allotted to Mg and the transition metals of the fourth period serves to indicate that Be and Mg are not to be considered as a kind of prototype of these groups." (p. 111)
- "The C group is shown as a separate segment. If one were not concerned by plane representation the rare earths could be represented as a loop or bulge above the surface of the plane. One might imagine that this group of metals is a sort of hernia of nature that has been excised so as to maintain a flat surface." (p. 112–113)

| Year: 1944 | PT id = 1238, Type = formulation |
Emerson's Long Chart Modified to Show Atomic Structure
Edgar I. Emerson 1944, A chart based on atomic numbers showing the electronic structure of the elements, J. Chem. Educ. 1944, 21, 5, 254.
A WWII chart showing neutronium over He, and a split d-block.
Thanks to René for the tip!
| Year: 1945 | PT id = 841, Type = element |
Discovery of Promethium
Pm ![]()
Promethium, atomic number 61, has a mass of 145 au.
Radioactive element: Pm is only found in tiny amounts in nature. Most samples are synthetic.
Promethium was first observed or predicted in 1942 by S. Wu, E.G. Segrè and H. Bethe and first isolated in 1945 by Charles D. Coryell, Jacob A. Marinsky, Lawrence E. Glendenin, and Harold G. Richter.
| Year: 1945 | PT id = 1118, Type = formulation 3D |
Talpain's Gnomonic Classification of the Elements
Talpain PL 1945, Gnomonic classification of elements, J.Phys. Radium 6, 176-181 (in French), https://doi.org/10.1051/jphysrad:0194500606017600
Talpain writes:
"To overcome the drawbacks presented by the various tables in rows and columns into which the classification of chemical elements is usually inserted, the author proposes a diagram in space, having the form of a double pyramid constructed according to a simple arithmetic law, inspired by Greek surveyors. Under these conditions, all the bodies belonging to the same chemical family are placed on the same column, and all those which have similar physical properties (magnetic, electrical, radioactive, crystallographic, rare earths, etc.) are grouped together. This same diagram also makes it possible to represent the electronic structure of the atoms, the quantified states of the electrons, the energy levels and the spectral lines of hydrogen. Perhaps spectroscopists will be able to use it to also represent the lines of other bodies."
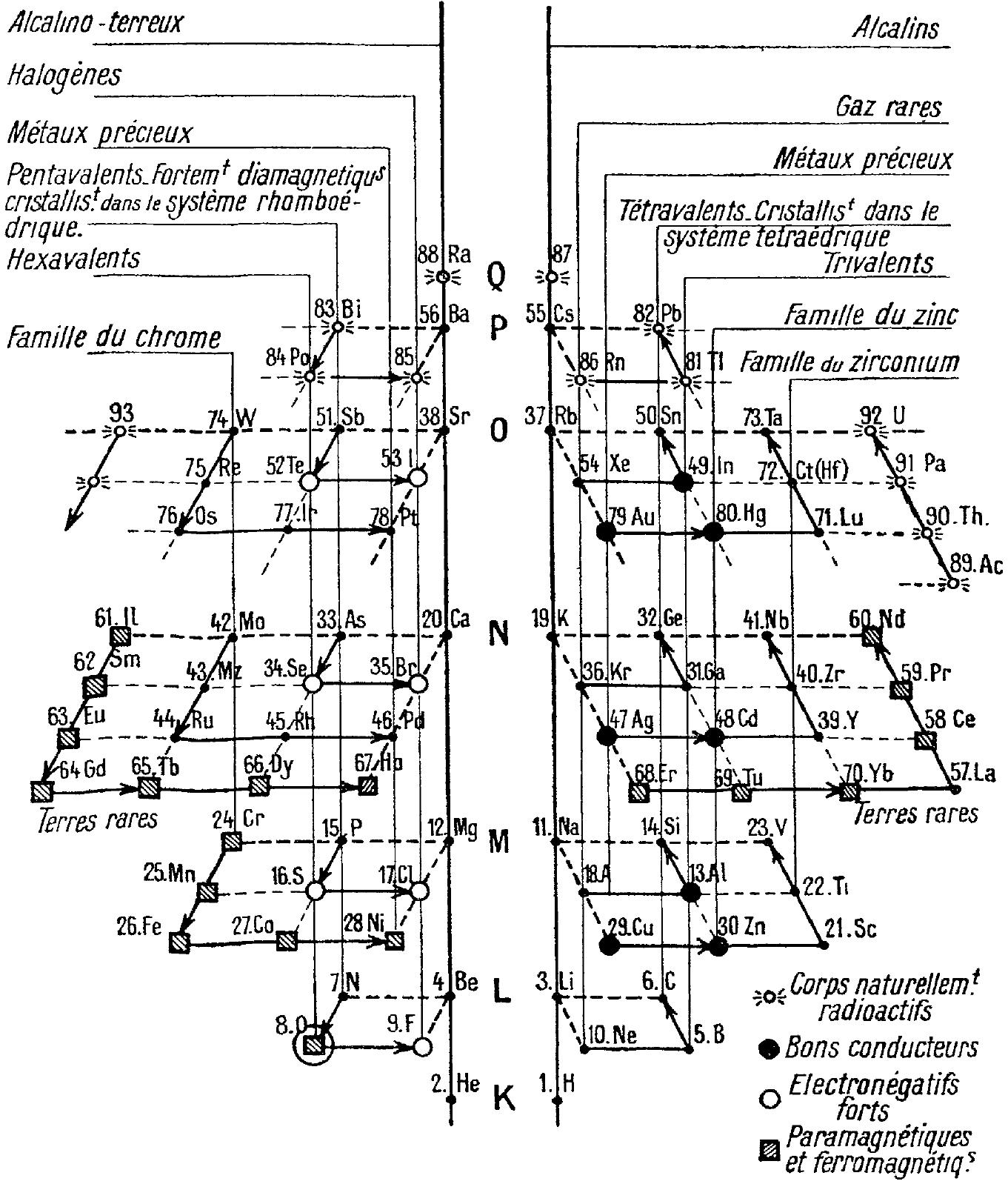
Thanks to René for the tip!
| Year: 1946 | PT id = 776, Type = formulation |
Achimof's System
Van Spronsen, on p. 157, says:
"Achimov's system took the form of a cross-section of a pyramid. He based his system on the principle that the lengths of the periods and the analogies in properties between the elements of these periods must be clearly demonstrated."
Achimov EI 1946 Zhur. Obshchei Khim., vol. 16, p. 961
Thanks to René for the tip!
| Year: 1946 | PT id = 1072, Type = formulation |
Yost & Russell's Periodic System
From D.M. Yost & H. Russell, Systematic Inorganic Chemistry of the Fifth-and-Sixth-group Nonmetallic Elements, Prentice-Hall, 1946, New York, p. 406.
René Vernon writes:
"Features of this peculiar periodic system:
- There are 11 main groups
- Group 3 features B-Al, followed by Sc-Y-La-Ac
- Group 4 bifurcates after C-Si into a Ti branch and a Ge branch; seemingly the Ti branch bifurcates after Zr into a Ce branch and a Hf branch
- Group 8 is Fe-Ru-Os
- Group 9 is Co-Rh-Ir
- Group 10 is Ni-Pd-Pt
- There are only 13 rare earths, running from Pr to Lu.
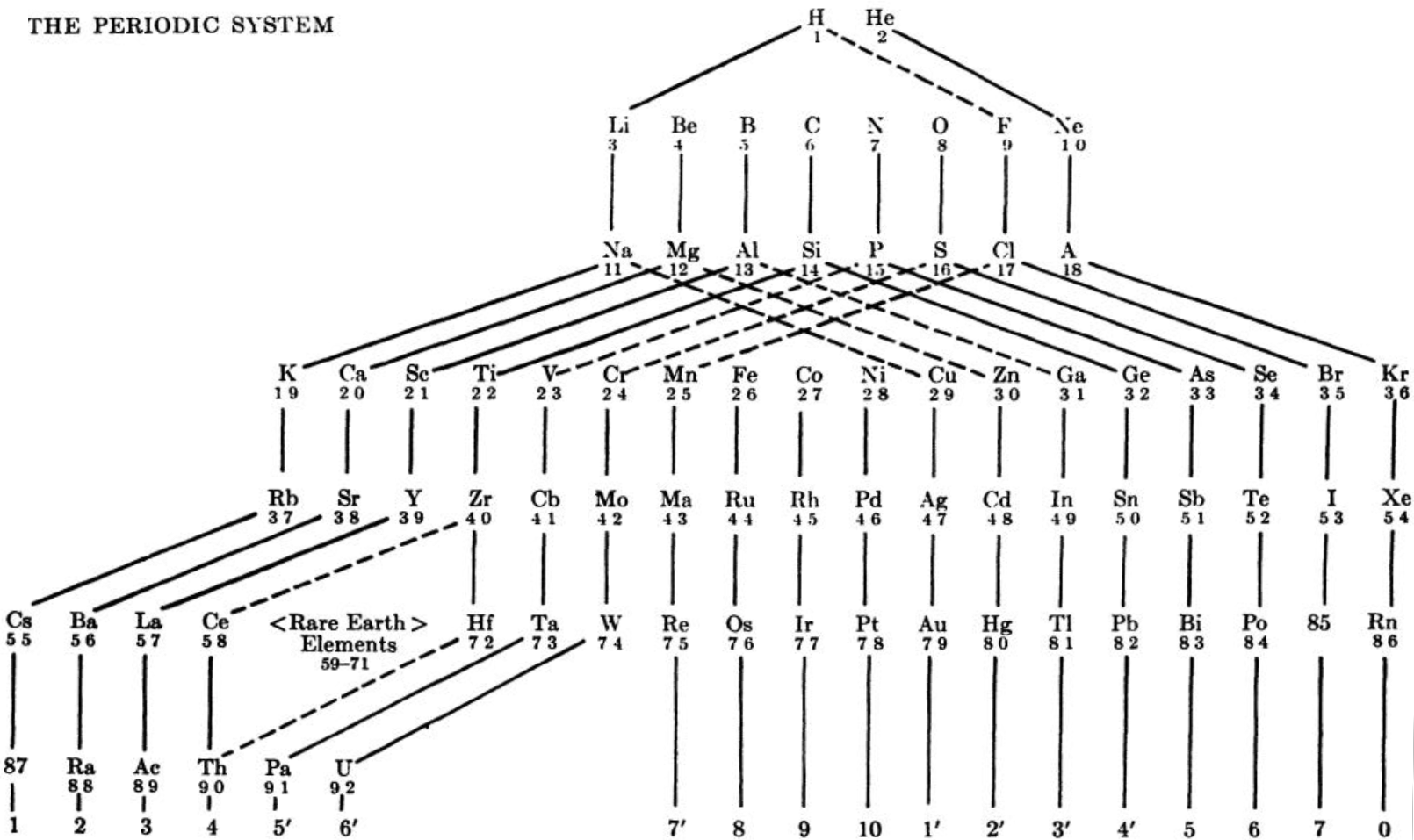
| Year: 1946 | PT id = 1088, Type = formulation |
Harrington's Crystal Chemistry of the Periodic System
R.H. Harrington, The Modern Metallurgy of Alloys, John Wiley & Sons, New York, p. 143 (1946)
René Vernon writes:
- The planar arrangement differs from the plan of his solid model, 'only as necessary to clarify the presentation'
- One the major features is that only two groups, at Si and Y, are considered to be 'truly' branched and that the latter 'is not usually considered in this manner'
- The smaller symbols, such as V under P, aren't necessary but are 'merely offered for consideration'
- Si shows a greater resemblance to Ge than it does to the closer Ti, while Y similarly shows greater resemblance to Lu than to La
- Stedman first drew his first version of this table sixteen years ago (= 1931)
- The neutron is included in group 0.
- Argon is still A; niobium Cb
- There's a blank space for Pm (discovered 1945).
- The main groups are recognisable, with the exception of group 3 as B-Al-Sc-Y-La. The other side of the table lists B-Al as being analogous to Sc-Y-La, rather than Ga-In-Tl.
The former option works better than the latter in terms of the quantitative smoothness of chemico-physical trend lines going down the group." - The equation of periods Š = 4 x n2;
- The equation of cycles/dyads S = 2(n21 + n22).
- Period
- Cycle
- Number of elements
- a harmonious whole, with a beginning and end
- regular and symmetrical
- without exception, it embraces all of its constituent members with simple mathematical expressions
- The position of H "Which [according to Ephraim] is difficult to place in this table in a satisfactory manner", outside of the main body of the periodic table, "remote from both Li and F, well removed from C, and above He and the inert gases"
- The old school location of B-Al in Group IIIa
- C-Si belong to both Ti-Zr-Hf-Th and Ge-Sn-Pb
- Rokhlin LL 2002, Magnesium Alloys Containing Rare Earth Metals: Structure and Properties, Taylor & Francis, London
- Shchukarev SA 1974, Neorganicheskaya khimiya, vol. 2. Vysshaya Shkola, Moscow (in Russian)
- Weaver EC & Foster LS 1960, Chemistry For Our Times. 3rd ed., McGraw-Hill, New York, p. 382
- Wiberg N 2001, Inorganic Chemistry, Academic Press, San Diego
- Wrigley AN, Mast WC & McCutcheon TP 1949, A laminar form of the periodic table, Part I, Journal of Chemical Education, 26(4), 216
- —— A laminar form of the periodic table, Part II, Journal of Chemical Education, 26(5), 248
- H over F
- the lanthanides under Y
- fifteen uranides under W (Hutton says they have, "properties increasingly similar to one another".)
- the complete block system according to Werner (1905)
- a horizontal Bohr line-system according to Spedding (1951)
- a period 0 containing the neutrino and neutron
- element number "00" for "v" suggests the neutrino has neither nuclear charge nor mass while "0" for Nn implies no nuclear charge
- regular period lengths of 2-2-8-8-18-18-32-32
- hydrogen has no direct relationship with a group, only secondary relationships with groups A1 and A7
- germanium, a semiconductor, is counted as a metal
- all groups numbered, where A = representative; B = transition
- C = Ln/An; analogous "transition" groups in the d-block (B8) and the f-block (C6)
- double periodicity among the Ln and An; and
- 25 columns wide i.e 18 + 32 = 50/2 = 25"
- Elegant and visually appealing overall design.
- Offers an impression of continuity through its (almost) spiral layout.
- In practice, this formulation does not resolve the discontinuity of periods any better than the conventional table; one still needs to complete one turn of the spiral and then mentally leap to the next, much as one moves from one row to the next in the flat form.
- Includes the He-over-Be placement, which remains controversial.
- Quantitative comparisons (group or period properties) become less readable; there’s no immediate visual sense of columns.
- Despite its elegance, the f-block placement appears somewhat awkward.
- Communicates feel more than data.
- It seems to imply relationships between groups on opposite sides of the p-, d- and f-blocks (for instance, between Sc-Y-Lu-Lr and Zn-Cd-Hg-Cn), whereas the actual correspondences run the other way; that is, between the early and later transition groups, such as 3–7 and 8–12. As Imyanitov (2018) observed: "In a generalised form, the properties of the early d? (f?) elements and their compounds are similar to those of the late d? + 5 (f? + 7)."
- Klechkovskii VM, Dokl. Akad. Nauk. SSSR, 135. 855 (1980). [In Russian]
- Klechkovskii VM, Zh. Eksperim. i. Teoret. Fiz., 41. 465 (1961). [In Russian]
- Which type of compounds certain elements will prefer to form under given conditions of mineral genesis (elementary substance, chalcogenide, oxide, oxysalt, etc.,)
- Whether the element will play a role of a cation or anion of a certain valency
- Which type of chemical bond the resulting mineral compound will have
- atomic number
- standard atomic weight
- ground-state electronic configuration
- element symbol
- element name
- discoverer and year of discovery
- melting point; boiling point
- critical temperature
- molar enthalpy of atomization
- molar enthalpy of fusion
- molar enthalpy of vaporization
- atomic energy levels of the outermost three orbitals
- formal oxidation states
- selection of standard reduction potentials
- first, second & third molar ionization energies
- Pauling electronegativity
- Allred-Rochow electronegativity
- molar electron affinity
- molar volume
- crystal structures
- polymorphic transition temperatures
- atomic radius
- effective ionic radii
- volumic mass (density)
- electrical resistivity
- thermal conductivity
- abundance in the solar system
- abundance in the Orgueil meteorite
- abundance in the solar photosphere
- abundance in the continental crust
- abundance in the primitive mantle
- abundance in the oceanic crust
- naturally occurring isotopes
- mass number and representative isotopic composition
- molar heat capacity
- Debye temperature
- coefficient of linear thermal expansion
- price; annual mining production
- world reserve base
- nuclear spin and NMR receptivity
- Mossbauer active nuclides
- physical (standard) state
- metallic character
- abundance in food (human daily intake)
- principal hazardous property
- Other information: Aufbau principle, quantum numbers, orbitals and sequence of orbital filling; trivial group names; drawings of crystal lattice structures; 12 plots of a chemical/physical property against atomic number; 9 plots of a property against another property; list of SI units and SI prefixes; list of other units and their conversion to SI; list of fundamental physical constants; scheme of fundamental particles; list of radioisotopes with half-life longer than 5 days, presenting half-life and mode(s) of decay, indicating cosmogenic isotopes and isotopes produced by U-235 fission, as well as radioisotopes used in geochronology, pharmacology and nuclear medicine.
- Formulations
- History
- Discovery
- Elements
- Isotopes
- Personalities
- Compounds
- etc.
- Most of the physical properties of Eu and Yb, "such as the atomic volumes, metallic radii, melting and boiling points, heats of sublimation, compressibilities, and coefficients of expansion are more like those of the alkaline-earth metals, Ca, Sr, and Ba, than those of the rare-earth metals" (Pauling 1960, p. 418; Gschneidner 1964, p. 286).
- Liquid ammonia dissolves certain alkali, alkaline earth, and Ln metals, and... combines with them to form solid compounds. Those metals whose compound-forming ability has been confirmed are Li, Ca, Sr, Ba, Eu and Yb. (Mammano (1970, p. 367)
- The lanthanides are sometimes regarded as trivalent versions of the alkaline earth metals (Evans 1982).
- The electron configurations of lanthanide cations are similar to those of alkaline earth metal cations, as the inner f- orbitals are largely or completely unavailable for bond formation; (Choppin & Rizkalla 1994)
- The lanthanide trivalent cations are essentially spherical and present an environment very similar to alkali and alkaline earth ions towards complex formation... the standard electrode potentials for the lanthanides have similar values and are comparable with the redox potentials of alkaline earth metals (Sastri et al. 2003)
- Ba-Eu-Yb have cubic crystalline structures whereas the rest of the Ln are hexagonal, or rhombohedral in the case of Sm (Russell & Lee 2005)
- There is a close alloying similarity between the lanthanides and Ca, Sr and Ba (Artini 2007)
- Lanthanides are effective mimics of calcium and can stimulate or inhibit the function of calcium-binding proteins (Brayshaw 2019)
- Lanthanide cations can substitute for Ca2+ and Sr2+ cations in host materials for solid state lasers (Ikesue 2013)
- There is a knight’s move relationship between Ca and La:
- The ionic radius of Ca2+ is 114 pm; that of La3+ is 117 pm
- The similarity in sizes means La3+ will compete with Ca2+ in the human body, and usually win on account of having a higher valence for roughly the same hydrated radius
- The basicity of La2O3 is almost on par with CaO2 Freshly prepared La2O3 added to water reacts with such vigour that it can be quenched like burnt lime (CaO)
- The electronegativity of Ca is 1.0; that of La is 1.1.
- Artini C (ed.) 2017, Alloys and Intermetallic Compounds: From Modeling to Engineering, CRC Press, Boca Raton, p. 92
- Brayshaw et al. 2019, Lanthanides compete with calcium for binding to cadherins and inhibit cadherin-mediated cell adhesion, Metallomics, vol. 11, no. 5, 2019, pp. 914–924
- Choppin GR & Rizkalla EN 1994, Solution chemistry of actinides and lanthanides, Handbook on the Physics and Chemistry of Rare Earths, pp. 559–590(560)
- Evans WJ 1982, Recent advances in the low valent approach to f-element organometallic chemistry, in McCarthy GJ, Silber HB and Rhyne JJ (eds), The Rare Earths in Modern Science and Technology, vol. 3, Plenum Press, New York, pp. 61–70(62)
- Gschneidner KA 1965, in Seitz F & Turnbull D (eds), Solid State Physics, vol. 16, Academic Press, New York, p. 286
- Ikesue A, Aung YL, Lupei V 2013, Ceramic Lasers, Cambridge University Press, Cambridge, pp. 26, 28
- Mammano N 1970, Solid metal ammonia compounds, in Metal–Ammonia Solutions, Proceedings of an International Conference on the Nature of Metal–Ammonia Solutions: Colloque Weyl II, pp. 367-393 (367), https://doi.org/10.1016/B978-0-408-70122-8.50030-4
- Pauling L 1960, The Nature of the Chemical Bond, 3rd ed., Cornell University Press, Ithaca, p. 418
- Pecharsky V 2016, Karl A. Gschneidner Jr (1930–2016), Nature Materials, vol. 15, no. 1059, https://doi.org/10.1038/nmat4751
- Russell AM & Lee KL 2005, Structure-property relations in nonferrous metals, John Wiley & Sons, Hoboken, inside cover
- Sastri et al. 2003, Modern Aspects of Rare Earths and their Complexes, Elsevier, Amsterdam, pp. 377, 878
- Sodium is actively practicing a "salt-formation" passing play with corrosive chlorine, who devours the delivery.
- Potassium, magnesium and barium are having an active metal huddle.
- The two hydrogens are hot desking.
- Iron is reading a weightlifting workout book.
- Platinum introduces gold to iridium, all three being noble metals.
- Lead, as a heavy frontier metal, is playing air guitar.
- Carbon is having a link up with frontier metal silver (masquerading as a transition metal); an unidentified frontier metal; and two "other" nonmetals.
- Silicon, as a metalloid and a semiconductor, is catching up on an assignment.
- Helium and neon, having each had a cup of chamomile tea during the break, are sleeping.
- The new system emphasizes oxidation states.
- The elements Eu and Yb fall directly below Ba, indicating the common oxidation state of 2+.
- Elements La, Gd, Lu fall in a column below Y, all having the dominant oxidation state 3+.
- Ce and Tb fall between Zr and Hf in keeping with their 4+ oxidation state. Pm (Z=61) lies immediately below Tc (Z=43), both without stable naturally occurring isotopes.
- The easily attained oxidation states of 2+ for Am and No, and 4+ for Bk are evident from the analogous positioning of the actinides.
- Hydrogen is a category of its own.
- The semimetals include selenium and astatine.
- There is no separate category for the halogen nonmetals.
- On page 100 the author refers to the inner occupation of the TM and Ln/An being particularly clear."
- FT Periodic Table: Element 1: LOVE
- FT Periodic Table: Element 2: SPACEWAR
- FT Periodic Table: Element 3: ROBOTS
- FT Periodic Table: Element 4: POP
- shell 6, s orbital - Cesium, Barium (Cs, Ba)
- shell 5, d orbital - Lanthanum (La)
- shell 4, f orbital - Cerium to Lutecium (Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu)
- shell 5, d orbital - Hafnium to Mercury (Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg)
- shell 6, p orbital - Thallium to Radon (Tl, Pb, Bi, Po, At, Rn)
- shell 7, s orbital - Francium, Radium (Fr, Ra)
- shell 6, d orbital - Actinium (Ac)
- shell 5, f orbital - Thorium (Th)
- Chemistry, Spectroscopy, and the Question of Reduction
- The Electronic Configuration Model, Quantum Mechanics and Reduction
- The Periodic Table and the Electron
- How Good is the Quantum Mechanical Explanation of the Periodic System
- Prediction and the Periodic Table
- Löwdin's Remarks on the Aufbau Principle and a Philosopher's View of Ab Initio Quantum Chemistry
- Mendeleev's Legacy
- The Role of Triads in the Evolution of the Periodic Table: Past and Present
- The Past and Future of the Periodic Table
- The Dual Sense of the Term "Elements", Attempts to Derive the Madelung Rule, and the Optimal Form of the Periodic Table, If Any
- Element symbol and element number Z = 0 – 118
- Period number n
- Group number G, related to the number of valence electrons
- Typical electronic valence configuration of the bound atoms ("neighbour configurations" such as s1p3 instead of s2p2 may be more important for some atoms in the group)
- Characteristic valence orbitals of highest angular momentum
- Chemical group name
- (Elements that do not belong to the group are put in parentheses.)
- The dashed lines indicate alternative or controversial group assignments, they are not meant to represent the authors' views.
- The reversal in atomic number order of Np to Am
- The return of the curides
- The placement of the Ln and the curides alongside the main table
- The assignment of the Ln and An to groups
- Triple periodicity among the Ln and heavy An
- Considers the fundamental nature of the periodic table to the physical sciences
- Explores the history of the discovery of trends among elements, to the construction of various forms of the table, and the growth of understanding of its meaning
- Touches on key ideas about both early atomic theory and quantum mechanics, showing how they have proved key to the meaning of the table
- Ideal for those who are curious to learn more about the periodic table and essential for any student of physics and chemistry
- Part of the Very Short Introduction series - over three million copies sold worldwide:
- Via MMSPT - All terms of the MMSPT are shifted to the right side without spaces.
- Via Janet Periodic Table - The first row of the Janet PT is deleted. - We remove 2 from all others 118 terms.
- The visual effect mirrors the look of Theodore Gray's series of posters, books, element cards and periodictable.com website and apps for the Apple iPod and iPad.
- Each element box is dominated by a Theodore Gray element photograph, with the element name, letter symbol, and atomic number relatively large, often overlapping the photo.
- The period numbers (below, right) are printed at the interface of the end/beginning of the periods, folded 90 degrees on the model, and the blocks and columns (old & new numbers), are identified below the data boxes - and in the case of the Actinoids, above.
- The element blocks connect at a central nexus (below, center), with the d- and f-blocks leaving, looping, and returning there, thus allowing the shorter period gaps above to be closed. For best visibility of the element data, these loops pinch together near the intersection. The p-block bends in a half-circle to join the s-block at the corner described above, with a patented 'downslant' where the element boxes gracefully sweep down a full box height (above) within this block to allow elimination of the "carriage return" effect: each period ending on the row above the next.
- The extended Hydrogen data box, a characteristic of all Alexander Arrangements, is more extended in this model, reaching for the multiple positions of the H box that are still under discussion among experts. The extra-extended Hydrogen box, illustrated by a composite image of a hydrogen cloud in space, (above, right) loops over the s- and p-blocks. Starting up from behind the corner of Helium & Lithium, inside the half-helical tube to loop over Helium, attach above Lithium, Beryllium and then Carbon as the loop descends (joining the ascending portion) over the data boxes of the s- & p-blocks, terminating in contact with Fluorine, Neon, and corner-on to Neon.
- The model size is the same as the previous Display Version of the AAE, but has fewer element data boxes, due to there being no photos of the lab created elements and for simplification of the educational application - introduction to property periodicity and organization of element data - the elements with atomic numbers over 94 are not included (see addendum).
- Where the f-block begins and ends, between Barium and Lutetium, the f-block is held perpendicular to the only flat segment of the element display by a pair of triangular braces, which also create the flat area, aligning the s-block with the 'pinch' of the d-block. This is particularly apparent from the bottom, when the model is supported from above. (see below)
- The first from about 10,000 BC to 1000 AD when 12 elements were discovered/used; one every 900 years or so.
- From 1669 until the present day when the other 106 have been rather steadily (and formally) discovered; one every couple of years.
- The last element to be made/discovered was in 2010.
- Periodic Trends Atomic Radius- When moving opposite to the zigzag line in a particular period, the atomic radius of the elements increase.
- Metallic Character- Metallic Character decreases when moving along the zigzag line in a particular period.
- Ionization Energy- When moving along the zigzag line in a particular period, the ionization energy increases. Electron Affinity- Electron Affinity increases when moving along the zigzag line in a particular period.
- H. Alderesey-Williams, Periodic Tales, Viking Press, 2011
- N.P. Agafoshin, Ley Periódica y Sistema Periódico de los Elementos de Mendeleiev, Ed. Reverté S.A., Barcelona, 1977
- I. Asimov, The Building Blocks of the Universe, Lancer Books, New York, 1966
- P.W. Atkins, The Periodic Kingdom, Basic Books, New York, NY, 1995
- O. Baca Mendoza, Leyes Geneticas de los Elementos Quimicos. Nuevo Sistema Periodico, Universidad Nacional de Cuzco, Cuzco, Peru, 1953
- P. Ball, A Guided Tour of the Ingredients, Oxford University Press, Oxford, 2002
- P. Ball, A Very Short Introduction to the Elements, Oxford University Press, 2004
- I. Barber, Sorting The Elements: The Periodic Table at Work, Rourke Publishing, Vero Beach, Florida, US, 2008
- R. Baum (ed), Celebrating the Periodic Table, Chemical & Engineering News, A Special Collector's Issue, September 8, 2003
- H.A. Bent, New Ideas in Chemistry from Fresh Energy for the Periodic Law, Author House, Bloomington IN, 2006
- J. Bernstein, Plutonium, Joseph Henry, Washington DC, 2007
- J. C.A. Boeyens, D.C. Lavendis, Number Theory and the Periodicity of Matter, Springer, Berlin, 2008
- N. Bohr, Collected Works Vol 4. The Periodic System (1920-1923), Nielsen J Rud (Editor), North Holland Publishing Company, 1977
- T. Bondora, The Periodic Table of Elements Coloring Book, Bondora Educational Media Publications, 2010
- D.G. Cooper, The Periodic Table, 3rd edition. Butterworths, London, 1964
- P.A. Cox, The Elements, Oxford University Press, Oxford, 1989
- P. Depovere, La Classification périodique des éléments, De Boeck, Bruxelles, 2002
- H. Dingle and G.R. Martin, Chemistry and Beyond: Collected Essays of F.A. Paneth, Interscience, New York, NY, 1964
- S. Dockx, Theorie Fondamentale du Systeme Periodique des Elements, Office Internationale de Librairie, Bruxelles, 1950
- A. Ducrocq, Les éléments au pouvoir, Julliard, Paris, 1976
- A. Ede, The Chemical Elements, Greenwood Press, Westport, CT, 2006
- J. Emsley, The Elements, 3rd edition. Clarendon, Oxford University Press, 1998
- J. Emsley, Nature's Building Blocks, An A-Z Guide to the Elements, Oxford University Press, Oxford, 2001
- P. Enghag, Encyclopedia of the Elements, Wiley-VCH, Weinheim, 2004
- D.E. Fisher, Much Ado About (Practically) Nothing, The History of the Noble Gases, Oxford University Press, New York, 2010
- I. Freund, The Study of Chemical Composition: An Account of its Method and Historical Development, Dover Publications, Inc., New York, NY, 1968
- J. García-Sancho & F. Ortega-Chicote, Periodicidad Química, Trillas, México, 1984
- A. E. Garrett, The Periodic Law, D. Appleton & Co., New York, 1909
- L. Garzon Ruiperez, De Mendeleiev a Los Superelementos, Universidad de Oviedo, Oviedo, 1988
- L. Gonik, C. Criddle, The Cartoon Guide to Chemistry, Harper Resource, New York, 2005
- M. Gordin, A Well-Ordered Thing, Dimitrii Mendeleev and the Shadow of the Periodic Table, Basic Books, New York, 2004
- T. Gray, The Elements: A Visual Exploration of Every Known Atom in the Universe, Black Dog & Leventhal, 2009
- D. Green, The Elements, The Building Blocks of the Universe, Scholastic Inc. New York, 2012
- R. Hefferlin, Periodic Systems and their Relation to the Systematic Analysis of Molecular Data, Edwin Mellen Press, Lewiston, NY, 1989
- D.L. Heiserman, Exploring the Chemical Elements and their Compounds, McGraw-Hill New York, 1991
- S. Hofmann, Beyond Uranium, Taylor & Francis, London, 2002
- F. Hund, Linienspektren und Periodisches System der Elemente, Verlag von Julius Springer, Berlin, 1927
- W.B. Jensen, Mendeleev on the Periodic Law: Selected Writings, 1869-1905, Dover, Mineola, NY, 2005
- S. Kean, The Disappearing Spoon, Little, Brown & Co., New York, 2010
- D.M. Knight, Classical Scientific Papers, Chemistry Second Series, American, Elsevier, New York, NY
- P.K. Kuroda, The Origin of the Chemical Elements, and the Oklo Phenomenon, Springer-Verlag, Berlin, 1982
- H.M. Leicester and H.S. Klickstein, A Source Book in Chemistry 1400-1900, 1st Edition, McGraw-Hill Book Company Inc., London, 1952
- M.F. L'Annunziata, Radioactivity, Introduction and History, Elsevier, 2007
- S.E.V. Lemus, Clasificación periódica de Mendelejew, Guatemalan Ministry of Public Education, Guatemala, 1959
- P. Levi, The Periodic Table, 1st American Edition. Schocken Books, New York, NY, 1984
- R. Luft, Dictionnaire des Corps Simples de la Chimie, Association Cultures et Techniques, Nantes, 1997
- J. Marshall, Discovery of the Elements, Pearson Custom Publishing, 1998
- E. Mazurs, Graphic Representation of the Periodic System During One Hundred Years, Alabama University Press, Tuscaloosa, AL, 1974
- D. Mendeleeff, An Attempt Towards A Chemical Conception of the Ether, translated by G. Kamensky. Longmans, Green, and Co., London, 1904
- D. Mendeleeff, The Principles of Chemistry, translated by G. Kamensky, 5th Edition, vol. 2. Longmans, Green, and Co., London, 1891
- L. Meyer, Modern Theories of Chemistry, 5th Edition, translated by P.P. Bedson, Longmans, Green, and Co., London, 1888
- L. Meyer, Outlines of Theoretical Chemistry, 2nd Edition, translated by P.P. Bedson and W.C. William. Longmans, Green, and Co., London, 1899
- F. Mohr, (E), Gold Chemistry, Wiley-VCH, 2009
- D. Morris, The Last Sorcerers, The Path from Alchemy to the Periodic Table, Joseph Henry Press, New York, 2003
- I. Nechaev, G.W. Jenkins, The Chemical Elements, Tarquin Publications, Norfolk, UK, 1997
- R.D. Osorio Giraldo, M.V. Alzate Cano, La Tabla Periodica, Bogota, Colombia, 2010
- M.J. Pentz, (General Editor), The Periodic Table and Chemical Bonding, Open University Press, Bletchley, Buckinghamshire, UK, 1971
- I.V. Peryanov, D.N. Trifonov, Elementary Order: Mendeleev's Periodic System, translated from the Russian by Nicholas Weinstein, Mir Publishers, Moscow, 1984
- J.S.F. Pode, The Periodic Table, John Wiley, New York, NY, 1971
- R.J. Puddephatt, The Periodic Table of the Elements, Oxford University Press, Oxford, 1972
- R.J. Puddephatt and P.K. Monaghan, The Periodic Table of the Elements, 2nd edition. Oxford University Press, Oxford, 1986
- H.-J. Quadbeck-Seeger, World of the Elements, Wiley-VCH, Weinheim, 2007
- E. Rabinowitsch, E. Thilo, Periodisches System, Geschichte und Theorie, Stuttgart, 1930
- R. Rich, Periodic Correlations, Benjamin, New York, 1965
- J. Ridgen, Hydrogen, The Essential Element, Harvard University Press, Cambridge, MA, 2002
- H. Rossotti, Diverse Atoms, Oxford University Press, Oxford, 1998
- D.H. Rouvray, R.B. King, The Periodic Table Into the 21st Century, Research Studies Press, Baldock, UK, 2004
- D.H. Rouvray, R.B. King, The Mathematics of the Periodic Table, Nova Scientific Publishers, New York, 2006
- G. Rudorf, The Periodic Classification and the Problem of Chemical Evolution, Whittaker & Co., London, New York, 1900
- G. Rudorf, Das periodische System, seine Geschichte und Bedeutung für die chemische Sysytematik, Hamburg-Leipzig, 1904
- O. Sacks, Uncle Tungsten, Vintage Books, New York, 2001
- R.T. Sanderson, Periodic Table of the Chemical Elements, School Technical Publishers, Ann Arbor, MI, 1971
- S. E. Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009
- E.R. Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, New York, 2007
- E.R. Scerri, Selected Papers on the Periodic Table, Imperial College Press, London and Singapore, 2009
- E.R. Scerri, A Very Short Introduction to the Periodic Table, Oxford University Press, Oxford, 2011; Also translated into Spanish and Arabic.
- E.R. Scerri, Le Tableau Périodique, Son Histoire et sa Signification, EDP Sciences, 2011, (translated by R. Luft); Japanese Translation by Hisao Mabuchi et. al.
- C. Schmidt, Das periodische System der chemischen Elementen, Leipzig, 1917.
- G.T. Seaborg, W.D. Loveland, The Elements Beyond Uranium, Wiley, New York, 1990
- M.S. Sethi, M. Satake, Periodic Tables and Periodic Properties, Discovery Publishing House, Delhi, India, 1992
- H.H. Sisler, Electronic Structure, Properties, and the Periodic Law, Reinhold, New York, 1963
- P. Strathern, Mendeleyev's Dream, Hamish-Hamilton, London, 1999
- R.S. Timmreck, The Power of the Periodic Table, Royal Palm Publishing, 1991
- M. Tweed, Essential Elements, Walker and Company, New York, 2003
- F.P. Venable, The Development of the Periodic Law, Chemical Publishing Co., Easton, PA, 1896
- M.E. Weeks, Discovery of the Elements, Journal of Chemical Education, Easton PA, 1960
- B.D. Wilker, The Mystery of the Periodic Table, Bethlehem Books, New York, 2003
- J. Van Spronsen, The Periodic System of the Chemical Elements, A History of the First Hundred Years, Elsevier, Amsterdam, 1969
- T. Zoellner, Uranium, Penguin Books, London, 2009
- A. Zwertska, The Elements, Oxford University Press, Oxford, 1998
- Nauchnyi arkhiv. Periodicheskii zakon, t. I, ed. B. M. Kedrov. Moscow: Izd. AN SSSR, 1953
- Periodicheskii zakon. Dopolnitel'nye materialy. Klassiki nauki, ed. B. M. Kedrov. Moscow: Izd. AN SSSR, 1960
- Periodicheskii zakon. Klassiki nauki, ed. B. M. Kedrov. Moscow: Izd. AN SSSR, 1958
- Top: Version with group 3 consisting of Sc, Y, Lu, and Lr.
- Middle: Version with group 3 consisting of Sc, Y, La, Ac. The sequence of increasing atomic number is anomalous with this assignment of elements to group 3, e.g., Lu (71), La (57), Hf (72).
- Bottom: Third option for incorporating the f-block elements into a long-form table. This version adheres to increasing order of atomic number from left to right in all periods, while grouping together Sc, Y, La and Ac but at the expense of breaking-up the d-block into two highly uneven portions :
- Three best data rich periodic tables
- Five formulations which show the development of the modern PT
- One, of many, interesting alternative formulations
- One example of the periodic table being used as an infographic template
- Since 1993 – and with its rather bland interface – WebElements has given access to vast quantities of in depth chemical data & information. This is the professional chemist's periodic table:
- Theo Gray's Photographic Periodic Table is undoubtedly the most attractive PT available in web space, but there is more. Clicking around the website gives access to a host of information, pictures & anecdotes from Theo's extraordinary and extensive collection of chemical elements:
- Ptable has a super-slick, and very fast interface. It is data/information rich and is available in 50 languages:
- There are several early listings of chemical substances, including Valentinus' Alchemy Table and Lavoisier's Table of Simple Substances (1789). In 1803 Dalton proposed that matter consists of discrete atoms that combine in fixed ratios, stoichiometry, to form chemical elements. Thus, Dalton's list of chemical elements, plus mass data, must be included in any top ten listing:
- If you examine the periodic tables from Antiquity to 1899, you will see that from about 1830 onwards, proto-periodic tables were coming thick and fast. Significant developments include: Daubeny's Teaching Display Board of Atomic Weights (1831), Chancourtois Telluric Helix (1862) and Newlands octaves (1864).
But, it was Mendeleev's Tabelle I that was first near complete periodic table formulation of the then known elements (no Group 18 rare gasses, note). Crucially, Mendeleev identified gaps and was able to make predictions about the chemical properties of the missing substances. Plus, Mendeleev promoted his ideas with great energy: - Werner's 1905 Periodic Table is remarkably modern looking. The formulation is a long form that separates transition metals and rare earths, but he guessed wrong on how many existed:
- Janet's Left Step formulation of 1928 is one for the purists as it clearly shows the chemical elements arranged into s, p, d & f-blocks of the recently developed quantum mechanical description of atomic structure:
- The modern (and commonly employed) periodic table is obtained by transforming Janet's Left Step into the modern long form periodic table by rearranging the blocks around. This transformational mapping is discussed in some detail here.
The long form and medium form PTs have electronegativity trending from top-right (electronegative) to bottom left (electropositive), and many aspects of periodicity corollate with electronegativity: atomic radius, first ionisation energy, etc.
Thus, the long form and medium form periodic tables are commonly used in the classroom: - Adobe Illustrator Shortcuts
- Adult Positions
- Airline Customer Reviews
- Beer Styles
- And, chosen more or less at random, European Nations:
- Include names, symbols, atomic numbers, weights, periods, groups, electrons/shell
- Include Lutetium (Lu) and Lawrencium (Lr) in the d-block if desired
- Related elements are color coded
- Print in color or black and white
- Print in A4 or US Letter page sizes
- Joseph Priestley and Antoine Lavoisier, whose discovery of oxygen – and radical interpretation of it – led to the modern science of chemistry
- Humphry Davy, who made electricity a powerful new tool in the search for elements
- Dmitri Mendeleev, whose Periodic Table brought order to the growing gaggle of elements
- Marie Curie, whose groundbreaking research on radioactivity cracked open a window into the atom
- Henry Moseley, whose investigation of atomic number redefined the Periodic Table
- Glenn Seaborg, whose discovery of plutonium opened up a whole new realm of elements, still being explored today.
- Location that remains near the Neutron Dripline of element.
- Location that remains very close to stable or long-lived isotopes of the element. Location that remains near the Proton Dripline of element.
- In the case of superheavy elements, we identify which Compound Nuclei are involved in the Hot Fusion reaction and which Compound Nuclei are involved in the Cold Fusion reaction.
- We see the r-process path and assess the r-process abundance.
- The pattern of abundance of chemical elements.
- We identify which elements are the product of exothermal fusion.
- We identify the location of isotope on the basis of two-neutron separation energy.
- Nuclear binding energy trend. Beta decay trend.
- We see the Straight Line of Nuclear Stability.
- Empirical Law discovered.
- Periodicity in the nuclear properties.
- We can compare the nuclear properties of an element with the nuclear properties of almost all the chemical elements.
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version.
- It adds color: YELLOW for the s-block, GREEN for the p-block, BLUE for the d-block and PURPLE for the f-block.
- It avoids being congested since it excludes the electronic configurations of the elements.
- It is updated and includes the atomic numbers 119 and 120.
- It shows that it is symmetrical around the vertical axis.
- The f-block, like all the other blocks, ends with even atomic numbers.
- H is over F, which is a smoother fit in terms of physicochemical trends down the group
- He is over Ne, which is a smoother fit etc
- group 3 has lanthanum in it
- the modern relationships Ti-Zr-Hf, V-Nb-Ta, Cr-Mo-W, and Mn-Tc-Re can still be traced
- the lanthanides and actinides are integrated into the main body of the table
- 15 lanthanides and 15 actinides(!)
- the old school arrangement of B-Al-Sc-Y-La can still be traced, as can the less smooth alternative B-Al-Sc-Y-Lu
- the 1s "block" starts at H; the s block proper at Li; p at B; d at Sc; f at Ce
- Beylkin G 2018, The periodic table of the elements with 4n2 n = 2,3... periods, https://arxiv.org/pdf/1901.02337.pdf
- Eric 2006, https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=20
- Johansson, B., Luo, W., Li, S. et al. 2014, Cerium; crystal structure and position in the periodic table. Sci Rep 4, 6398. https://doi.org/10.1038/srep06398
- Gregory Beylkin: https://en.wikipedia.org/wiki/Gregory_Beylkin
- Sc, Y, La, Ac
- Sc, Y than a gap for the lanthanides & a gap for the actinides
- Sc, Y, Lu, Lr
- Toma, H. E. IYPT 2019 International Year of the Periodic Table of the Chemical Elements. Quimica Nova 42, 468–472 (2019).
- Toma, H. Estrutura atômica, ligações e estereoquímica. (Edgard Blucher, 2018).
- Toma, H. Química bioinorganica e ambiental. (Edgard Blucher, 2015).
- Toma, H. Green Processing of Strategic Elements Based on Magnetic Nanohydrometallurgy. Green Chem. 29, 948–959 (2015).
- Hydrogen (H), boron (B), carbon (C), calcium (Ca), phosphorous (P), potassium (K), magnesium (Mg), vanadium (V), manganese (Mn), iron (Fe), cobalt (Co), copper (Cu), zinc (Zn), selenium (Se), rubidium (Ru), molybdenum (Mo), and cesium (Cs) are commonly found in supplements readily available to the public and are illustrated as such. Helium (He) is crucial in the operation of MRI machines.
- Lithium (Li) as lithium carbonate is the most common treatment of bipolar disorder.
- Beryllium (Be) foil is used as shielding in radiographic instruments.
- Nitrogen (N), as nitrous oxide, is a common anesthetic.
- Oxygen (O) has many medical uses, including anesthetics and resuscitation, and is illustrated here for use in ventilation.
- Fluorine (F) and tin (Sn) as stannous fluoride are a common ingredient in toothpaste.
- Sodium (Na) and chlorine (Cl) are used as NaCl in saline solutions.
- Aluminum (Al) compounds are a common active ingredient in antiperspirant deodorants.
- Silicon (Si) is used in antacid products.
- Sulfur (S) is illustrated as campden tablets, which are used for sterilization in beer fermentation.
- Argon (Ar) lasers are used in eye surgery.
- Zirconium (Zr) is used in immuno-positron emission tomography (PET) imaging while scandium (Sc) is a candidate for the same technique.
- Titanium (Ti), palladium (Pd), niobium (Nb), nickel (Ni), and tantalum (Ta) are used in medical implants.
- Chromium (Cr) is shown as Cr(III) picolinate, which is a controversial supplement used in lowering insulin resistance.
- Gallium (Ga), yttrium (Y), technetium (Tc), lanthanum (La), astatine (At), and actinium (Ac) are all used in nuclear medicine.
- Arsenic (As), as As(III) trioxide, is used to treat certain forms of leukemia.
- Bromine (Br) as KBr is an active ingredient in canine seizure medication.
- Krypton (Kr) was used in lung ventilation studies but has since been phased out.
- Strontium (Sr) is used in Sensodyne® toothpaste.
- Rhodium (Rh), ruthenium (Ru), and rhenium (Re) complexes are used as anticancer agents.
- Silver (Ag) is used in antibacterial ointments.
- Indium (In) is used in white blood cell scans.
- Antimony (Sb) is used in leishmania medicine.
- Barium (Ba) is used in X-ray imaging of the gastrointestinal tract.
- Tungsten (W) is used in shielded syringes.
- Iridium (Ir) is used in brachytherapy.
- Gold (Au) was used as a treatment for rheumatoid arthritis but has been phased out.
- Mercury (Hg) is used in dental amalgams.
- Lead (Pb) is used in X-ray aprons.
- Bismuth (Bi) is used in stomach ulcer medicine.
- Neon (Ne), germanium (Ge) cadmium (Cd), tellurium (Tl), hafnium (Hf), osmium (Os), polonium (Po), francium (Fr), radon (Rn), and radium (Ra) although most of these are toxic elements for human life, some of these elements are under development as potential agents for disease treatment but to our knowledge they are not currently used for beneficial applications in medicine.
- This table is based on two tables by Fernelius (1986), one of which is a portrait version of Bohr's 1922 table, and the second of which is a conventional table highlighting oxidation number trends in groups 4 to 10, and most of the p-block. Ref. Fernelius WC , 'Some reflections on the periodic table and its use', Journal of Chemical Education, vol. 63, no. 3, pp. 263–266 (1986).
- In my table, the transition metals are in groups 4 to 11. As per Mark's Leach's email: "...It's the 'incomplete d-subshell' that gives rise to properties such as: variable oxidation state, catalytic behaviour, d-orbital splitting and [thus] coloured ions/compounds."
- I've included oxidation number details for some elements. I've tried a different way of showing the composition of a bifurcated group 3, which is more in keeping with Bohr's table.
- The horizontal bar of the "T" is for Sc → Ti; the downward bar is for Y → Lu-Lr.
- Thus, Group 3 as Sc-Y-Lu-Lr could be called group 3T.
- La-Ac and Lu-Lr are duplicated in a greyed-out style to make it clearer where the lanthanides and actinides fit into the main body of the table.
- The inner transition metals are clearly delineated. Analogously to the transition metals they're all capable of forming ions with incomplete f sub-shells.
- The early actinides resemble the transition metals.
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version (pdf).
- The "inside corner below" is like looking at the junction of a floor and two walls in the corner of a room.
- The "outside corner above" is like looking up at the underside of an overhanging corner of a building.
- The "outside corner below" is like looking down on the corner of a large box.
- The "inside corner above" is like looking at the junction of walls and a ceiling in a room.
- A collection of 14 edited papers from historians of chemistry, philosophers of chemistry, and chemists with epistemological and educational concerns
- Contains educational debates concerning how to teach and present the concept of elements
- Provides a beneficial, scholarly, unique, and understandable overview of the current debate on the chemical elemen.
- Very good correspondence with natural categories
- Largely linear trends seen along main groups; two switchbacks seen in group 13; also falloffs (6p sub-shell) seen in groups 14-17
- First row anomalies seen for Li (in amphoteric territory), Be (ditto), C (misaligned), N (in noble gas territory), O (misaligned), F (ditto) and He (ditto)
- For group 13, the whole group is anomalous, no doubt due to the scandide contraction impacting Ga and the double whammy of the lanthanide and 5d contraction impacting Tl
- Nitrogen was called a noble gas before the discovery of the real noble gases and appropriately enough falls into that territory
- Rn is metallic enough to show cationic behaviour and falls just outside of noble gas territory
- F and O are the most corrosive of the corrosive nonmetals
- The rest of the corrosive nonmetals (Cl, Br and I) are nicely distributed, across the border from F
- The rest of the simple and complex anions, funnily enough, comprise the intermediate nonmetals
- The metalloids are nicely aligned; Ge falls a little outside of the metalloid line, being still occasionally referred to as a metal; Sb, being the most metallic of the metalloids falls outside the border; At is inside; Po is just outside
- Pd is located among the nonmetals due to its absence of 5s electrons; see here
- The proximity of H to Pd is astonishing given the latter's capacity to adsorb the former
- The post-transition metals (PTM) form an "archipelago of amphoterism" bounded by transition metals: Ni and C to the west; Fe and Re to the south; V, Tc and W to the east; noble metals to the north
- Curiously, Zn, Cd, and Hg are collocated with Be, and distant from the PTM and the TM proper (aside from Mn)
- Zn is shown as amphoteric, which it is. Cd is shown as cationic but is not too far away from amphoteric territory; it does show amphoterism, reluctantly; Hg is shown as amphoteric which is the case, weakly, for HgO, as is the congener sulfide HgS, which forms anionic thiomercurates (such as Na2HgS2 and BaHgS3) in strongly basic solutions
- The ostensibly noble metals are nicely delineated; Ag is anomalous given its greater reactivity; Cu, as a coinage metal, is a little further away
- The proximity of Au and Pt to the halogen line is remarkable given the former's capacity to form monovalent anions
- The ferromagnetic metals (Fe-Co-Ni) form a nice line
- The TM from groups 4-12 form switchback patterns e.g. Ti-Zr and the switchback to Hf
- The refractory metals, Nb, Ta, Mo, W and Re are in a wedge formation
- Tc is the central element of the periodic table in terms of mean radius and EA values; V is close, Cr is a little further away
- Ti is just inside the basic cation line; while Ti(IV) is amphoteric, Ti3+ is ionic
- Sc-Y-La shows a main group pattern up to La, when there is a switchback to Ac
- Sc-Y-Lu-Lr shows a TM switch back pattern
- La, and to lesser extent Ce are rather separated from the rest of the Ln, consistent with Restrepo and here.
- Sc and Lu are close to the amphoteric territory and are both in fact, weakly amphoteric
- The post-cerium Ln and An (but for Th) all fall within basic cation territory
- EA values for the An are estimates and need to be treated with due caution
- The light actinides (Th to Cm) occupy a tight locus, with the exception of Th, where the 5f collapse is thought to occur, and Pu, which sits on the border of 5f delocalisation and localisation
- While the light actinides U to Cm are shown as being cationic they are all known in amphoteric forms
- The heavy actinides, Bk to Lr, are widely dispersed
- All the Ln, bar Tm, are located within close proximity of the light An locus; Tm is the least abundant stable Ln
- The gap between La and Ce, and rest of the Ln is consistent with Restrepo's findings and here
- Nobelium in this edition of the chart falls off the bottom, having a radius 1.58 (cf Es) and an EA of -2.33
- There is an extraordinary alignment between He and the Group 2 metals
- Magnesium is on the cationic-amphoteric boundary; some of its compounds show appreciable covalent character
- Li, being the least basic of the alkali metals, is located just outside the alkalic zone; Li compounds are known for their covalent properties
- The reversal of the positions of Fr and Cs is consistent with Cs being the most electronegative metal
- A similar, weaker pattern is seen with Ba and Ra.
- The results are similar to the orbital radii x EA plot, although not quite as clear, including being more crowded
- Very good correspondence with natural categories
- Largely linear trends seen along groups 1-2, 17 and 15-18 (Ne-Rn)
- First row anomaly seen for He (or maybe not since it lines up with the rest of group 2)
- For group 13, the whole group is anomalous
- For group 14 , the whole group is anomalous no doubt due to the scandide contraction impacting Ge and the double whammy of the lanthanide and 5d contraction impacting Pb
- F and O are the most corrosive of the corrosive nonmetals
- The rest of the corrosive nonmetals (Cl, Br and I) are nicely aligned with F
- The intermediate nonmetals (IM) occupy a trapezium
- Iodine almost falls into the IM trapezium
- The metalloids occupy a diamond, along with Hg; Po is just inside; At a little outside
- Rn is metallic enough to show cationic behaviour and falls into the metalloid diamond
- Pd is located among the nonmetals
- The proximity of H to Pd is again (coincidentally?) curious given the latter's capacity to adsorb the former
- The post-transition metals occupy a narrow strip overlapping the base of the refractory metal parallelogram
- Curiously, Zn, Cd, and Hg (a bit stand-off-ish) are collocated with Be, and relatively distant from the PTM and the TM proper
- The ostensibly noble metals occupy an oval; curiously, W is found here; Ag is anomalous given its greater reactivity; Cu, as a coinage metal, is a little further away
- Au and Pt are nearest to the halogen line
- The ferromagnetic metals (Fe-Co-Ni) are colocated
- The refractory metals, Nb, Ta, Mo, W and Re are in a parallelogram, along with Cr and V; Tc is included here too
- Indium is the central element of the periodic table in terms of mean orbital radius and EN; Tc is next as per the EA chart
- The reversal of He compared to the rest of the NG reflects #24
- All of the Ln and An fall into an oval of basicity, bar Lr
- The reversal of the positions of Fr and Cs is consistent with Cs being the most electronegative metal
- A similar, weaker pattern is seen with Ba and Ra.
- About the Author
- Introduction
- The Periodic Table Exploration Begins!
- Isotopes and Nuclear Patterns
- Selected Trends in Atomic Properties
- First Period Problems
- The Group 3 Problem
- Categorizations of the Elements
- Isoelectronicity
- Group and Period Patterns among the Main Group Elements
- Patterns among the Transition Metals
- Group (n) and (n+10) Relationships
- Chemical "Knight's Move" Relationship
- Isodiagonality
- Lanthanoids, Group 3, and Their Connections
- Actinoid and Post-Actinoid Elements
- Pseudo-Elements
- Index
- s 20
- p –35
- d 125
- 4f 46,000
- 5f 522
- Ce is known at +4, Pr is known as +5, and I recall seeing some speculation about the possibility of Nd +6. (Pm +7 may be overreach.)
- Tl is lined up under Au even though Tl prefers +1. That said Au is not adverse to +1.
- I stopped at Hs since the limits of SHE chemistry just about runs out there.
- The dividing line between metals and nonmetals is 73 element box sides long.
- Dias JR 2004, "The periodic table set as a unifying concept in going from benzenoid hydrocarbons to fullerene carbons", in DH Rouvray & RB King (eds.), The periodic table: into the 21st century, Institute of Physics Publishing, Philadelphia, pp. 371–396 (375)
- Fernelius WC 1982, "Hafnium," J. Chem. Educ. vol. 59, no. 3, p. 242
- Greenwood NN & Earnshaw A 2002, Chemistry of the elements, 2nd ed., Butterworth-Heinemann, Oxford, p. 1148
- Habashi F 2010, "Metals: typical and less typical, transition and inner transition", Foundations of Chemistry, vol. 12, pp. 31–39
- Lee JD 1996, Concise inorganic chemistry, 5th ed., Blackwell Science, Oxford, p. 753
- Kornilov II 1965, "Recent developments in metal chemistry", Russian Chemical Reviews, vol. 34, no. 1, p. 33
- Küpfer YJ 1954, "Rhodium uses in plating", Microtecnic, Agifa S.A., p. 294 Niedenzu K & Dawson JW 1965, Boron-nitrogen compounds, Springer, Berlin, preface
- Oshe RW (ed.) 1985, "Handbook of thermodynamic and transport properties of alkali metals", Blackwell Scientific, Oxford, p. 987
- Paine et al. 2005, "Recent developments in boron-phosphorus ring and cage chemistry", in Modern aspects of main group chemistry, M Lattman et al. (eds.), ACS Symposium Series, American Chemical Society, Washington DC, p. 163
- Rayner-Canham G 2020, The periodic table: Past, present, and future, World Scientific, Singapore
- The traditional form of periodic table is a hybrid of an electronic and a chemistry based table.
- An electronic or physics-based table would show (a) He over Be; and (b) group 3 as Sc-Y-Lu-Lr; and (c) group 13 as B-Al-Ga-In-Tl
- A chemistry-based table would show (d) He over Ne; and (e) B-Al over Sc-Y-La-Ac.
- What we have instead is a hybrid table with 1(c) and 2(d). It is not as symmetric or tidy as the pure Lu form; neither is it as irregular as the form with three split blocks.
- Rang (1893)
- Gooch & Walker (1905)
- Cuthbertson & Metcalfe (1907)
- Baur (1911)
- Rydberg (1913)
- Black & Conant (1920)
- Lewis (1923)
- Hubbard (1924)
- Deming's table (1925), which popularised the medium-long form
- Antropoff (1926)
- LeRoy's table (1927)
- Irwin (1939)
- Seaborg (1945), with B left in group 13
- Yost & Russell (1946)
- Coryell (1952)
- Pauling's table (1960)
- Habishi's Metallurgist's Periodic Table (1992), Habishi leaves B in group 13
- H and P are almost on top of one another
- The proximity of Be to the post-transition metals, and its relative scarcity in the crust
- The metalloids, with their intermediate values of electronegativity, go down the middle. At the same time they span nearly the full range of abundance.
- B-Ga-Sc-Y-La are in a row
- N falls along the halogen line
- The abundance of O and Si, which we see in the form of silica
- F is more abundant in the crust than 85 percent of metals
- Al is the most abundant metal. Al and Fe are in the same vicinity: "Curiously, the chemistry of aluminium also resembles that of the iron(III) ion... These similarities may be ascribed to the same 3+ charge and near-identical ion radii (and hence charge density)." (Rayner-Canham 2020, p. 191)
- The abundance of Ar compared to the rest of the noble gases. Apparently this is influenced by the radioactive decay of potassium-40 in Earth's core, which is considered one of the main sources of heat powering the geodynamo that generates Earth's magnetic field. It has been suggested that a large amount of Ar may be present in the core, as the compound ArNi with an L11 Laves structure (similar to an intermetallic phase, and related to a cubic close packed lattice). ArNi is stabilised by notable electron transfer from Ni to Ar, changing their electron configurations toward 3d7 and 4s1. (Adeleke et al. 2019)
- Ti, a light yet strong metal, is about 2,500 times as abundant as Sn, a weak heavy metal
- Zn is an outlaw post-transition metal
- The most active 4d-5d transition metals (Zr, Hf) occupy a boundary overlap with the rare earth metals
- Ag, which has a largely main-group chemistry, is located in the PTM region. It is about 20 times as abundant as the noble meals
- Re is an outlaw noble metal
- Adeleke AA, Kunz M, Greenberg E, Prakapenka VB, Yao Y, Stavrou R 2019, A high-pressure compound of argon and nickel: Noble gas in the Earth's core?, ACS Earth and Space Chemistry, vol. 3 no. 11, pp. 2517-2544, https://pubs.acs.org/doi/10.1021/acsearthspacechem.9b00212
- Rayner-Canham G, 2020, The periodic table: Past, present, future, World Scientific, Singapore
- Metals with lower EN, i.e. < 1.7, or active nonmetals with higher EN, tend to be concentrated in silicate or oxide phases that are more easily found in the crust due to their lower density, and hence have higher abundances.
- Metals with moderate EN 1.7 to 2.1, say the later transition metals and post-transition metals, tend to form sulfide liquid phases; are less easily found in the crust due to their relatively higher densities; and are less abundant by about two orders of magnitude compared to the metals found in silicate or oxide phases.
- Metals with EN > 2.2, i.e. the noble metals, have an affinity for a metallic liquid phase, and are depleted in the crust since they generally sank to the core and hence have very low abundances. They are about two orders of magnitude less abundant than the sulfide metals.
- Cox PA 1997, The elements: Their origin, abundance and distribution;
- Gill R 2014, Chemical fundamentals of geology and environmental geoscience;
- White WA 2020, Geochemistry
- For the nonmetals, the relative average abundance proportions are about 5: 700: 250: 1 for, respectively, the metalloids; the core nonmetals H, C, N, P, S, and Se; the halogen nonmetals; and the noble gases. Si and O were left out as outliers, in terms of their massive abundances.
- Thus, metalloids aside, the abundance of the nonmetals tends to fall with increasing EN. I don't know what's going on with the metalloids.
- The chart may prompt some further appreciative enquiry:
- In the case of exceptions to the initial three generalisations why do these occur?
- Why is Li so rare, compared to the other alkali metals?
- Why is Si good at forming a planetary crust?
- Why do the metalloids span such a wide range of abundances?
- If H is supposed to make up ca. 74% of the universe why does it have the same abundance in the Earth's crust as P?
- In what form is H found in Earth's crust—water, hydroxides?
- If H is supposed to make up ~ 74% of the universe why does it have the same abundance in the Earth's crust as P?
- Are there any chemical similarities between H and P, given both have some metalloidal character? The have virtually identically electron affinities. H is sometimes positioned above B due to chemical similarities. It then forms a diagonal relationship with C, which in turn has a diagonal relationship with P, which has a diagonal relationship with Se e.g. P reacts with Se to form a large number of compounds characterised by structural analogies derived from the white phosphorus P4 tetrahedron.
- The rare earth metals are relatively rare, having an average abundance of 1% that of the 3d metals. That being so, why is their rareness sometimes questioned? Why does the crustal abundance of the REM plummet by two orders of magnitude towards the end of the lanthanides?
- There is symmetry in this version.
- The physiochemical relationship of He to Ne is retained.
- There is a loss of physiochemical regularity in placing He over Be. Even if helium can be enticed to become chemically active, it will still be very much better located in group 18.
- While the d, p, and s blocks start with the appearance of the relevant electron, there is a loss of consistency with La at the start of the f-block. This is confusing to students since there is no such inconsistency in the La form.
- In terms of predominant differentiating electrons in each block, this form is less consistent than an La table.
- There is one less form of "element block-type" symmetry, than in the La form.
- The table is made up of 67 cubes stacked onto each other, having three sides exposed(top, left and right)
- The top faces contain s and p-block elements
- The left side faces contain d block and right side faces contain f block
- There are two different Major Groups: A and B
- Major Group A is divided into 14 minor groups (from -1 to 12) and Major group B is divided into 8 minor groups (from 3 to 12)
- Major Group A applies to s, p & f-block. Major Group B is exclusively for d-block.
- To go down a group we follow the arrow and descend the stair in the given direction
- Although being 3 dimensional, it can be easily represented in 2 dimensions in the form of trisected hexagons
- All the elements of the same period lie in the same line (unlike MPT where f-block elements had to be depicted separately due to lack of space).
- Viewing the table from 3 different directions makes only one or two blocks visible:
1. From top: s & p-block
2. From left: d-block
3. From right: f-block - This helps in diffrentiating between the blocks easily
- The disturbing gap between s and p-block of traditional periodic table is not simply there. All advantages of Modern Periodic Table remain conserved.
- Makeev A.K. Julius Lothar Meyer was the first to build a periodic system of elements // European applied sciences, No. 4 2013, (April) volume 2. - pp. 49-61. ISSN 2195-2183.
- A.K. Makeev. Self-reproduction of matter // Materials of the international scientific-practical conference: "Prospects for the Development of Modern Science" – Jerusalem, Israel: Regional Academy of Management, 2016. – 535 p. P. 213-220. UDC 001.18 BC 72 P 93 ISBN 978-601-267-398-2 https://drive.google.com/file/d/0B_W2hkSE3iXram5DX1FoX3NsLVE/view?resourcekey=0-OFX6dMXY-xOGnOLDyxFvLQ
- A.K. Makeev. The essence of time. // Prose.ru http://proza.ru/2015/11/18/796
- A.K. Makeev. What is the natural ending of periods? // Prose.ru https://proza.ru/2019/09/28/115
- A.K. Makeev. Is hydrogen a dielectric gas or an alkali metal? // Prose.ru https://proza.ru/2016/09/28/1721
- A.K. Makeev. Do vacuum and ether participate in gravity? // Prose.ru https://proza.ru/2019/04/16/1893
- Bjerrum, N (1936). Bjerrum’s Inorganic Chemistry. London: Heinemann
- Hein, M; Arena, S (2013). Foundations of College Chemistry. Hoboken: John Wiley & Sons. pp. 226, G-6. ISBN 978-1-118-29823-7.
- Oderberg DS 2007, Real Essentialism, Routledge, New York, ISBN 978-1-134-34885-5
- Vernon R 2013, "Which elements are metalloids?", Journal of Chemical Education, vol. 90, no. 12, 1703?1707, doi:10.1021/ed3008457
- Deming
- The literature since his time, as shown
- The expected behaviour of the super-heavy elements
- The smoothness of Z vs physiochemical property trendlines going down groups, for up to 40 physiochemical properties
- Achimov EI 1946, Zhur. Obshchei Khim., vol. 16, p. 961; https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=776
- Baca Mendoza O 1953, Leyes Genéticas de los elementos Químicos, Nuevo Sistema Periódico, National University of Cusco, https://www.meta-synthesis.com/webbook/35_pt/Mendoza_PT.pdf, accessed May 12, 2024
- Gutiérrez-Samanez JA 2020, Binódic periodic system: a mathematical approach, Found Chem, vol. 22, pp. 235–266 (255)
- Janet C1928, Essais de classification hélicoïdale des éléments chimiques. Imprimerie Départementale de l’Oise, Beauvais, https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=152
- Jensen WB 1986, Classification, symmetry and the periodic table, Computers & Mathematics with Applications, vol. 12B, no. 12, pp. 487–510 (508)
- Rydberg JR 1913, Untersuchungen über das system der grundstoffe, Lunds Univ. Ärsskrift, vol. 9, no. 18. In French: 1914, Recherches sur le système des éléments, Journal de Chimie Physique, vol. 12, p. 585
- Saz E 1931, Iberica, vol. 35, p. 186
- Wemer A 1905, Beitrag zum Aufbau des periodischen Systems, Ber. Deut. Chem. Ges, vol. 38, pp. 914–921, 2022–2027
"The numbers below each element symbol refer to the crystal: 1 = FCC, 9 = graphite structure, 11 = orthorhombic, etc. Extra numbers are for structures at higher temperatures.
"The wriggly lines between groups 3 and 4, and 11 and 12 refer to a gradation between the classes involved. Wikipedia calls these linking or bridging groups
"Harrington's class names are novel. [Who would have thought of the elements of groups 1 to 3 as being called the "salts of electrons"?] Then again, "in view of the extensive role that electrons play as anions" Dye (2015) asked: "where should electrons be placed in the periodic table?" (Note: In 1946 Achimof tried answering this, with an electron as element -1 above H and a neutron as element 0 above He.)
"Aluminium appears in group 3 and group 13 since, according to Harrington, it has the crystalline structure of a true metal. This is not quite true since its crystalline structure shows some evidence of directional bonding.
"For the transition metals as "wandering bonds", Harrington writes that the metallic bond is spatially undirected and that it may operate between any given atom and an indefinite number of neighbours" (p. 145). Since A-metals are better called, in his mind, "salts of electrons" [and B-metals show signs of significant directional bonding] the transition metals are therefore called by him as wandering bonds. This becomes confusing, however, given d electrons in partially filed d-orbitals of transition metals form covalent bonds with one another.
"Counting boron as a pseudo metals looks strange.
"Germanium is counted as a metal: "...the electrical conductivit[y]... [is] sufficiently high to show that the outer electrons are very loosely held and the linkage must be partly metallic in character." (p. 148). In fact the electrical conductivity of high purity germanium, which is a semiconductor, is around 10–2S.cm–1. Compare this with antimony, at 3.1 x 104S.cm–1
"Tin has brackets around it to show its "renegade" status, "with its white form behaving largely as would a True Metal, whereas its grey form is more non-metallic than metallic." White tin actually has an irregularly coordinated structure associated with incompletely ionised atoms.
"Thallium and lead have brackets around them since their crystalline structures are supposedly like those of true metals. This is not quite right. While both metals have close-packed structures they each have abnormally large inter-atomic distances that have been attributed to partial ionisation of their atoms.
"The B-subgroup metals are divided into pseudo metals and hybrid metals. The pseudo metals (groups 11 and 12) behave more like true metals than non-metals. The hybrid metals As, Sb, Bi, Te, Po, At – which other authors would call metalloids – partake about equally the properties of both. According to Harrington, the pseudo metals can be considered related to the hybrid metals through the carbon column.
"The location of the dividing line between metals and nonmetals, running as it does through carbon to radon is peculiar. The line is usually shown running through boron to astatine."
| Year: 1947 | PT id = 85, Type = formulation spiral |
Stedman's Design
In his article Stedman says:
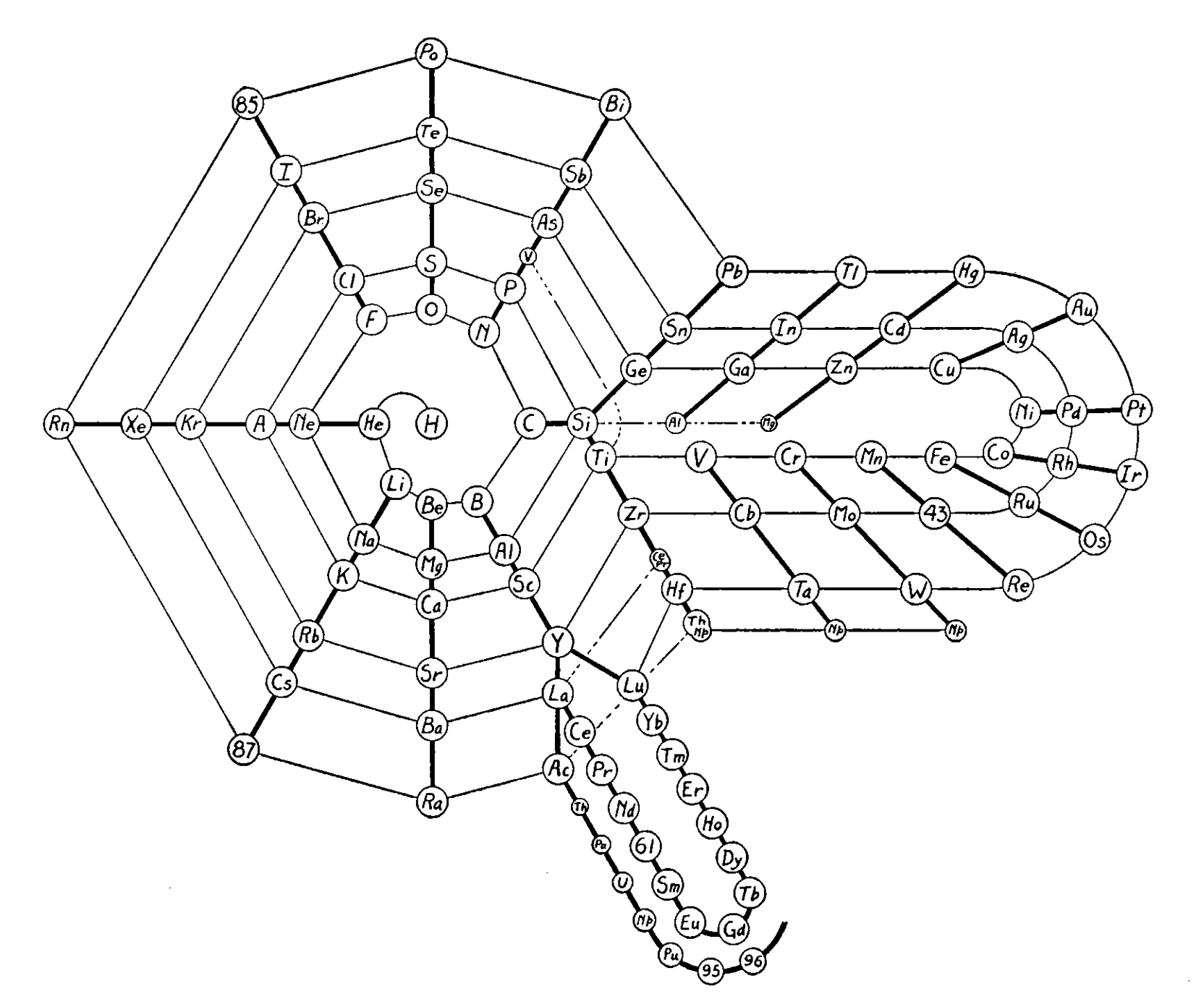
Thanks to René for the tip!
| Year: 1947 | PT id = 1166, Type = formulation |
Ageev's Crystalline Structures of The Elements
Ageev NV 1947, The nature of the chemical bond in metal alloys (Izdvo Akad. Nauk SSSR, Moscow/Leningrad, p. 10
René Vernon writes:
"In this curious 18-column table, showing the crystalline structures of the elements, Ageev locates the predominately non-metallic groups on the left and the remaining groups on the right.
"It's odd that he located boron and aluminium on the far left over gallium, rather than over scandium. I suppose he did this so that gallium, indium, and thallium would not be mistaken for d-block metals.
"Reading from left to right then, Ageev's table could be said to be made up of five blocs:"
[1] the nonmetallic bloc
[2] the alkaline bloc
[3] the inner transition bloc
[4] the transition metal block
[5] a post-transition metallic bloc
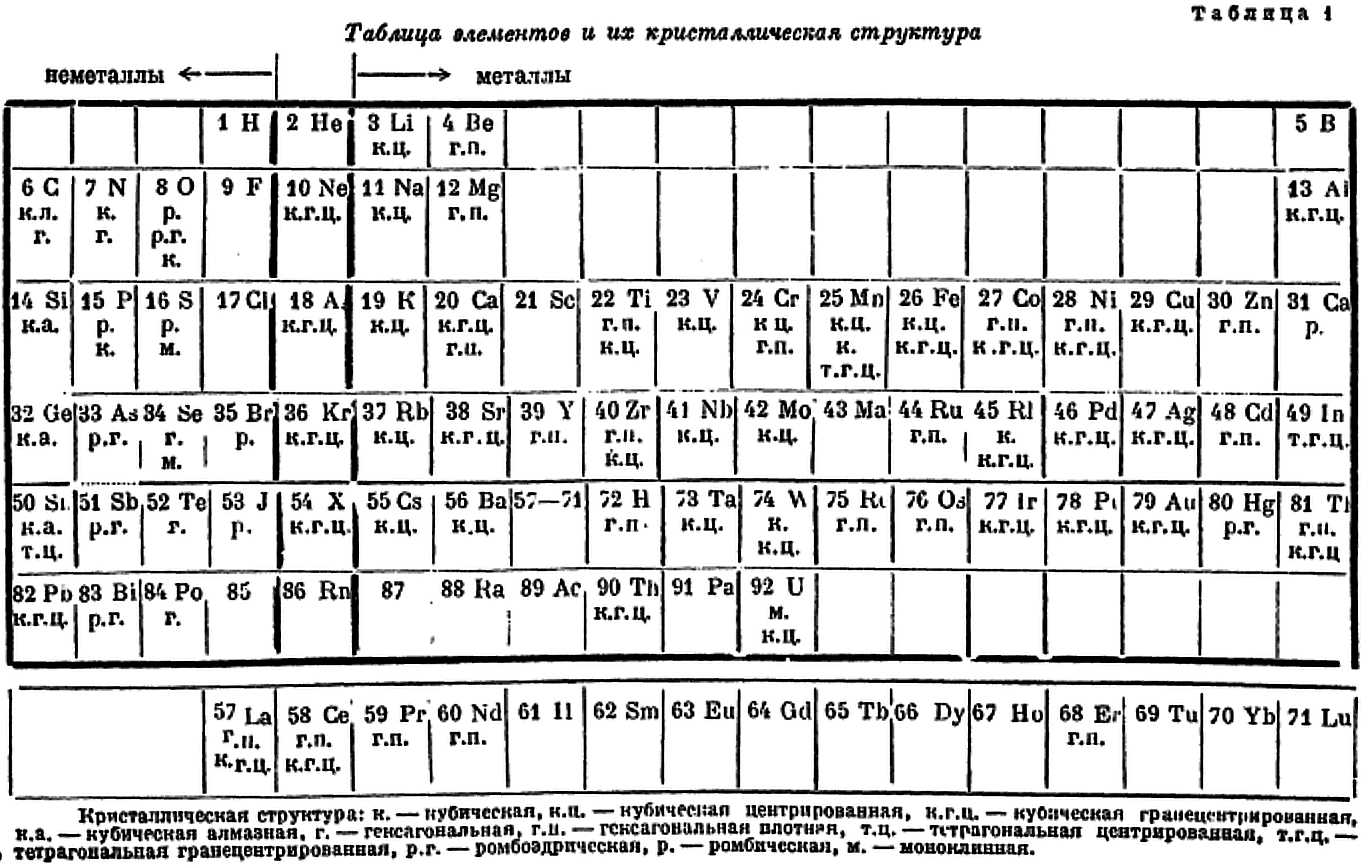
| Year: 1947 | PT id = 1243, Type = formulation |
Science Service: Two Periodic Tables
A two-sided Science Service periodic table from 1947. The one is listed as "After Bohr", the other as "After Mendeleeff".
René Vernon writes:
"Here’s a slightly odd table (with two sides):
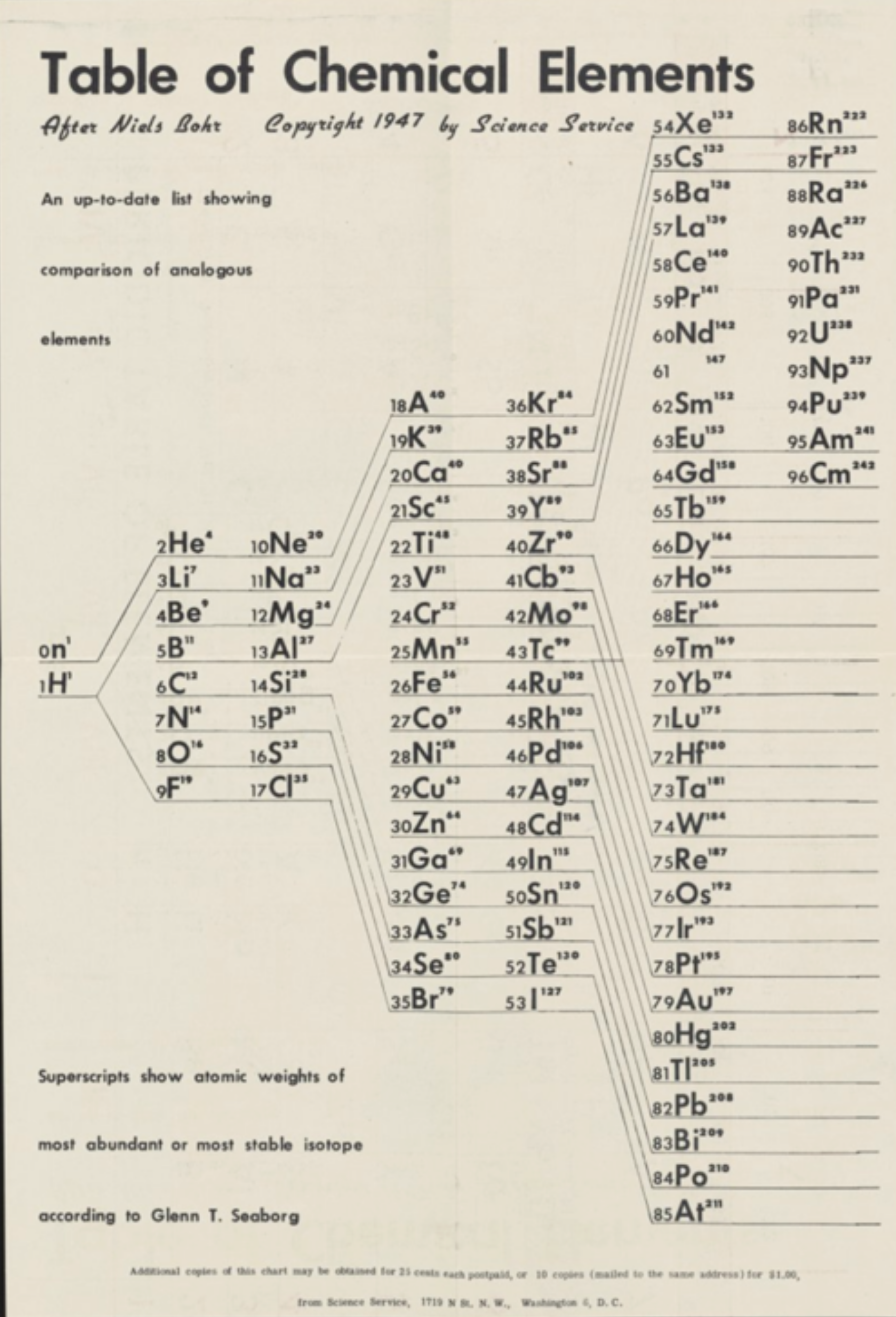
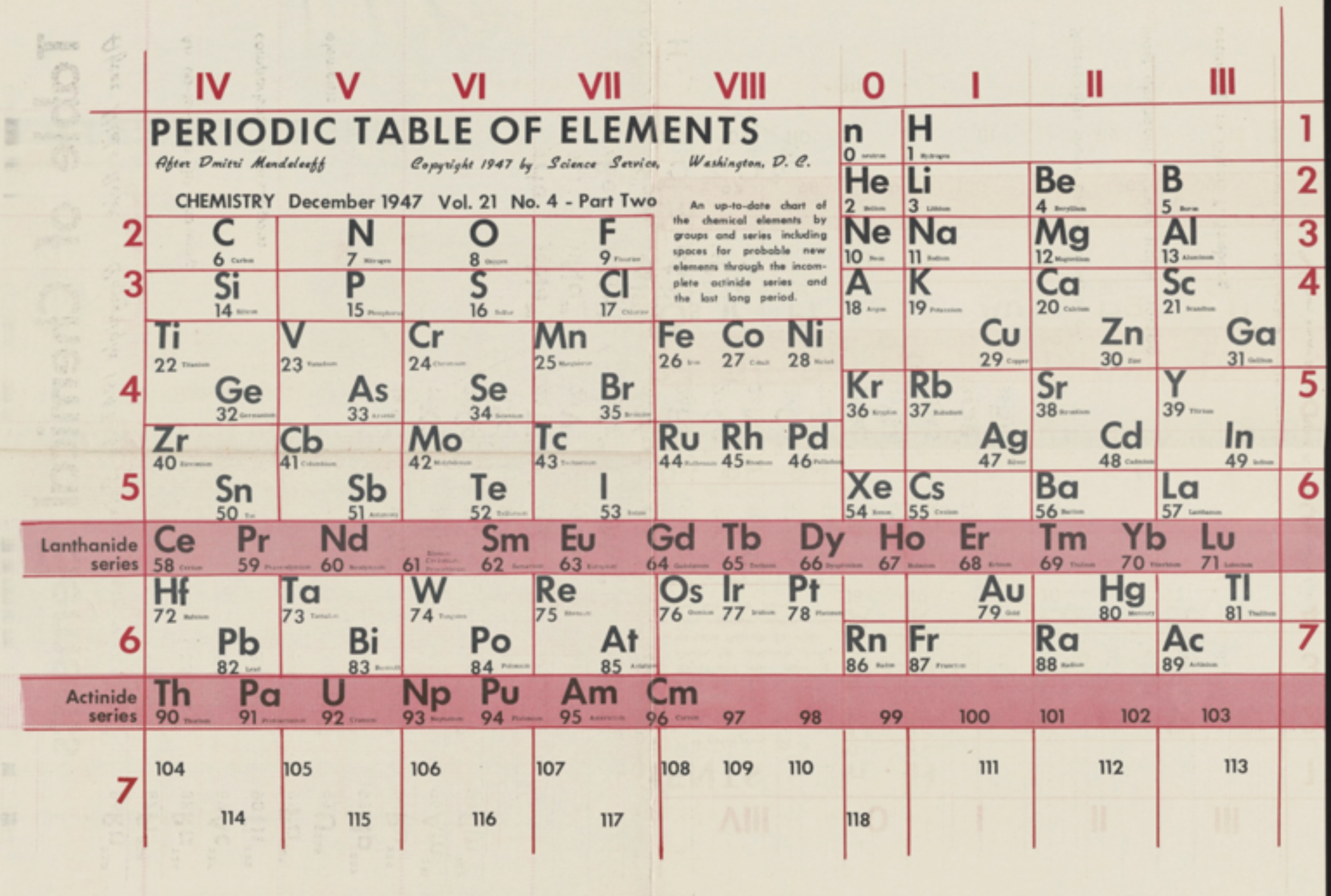
| Year: 1949 | PT id = 1052, Type = formulation |
Catalán's Periodic System/Sistema Periodico Ampliado
Two versions of Catalán's Periodic System/Sistema Periodico Ampliado. The first from Moore, Charlotte E., Atomic Energy Levels, National Bureau of Standards, Circular no. 467 (Washington, D. C.: U. S. Government Printing Office, 1949), vol. 1, Table 25. and the second as referenced here: http://www.miguelcatalan.net/pdfs/bibliografia/biblio09.pdf.
René Vernon, who provided the graphics, writes:
"I feel the footnote along the base of the first table could merit better attention being drawn to it. It says:
This arrangement is by Catalán. The electrons indicated in column two that are connected by braces have approximately the same binding energy. Consequently, for some elements one type of electron is preferred over another in the normal configuration, as for example, Cr, Cb, Pd, La, Ac, Th.
"The connecting braces hone in on the source of much of the controversy concerning notions of an ideal, optimal, better, this or that, or fundamental periodic table. I can't recall seeing a table with such a feature. For the second table, turning it on its side (attached) reminds of the ADOMAH [formulation].
Click on the images to enlarge:
Thanks to René for the tip!
| Year: 1949 | PT id = 295, Type = formulation 3D |
Wrigley's Lamina System
In two papers: A.N. Wrigley, W.C. Mast, and T.P. McCutcheon, "A Laminar Form of the Periodic Table, part 1," Journal of Chemical Education 26 (1949): 216-218 and A.N. Wrigley, W.C. Mast, and T.P. McCutcheon, "A Laminar Form of the Periodic Table, part 2: Theoretical Development, and Modifications," Journal of Chemical Education 26 (1949): 248-250 a Laminar Periodic Table is introduced. (Thanks to Ann E. Robinson for this informaton & references.)

This formulation was discussed and re-drawn by van Spronsen in 1969:
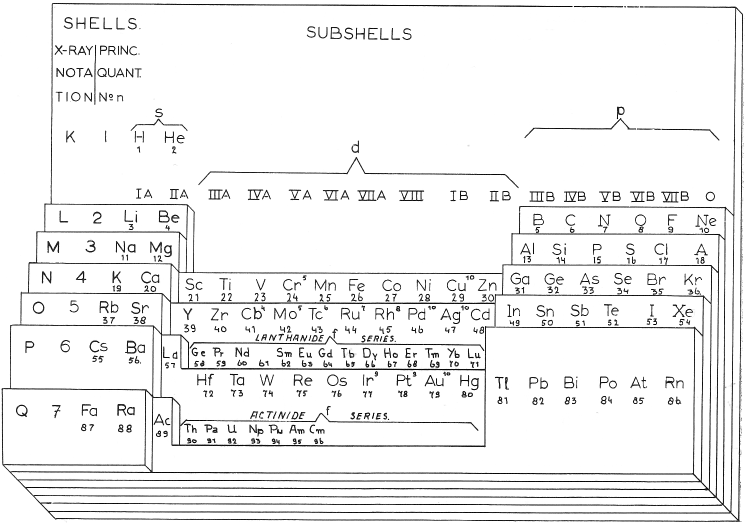
There is a Russian publication "100 Years of Periodic Law of Chemical Elements", Nauka 1969. On page 87 there is a formulation that appears to be a version of the van Spronsen re-drawing. The caption says: "Volumetric Model of 18-period Long System of D.I.Mendeleev." after Riggli (1949). (Thanks to Larry T for this.)
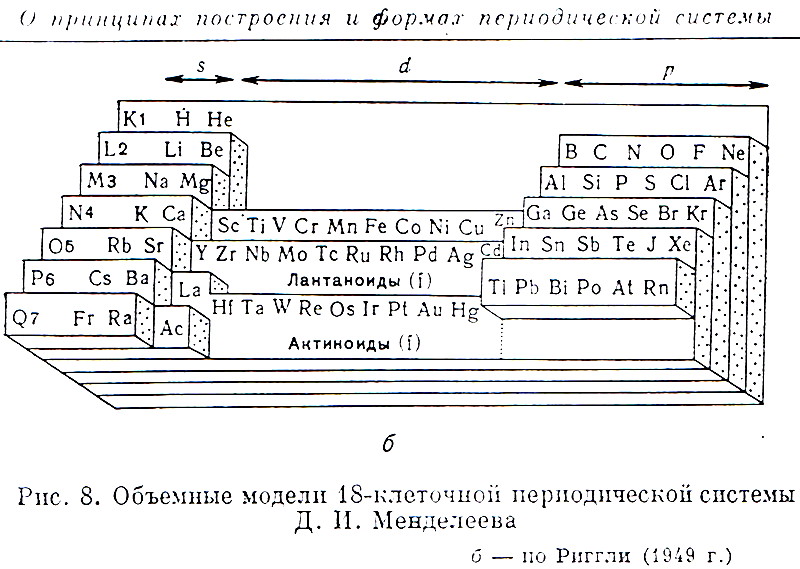
| Year: 1949 | PT id = 1018, Type = formulation 3D |
Scherer's Student Model of Spiral Periodic Chart
George A. Scherer, New Aids for Teaching the Periodic Law, School Science and Mathematics, vol. 49, no. 2 (1949).
René Vernon writes:
"This is a Left-Step periodic table with a split d-block, that can be rearranged into a cylinder. Students were expected to keep a copy of the two halves of the table in their note books, for reassembly as required. It was a clever way of introducing the 32-column form, and the transition from 2D to 3D (that faded into obscurity)":
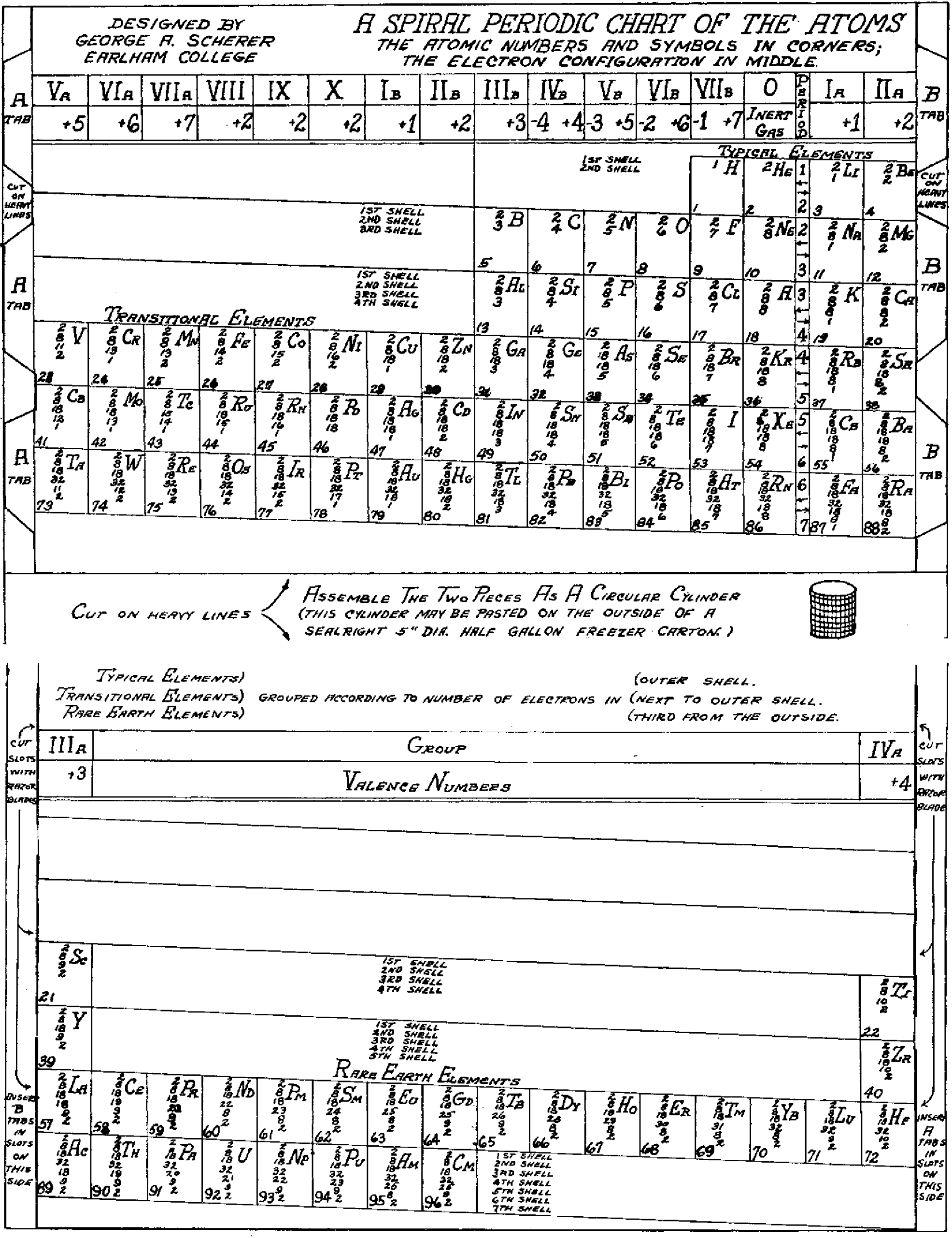
Thanks to René for the tip!
| Year: 1950 | PT id = 14, Type = formulation |
The modern periodic table is based on quantum numbers and blocks, here.
A periodic table can be constructed by listing the elements by n and l quantum number:
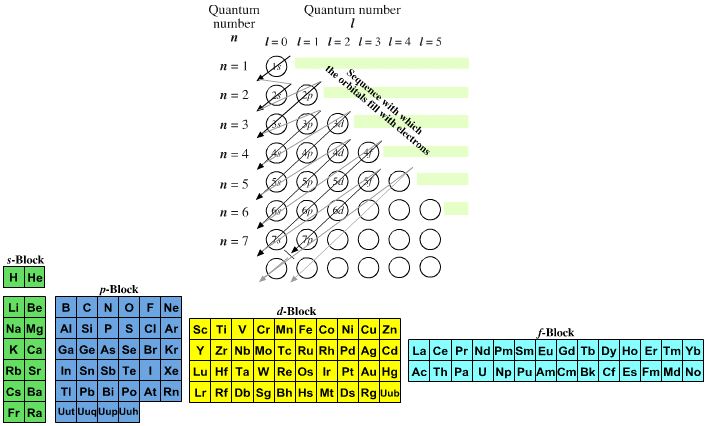
The problem with this mapping is that the generated sequence is not continuous with respect to atomic number atomic number, Z: Check out the sequence Ar to K, 18 to 19.
Named after a French chemist who first published in the formulation in 1929, the Janet or Left-Step Periodic Table uses a slightly different mapping:
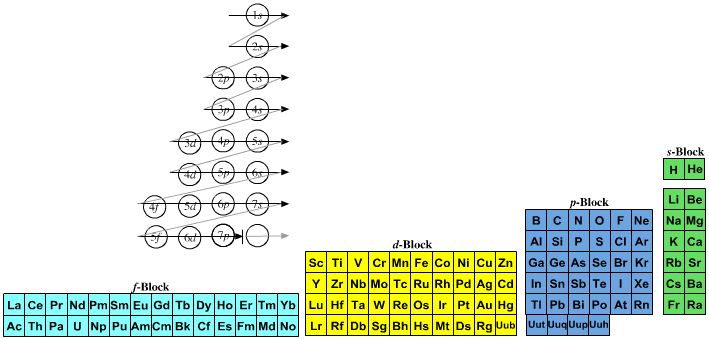
While the Janet periodic table is very logical and clear it does not separate metals from non-metals as well as the Mendeleev version, and helium is a problem chemically.
However, it is a simple mapping to go from the Janet or Left-Step periodic table to a modern formulation of Mendeleev's periodic table:
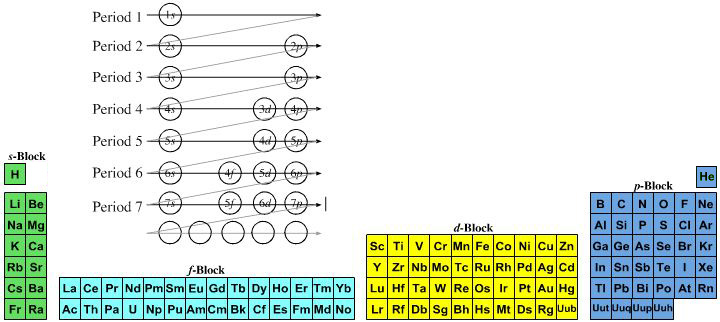
On this page web, "full" f-block included periodic tables are shown wherever possible, as above.
However, the periodic table is usually exhibited in book and on posters in a compressed form with the f-block "rare earths" separated away from the s-block, p-block and d-block elements:
However, the compression used introduces the well known problem known as a "fence post error".
The effect is that:
La and Ac: move from f-block to d-block
Lu and Lr: move from p-block to f-blockChemically, the elements can be fitted in and classified either way. Many thanks to JD for pointing the situation with the periodic table is a fence post error.
Mark Winter's Web Elements project, here, uses the formulation shown below:
Interestingly, the IUPAC periodic table separates out 15 lanthanides, La-Lu, and 15 actinides, Ac-Lr by leaving gaps in period 3 under Sc & Y:
This corresponds to:
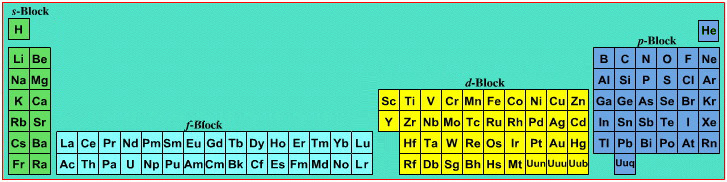
By Mark Leach
| Year: 1950 | PT id = 1080, Type = formulation |
Sidgwick's Periodic Classification (Mendeleeff)
From N.V. Sidgwick, Chemical Elements and Their Compounds, vol. 1, Oxford University, London, p. xxviii (1950).
René Vernon writes:
"In this curious table the Lanthanides are located in group IIIA while the Actinides have been fragmented.
Instead:
• Ac, Th, and Pa are located in groups IIIA, IVA and VA under Lu, Hf, and Ta, respectively
• The uranides, U, Np, Pu, Am, and Cm, are located in group VIA, under W."
Sidgwick writes:
"This subgroup (VIA) consists of Cr, Mo, W, and U, to which the 'uranide' elements, Np, Pu, Am, and Cm (which might be assigned to any Group from III to VI) must now be added." (p. 998)
"...the trans-uranium elements 93–6... for the first time give clear evidence of the opening of the 'second rare earth series', the 'uranides', through the expansion of the fifth quantum group from 18 towards 32." (p. 1069)
"The question whether the fifth quantum group of electrons which is completed up to 18 in gold begins to expand towards 32, as the fourth does in cerium, has now been settled by the chemical properties of these newly discovered elements. In the Ln the beginning of the expansion is marked by the main valency becoming and remaining 3. With these later elements of the seventh period there is scarcely any sign of valencies other than those of the group until we come to uranium... Up to and including uranium, the group valency is always the stablest, but beyond this no further rise of valency occurs, such as we find in rhenium and osmium. Hence the point of departure of the new series of structures (corresponding to lanthanum in the first series) is obviously uranium, and the series should be called the uranides. (p. 1092):
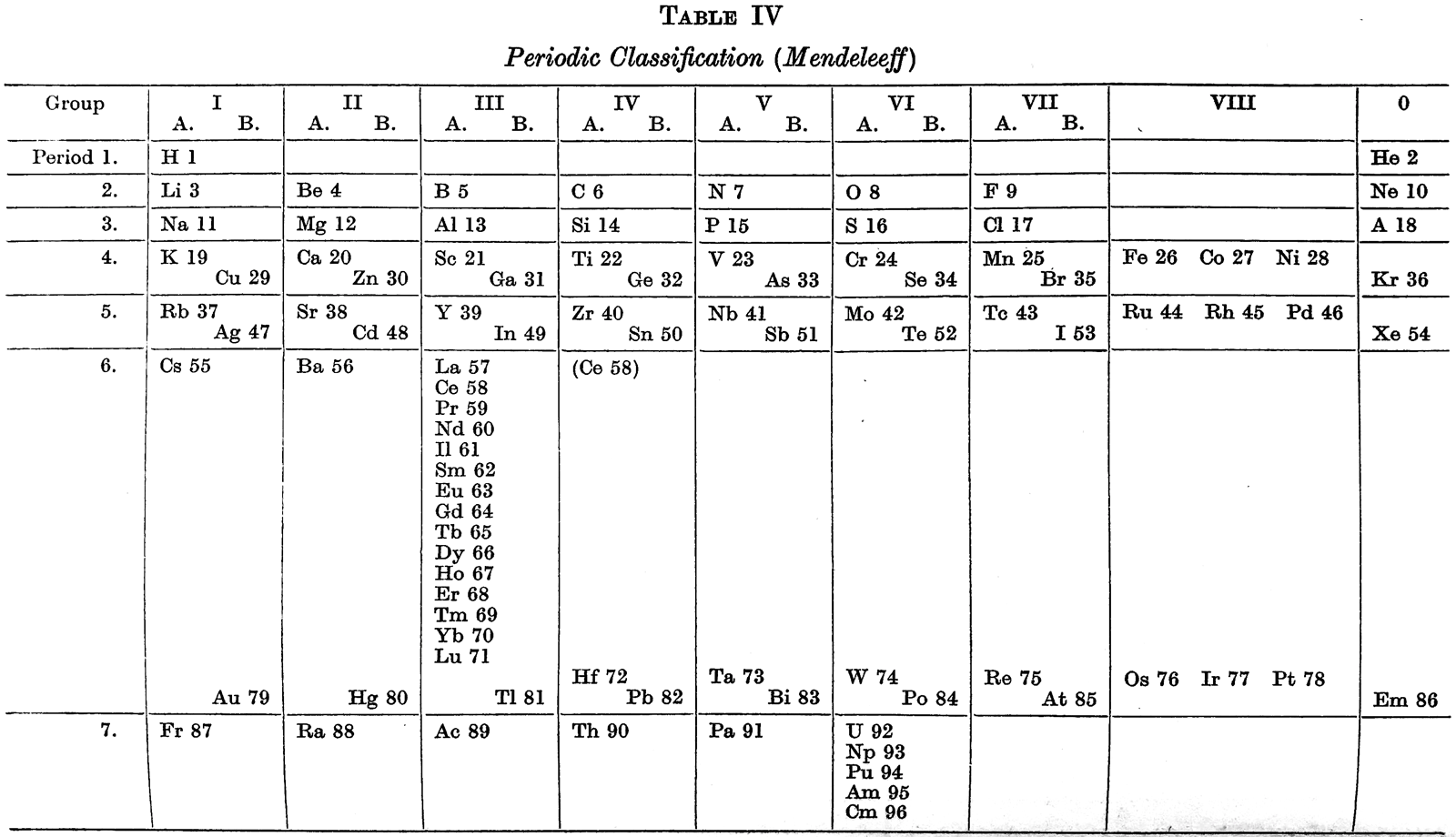
| Year: 1950 | PT id = 1119, Type = formulation 3D |
McCutchon's Simplified Periodic Classification of the Elements
McCutchon KB, A simplified periodic classification of the elements, Journal of Chemical Education, vol. 27, no. 1, pp. 17–19 (1950)
This 3-dimensional table has two double-sided flaps attached. The top flap is the f bock. Under that is the d block.
The superscripts denote the number of d electrons an element has. Thus, La1 is shown as being an f1 element. But it has a 1 superscript, meaning that the f electron count is reduced by 1 and the d electron count is 1.
René Vernon writes:
"On group 3, McCutchon cryptically says: The proposed arrangement brings out certain known facts about the tertiary elements which are rarely shown by other arrangements. For example, it suggests, correctly, that the resemblance between yttrium and lutecium is greater than that between yttrium and lanthanum. It classifies lanthanum but not lutecium as a rare earth, in accordance with their chemical properties (which also contradict spectrographic evidence at this point). It also demonstrates the tetravalence of both cerium and thorium, and that thorium and protactinium show a resemblance in chemical properties to zirconium and niobium, as well as to hafnium and tantalum."
I say "cryptically" because McCutchon presents no further evidence in support of his assertion that the resemblance between Y and Lu is greater than between Y and La. He may have had in mind the fact that Lu is more often found in ores of Y than is the case for La... and I don't understand his reference to spectrographic evidence.
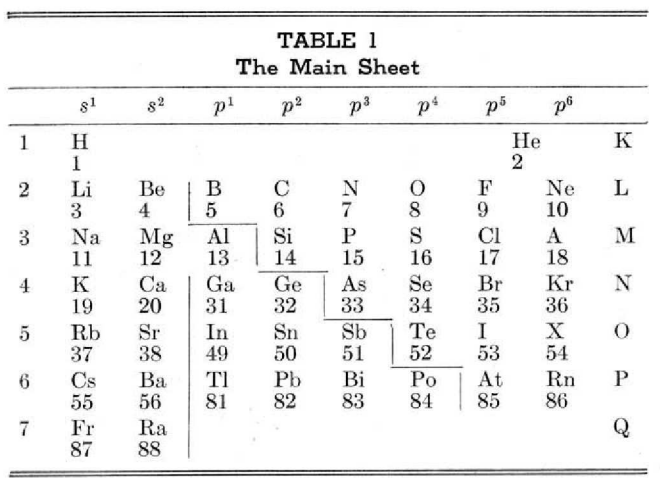
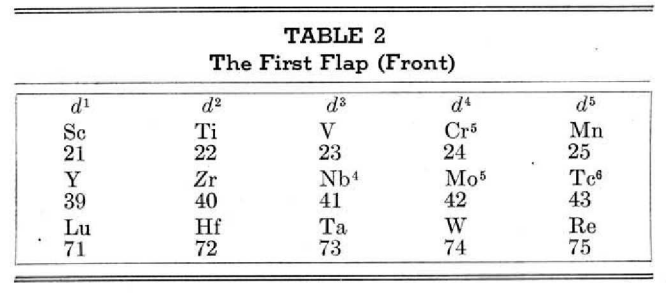
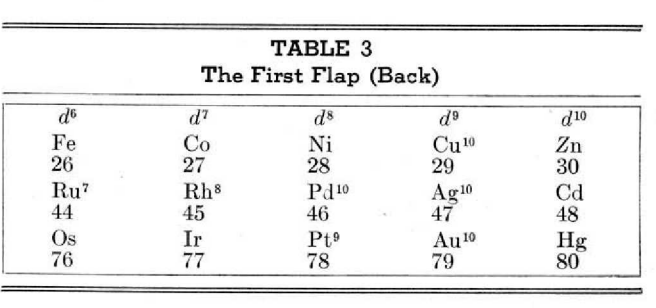
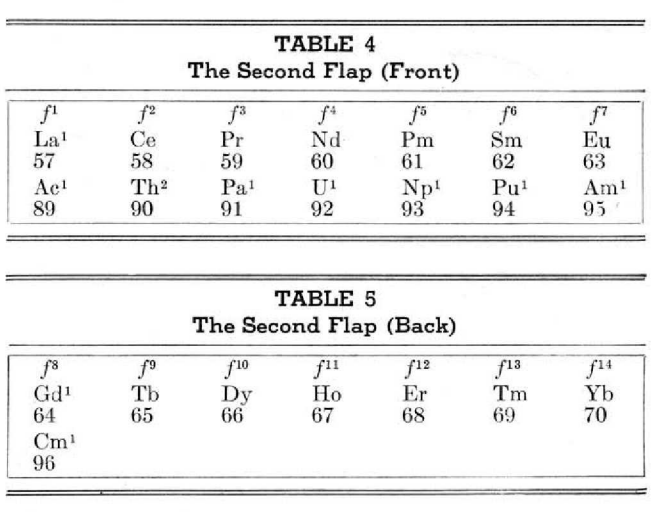
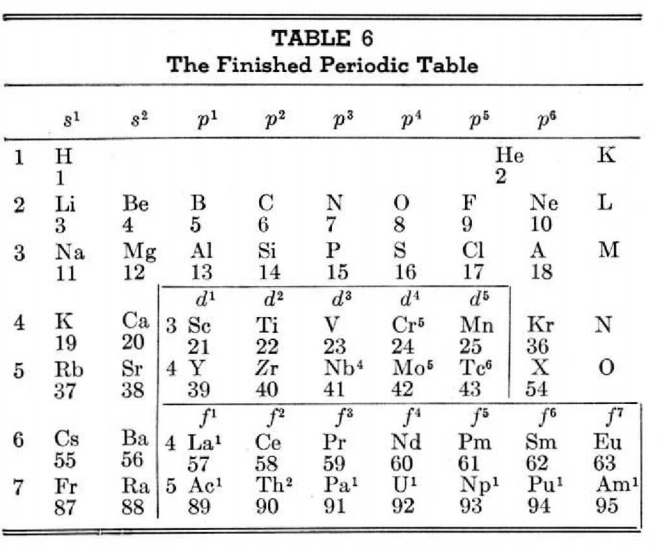
| Year: 1951 | PT id = 1324, Type = formulation |
Kapustinsky's Structure of The System of Elements
René Vernon writes:
Kapustinsky AF 1951, Structure of the periodic table of chemical elements (in Russian), Proceedings of the USSR Academy of Sciences, vol. 81, no. 1, pp. 47–50
Below the title Kapustinsky gives two equations that he says determine the structure of the system:
The legend at the bottom left is:
Kapustinsky refers to the periodic system of elements in terms of its emergence (proto-elements), formation (typical elements), and disintegration (synthetic elements). Kapustinsky refers to e, n, H, He as "proto-elements".
The electron and the neutron are not chemical elements but are elements in the sense of each being a rudiment, which means a beginning; an initial or imperfect form or stage. As Kapustinsky says, the properties of ordinary elements are not yet associated with them.
H and He can be considered "proto-elements" in the sense that they were the first building blocks from which heavier elements were later formed through nucleosynthesis in stars. Kapustinsky says that the system is thus:

| Year: 1951 | PT id = 1245, Type = formulation |
Friend's Updated Periodic Table
René Vernon writes:
"This 1951 table succeeds Friend’s table of 1926. Notice how Pu, Am, and Cm have been assigned to group VIII. The splitting of the Ln across two periods is bizarre."
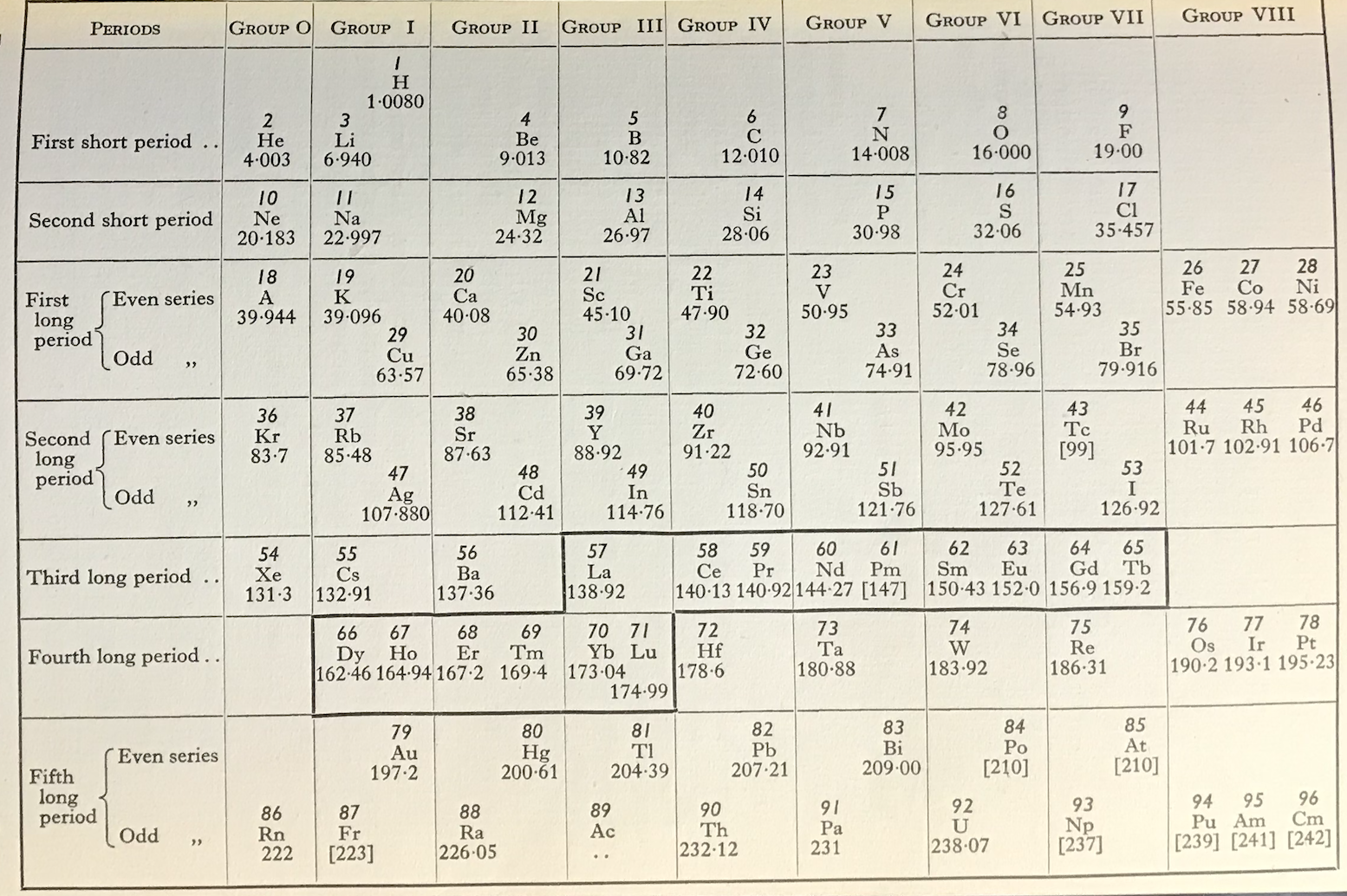
| Year: 1951 | PT id = 1272, Type = formulation |
Spedding's Rare Earths Periodic Table
Ref: Spedding FH 1951 The Rare Earths, Scientific American, vol. 185, no. 5, pp. 26–31
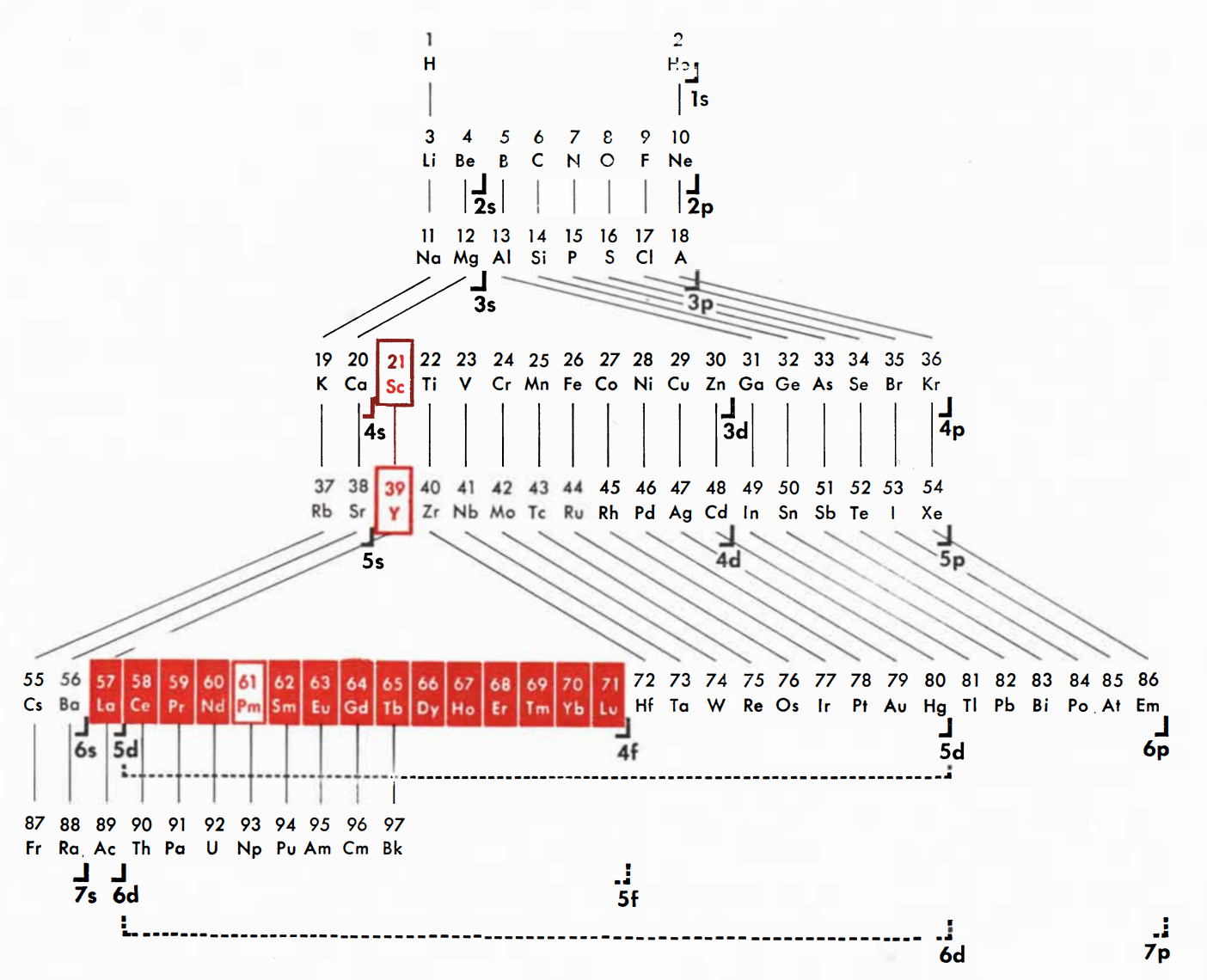
Thanks to René for the tip!
| Year: 1952 | PT id = 1054, Type = formulation |
Coryell's Periodic Table in Long Form
Charles D. Coryell The periodic table: The 6d-5f mixed transition group, J. Chem. Educ., vol. 29, no. 2, pp. 62–64 1952.
Coryell (1912–1971), was an American chemist involved in the discovery of promethium.
René Vernon writes:
"In Coryell's table, just two elements are shown as having two solid 'tie lines':
Yttrium: to La-Ac and to Lu-Ac
Silicon: to Ti-Zr-Hf and to Ge-Sn-Pb.
"These days Ti-Zr-Hf-Rf is deemed to make-up group 4 (rightly so given group 4 is the first to exhibit characteristic transition metal properties) whereas C-Si-Ge-Sn-Pb-Fl is deemed to make-up group 14.
The solid tie lines Coryell shows between Hf-Th, Ta-Pa, and W-U would now be rendered in broken form.
If Coryell's table was mapped to a 32- or 18-column form, group 3 would presumably be shown as bifurcating after Y.
The circle around indium is possibly a typo(?): indium has two stable isotopes, In-113 (4.29%) & In-115 (95.71%)... actually, In-155 has a half-life of 4.4x1014 years."
| Year: 1952 | PT id = 988, Type = formulation |
Hakala's Periodic Law in Mathematical Form
Reino Hakala published a paper, The Periodic Law in Mathematical Form, J.Phys.Chem., 1952, 56(2) 178-181. It is argued that: "Janet's [left-step] best meets these requirements".
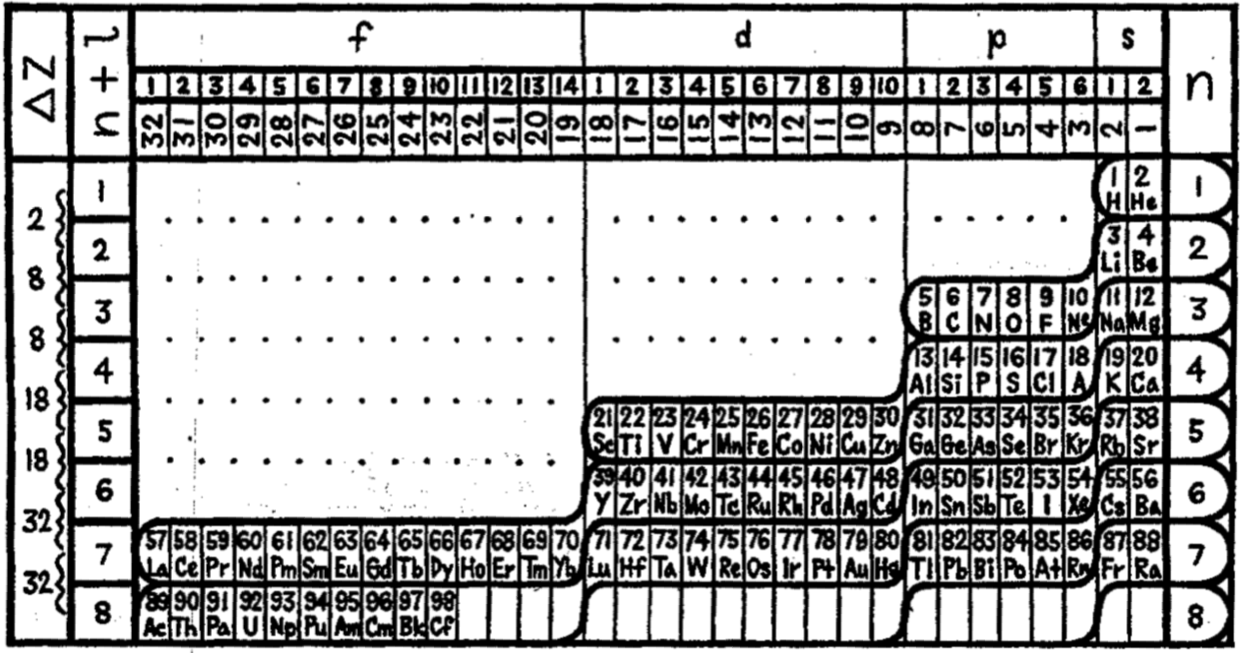
Thanks to René for the tip!
| Year: 1953 | PT id = 1387, Type = formulation 3D spiral |
Kapustinsky's Pyrimid
Kapustinsky, A. F. (1953). Periodicity in the structure of the electron envelopes and nuclei of atoms Communication 1. Periodic system of the elements and its connection with the theory of numbers and with physicochemical analysis. Bulletin of the Academy of Sciences of the USSR Division of Chemical Science, 2(1), 1–9. Paper as pdf.
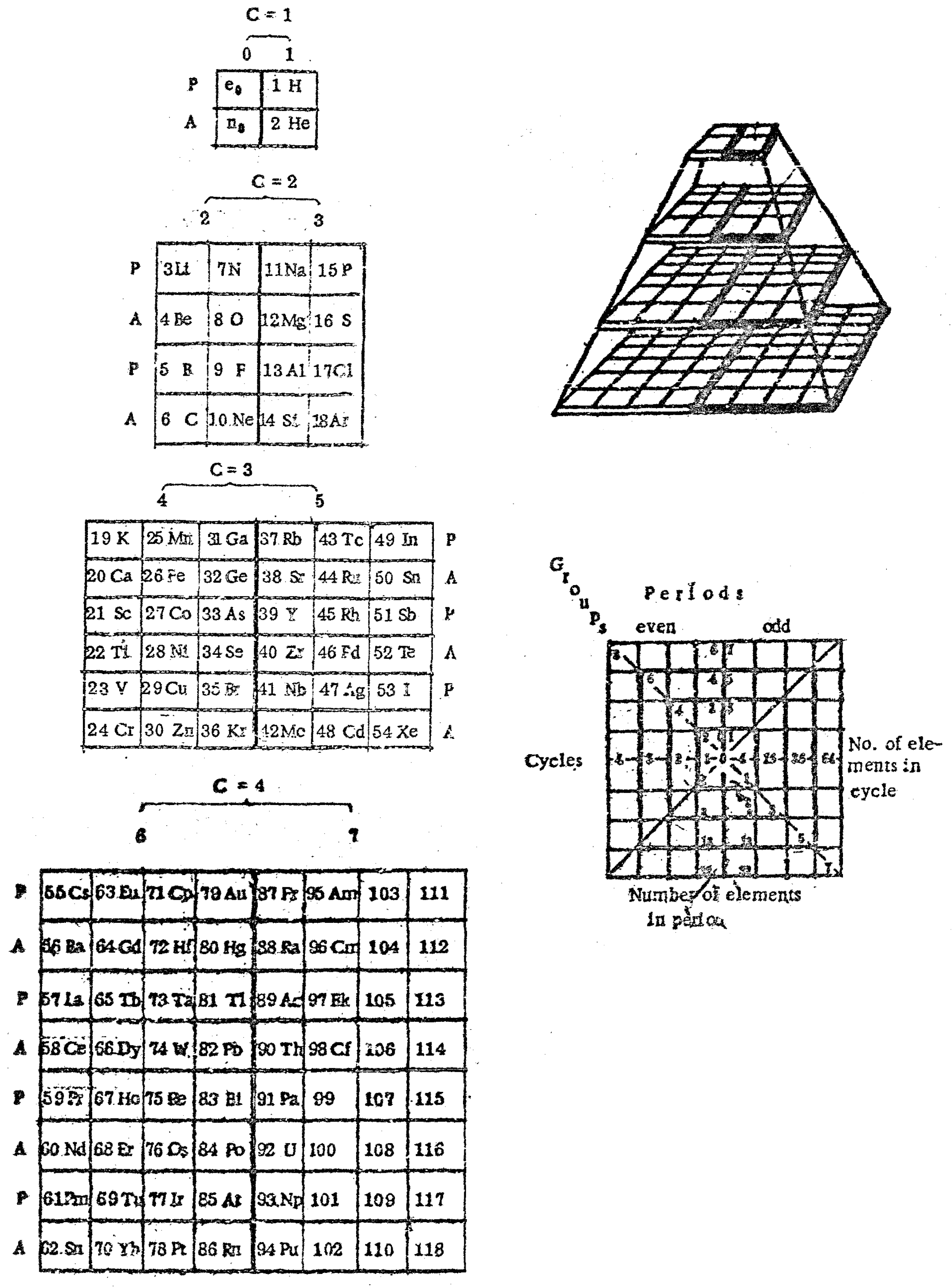
Thanks to René Vernon for the tip!
| Year: 1953 | PT id = 397, Type = formulation |
Chaverri-Rodríguez Tabla Periódica de los Elementos Químicos
Spanish to English translation from here.
Click here to see a larger version.
Originally published: Tabla Periódica de los Elementos. J. Chem. Educ. 1953, 30, 632-633
"The arrangement of the Periodic Table of the Elements according to Gil Chaverri-Rodríguez mainly takes into account the electronic structure of an element in determining the element's position in the table. It takes into account the different periods of elements have different length, because the first is of two elements, then followed by two periods of eight elements each, then two periods of 18 elements each, then a period of thirty-two elements and finally a seventh period incomplete.
"The table takes into account the important fact that, despite the variable length, the first two elements and the last six items in each period respectively have similar properties, forming the eight groups or columns of representative elements with similar chemical properties. The elements that constitute the Series Transition and Rare Earth Series are arranged in rows, in locations that correspond to how energy sublevels are filled that characterize these elements. Each element corresponds to a specific place and only in a box in the table, with no need to drop items off the table, at the foot of it, as in previous arrangements.
"With the information provided by the Board, you can deduce the electronic structure of a component, from its placement on the table, except the few cases that have small irregularities. In general, the Table is a settlement based on the electronic structure of chemical elements and this criterion determines its position in the array.":
| Year: 1954 | PT id = 1317, Type = formulation |
New Periodic Table of the Elements Based on the Structure of the Atom
Tomkeieff SI, 1954, A New Periodic Table of the Elements Based on the Structure of the Atom, Chapman & Hall, London.
Thanks to René Vernon for the tip, who writes:
It is a helix wrapped on the surface of a cone. The shadow on the left is from the edge of my hand holding down the table; the shadow on the right is from the edge of a different book, again used to hold down the table into some semblance of flatness.
Mazurs said: "This is not a very successful table".
First, there is the cumbersome nature of a table on a cone, Secondly, see how the eight main group numbers at the top are sort of mushed into the 18 A and B series group numbers. This does not work well.
The colour scheme shows the dominant acid-base properties of the elements:
Dark blue — strong bases
Light blue — weak bases
Light red — weak acids
Dark red — strong acids
White — Inert gasesSince nonmetals never form basic oxides it is interesting to note that the (23) nonmetals fall on the right side of the table:
H He
B C N O F Ne
Si P S Cl Ar
Ge As Se Br Kr
Sb Te I Xe
Rn[Water is amphoteric; hydrogen peroxide is weakly acid.]
While the underlined elements are sometimes called metalloids, it is has been known for over 100 years that metalloids predominately behave chemically like nonmetals.
Astatine would’ve been a nonmetal but for relativistic effects. Immediately following its production in 1940, early investigators considered it a metal.

| Year: 1954 | PT id = 1255, Type = formulation |
Ephraim's Periodic Classification
Ephraim F 1954, Inorganic Chemistry, 6th ed., Oliver and Boyd, London (revised by PCL Thorne and ER Roberts)
René Vernon writes that items of interest include:
| Year: 1956 | PT id = 1216, Type = formulation |
Sistema Periodico de Los Elementos (after Antropoff)
Mario Rodríguez Peña, PhD translates the spanish text on the Archive.org website:
"Periodic System of Elements, type Antropoff., 1956 Antropoff's periodic table was designed in Bonn (Germany) in 1926: https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=26 It was disused after the WWII (1945) in most of the countries, except Spain. This was dated in 1956 because Mendelevium (101) was discovered and accepted by IUPAC in 1955 and in 1957, the element symbols of Argon (18), Xenon (54), Einstenium (99) and Mendelevium itself changed to the current Ar, Xe, Es and Md, respectively."
| Year: 1956 | PT id = 976, Type = formulation |
Remy's Long Period Form Periodic Table
From H. Remy's 1956, Treatise on Inorganic Chemistry, Vol. 1, (Introduction and main groups of the periodic table), Elsevier, Amsterdam, p. 4, is what Remy calls a "Long-Period Form of the Natural System of the Elements".
This is a semi-lanthanide/actinide formulation, with Th-Pa-U shown as 6d metals, and the remaining actinides (Np, etc.) shown as transuranic counterparts to Pm, etc. The layout of Remy's table was based on ideas by Haissinsky in competition with Seaborg's formulation of 1945.
In the appendix there is a second "Table II" version of this formulation with shorter periods.
Thanks to René for the tip!
| Year: 1956 | PT id = 1009, Type = formulation |
Walker & Curthoys' New periodic Table Based of Stability of Atomic Orbitals
By W. R. Walker and G. C. Curthoys, A new periodic table based on the energy sequence of atomic orbitals, J. Chem. Educ., 1956, 33 (2), p 69.
The abstract states:
"Since the theory of atomic and molecular orbitals has proven to be of such value in interpreting the data of inorganic chemistry, it is hoped that a new periodic table based on the energy sequence of atomic orbitals will be an aid to the further systematizing of chemical knowledge."
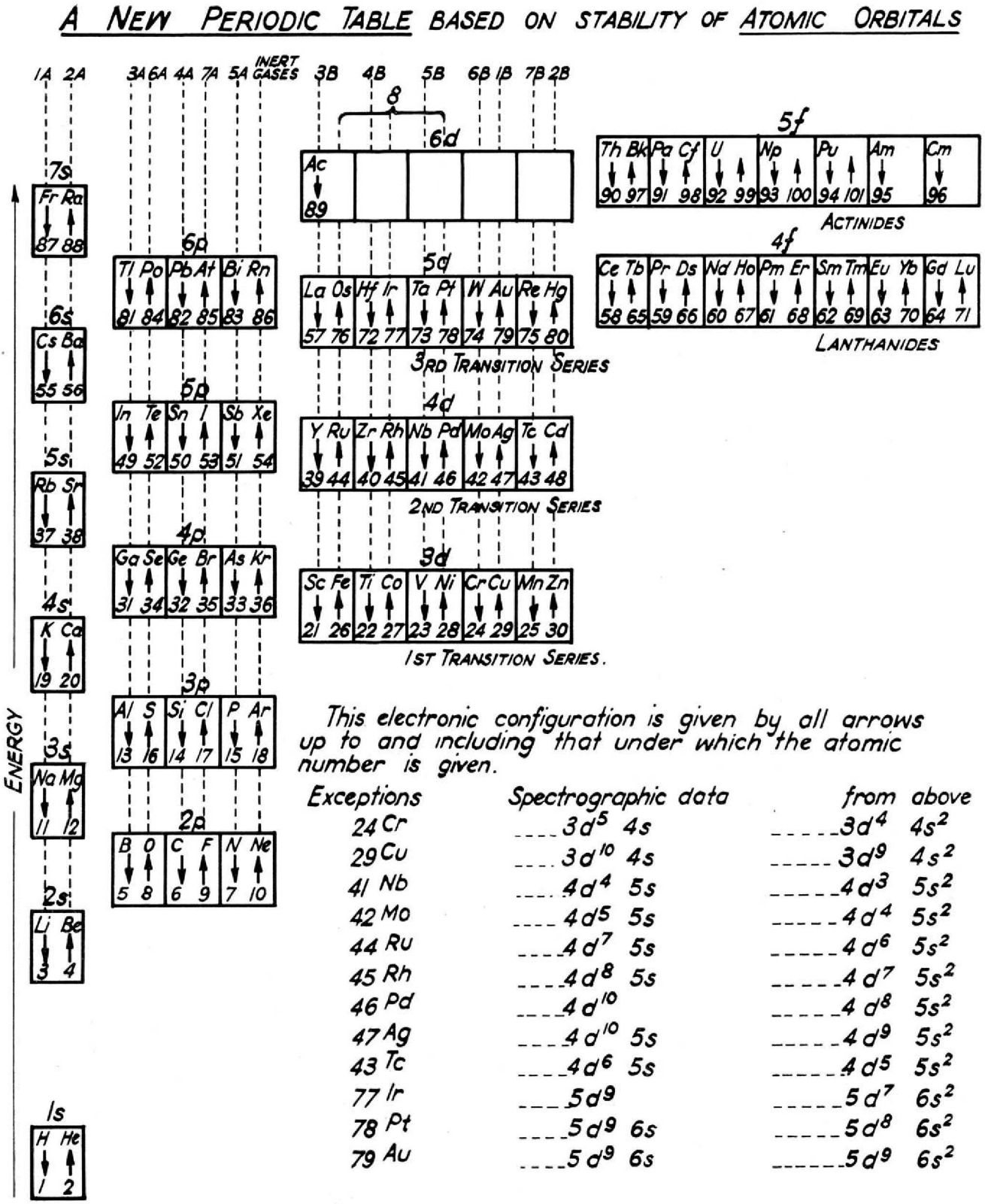
Thanks to René for the tip!
| Year: 1957 | PT id = 1079, Type = formulation |
Laubengayer's Long Periodic Table
From A.W. Laubengayer, General Chemistry, revised ed., Holt, Reinhart and Winston, New York (1957).
René Vernon writes:
"In this busy table the author appears to show three of each of groups I to VII (e.g group I; group IA; group IB) and one group VIII, and one group 0, for a total of 23 groups and subgroups."
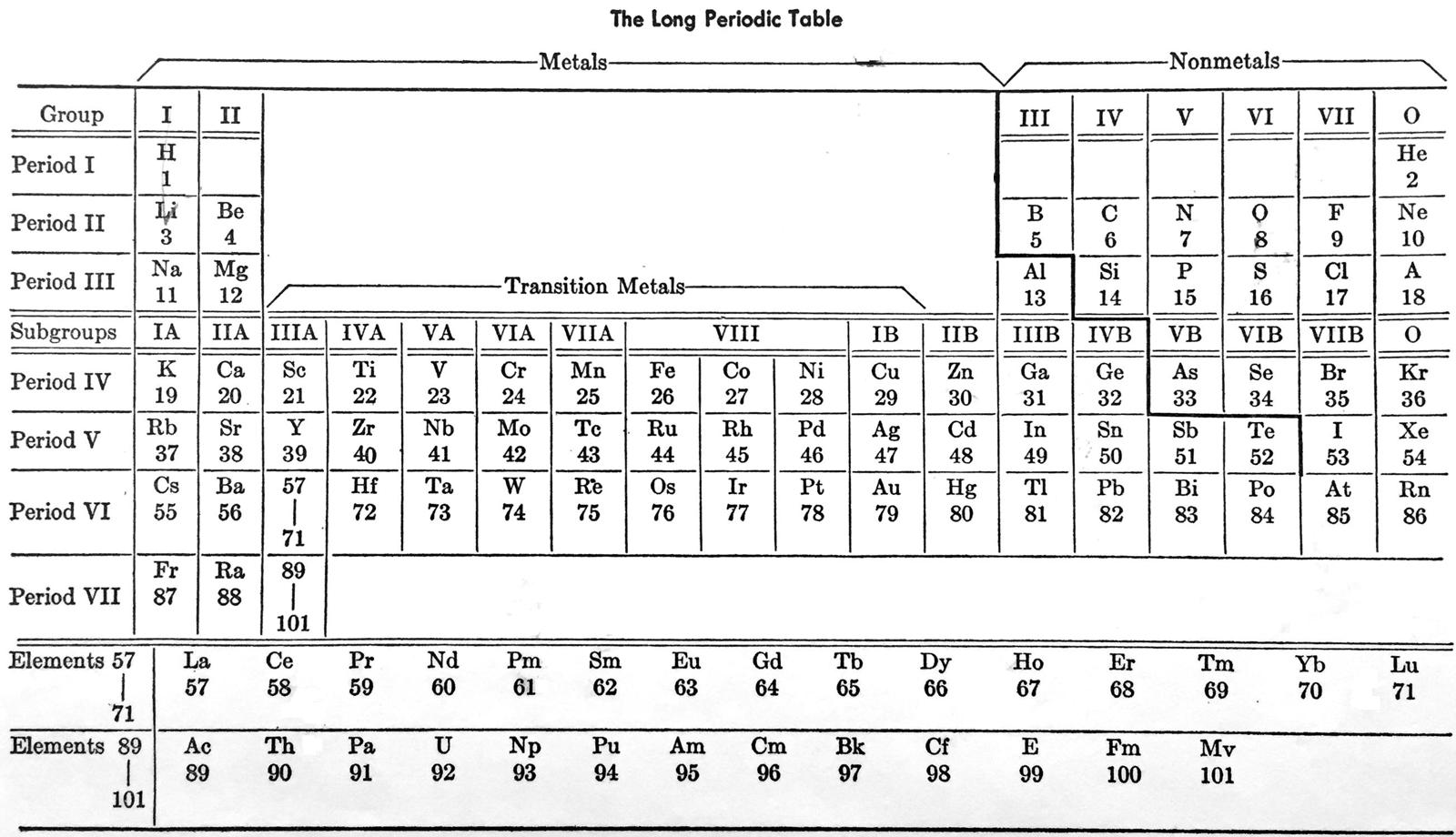
| Year: 1958 | PT id = 1148, Type = formulation |
Landau & Lifshitz's Periodic System of Mendeleev
L.D. Landau & E.M. Lifshitz, Quantum Mechanics (Volume 3 of A Course of Theoretical Physics), pages 255-258. (Note: First published in English in 1958, the link is to the 1963 3rd ed. of the English version translated from Russian.)
René Vernon writes:
The authors discuss aspects of the periodic system of D I Mendeleev. The electron configurations of hydrogen & helium are briefly noted. This is followed by three tables setting out the electron configurations of the s, p, d & f elements.
Some extracts from the text follow:
"The elucidation of the nature of the periodic variation of properties, observed in the series of elements when they are placed in order of increasing atomic number, requires an examination of the peculiarities in the successive completion of the electron shells of atoms." (p. 252)
"Many properties of atoms (including the chemical properties of elements...) depend principally on the outer regions of the electron envelopes." (p. 254)
"The elements containing complete d and f shells (or not containing these shells at all) are called elements of the principal groups; those in which the filling up of these states is actually in progress are called elements of the intermediate groups. These groups of elements are conveniently considered separately." (p. 254)
"We see that the occupation of different states occurs very regularly in the series of elements of the principal groups: first the s states and then the p states are occupied for each principal quantum number n. The electron configurations of the ions of these elements are also regular (until electrons from the d and f shells are removed in the ionisation): each ion has the configuration corresponding to the preceding atom. Thus, the Mg+ ion has the configuration of the sodium atom, and the Mg++ ion that of neon." (p. 255)
"Let us now turn to the elements of the intermediate groups. The filling up of the 3d, 4d, and 5d shells takes place in groups of elements called respectively the iron group, the palladium group and the platinum group. Table 4 gives those electron configurations and terms of the atoms in these groups that are known from experimental spectroscopic data. As is seen from this table, the d shells are filled up with considerably less regularity than the s and p shells in the atoms of elements of the principal groups. Here a characteristic feature is the 'competition' between the s and d states."
"This lack of regularity is observed in the terms of ions also: the electron configurations of the ions do not usually agree with those of the preceding atoms. For instance, the V+ ion has the configuration 3d4 (and not 3d24s2 like titanium) ; the Fe+ ion has 3d64s1 (instead of 3d54s2 as in manganese)."
"A similar situation occurs in the filling up of the 4f shell; this takes place in the series of elements known as the rare earths. † The filling up of the 4f shell also occurs in a slightly irregular manner characterised by the 'competition' between 4f, 5d and 6s states."
"† In books on chemistry, lutetium is also usually placed with the rare-earth elements. This, however, is incorrect, since the 4f shell is complete in lutetium; it must therefore be placed in the platinum group."
"The last group of intermediate elements begins with actinium. In this group the 6d and 5f shells are filled, similarly to what happens in the group of rare-earth elements." (p. 256–257)
The authors exclude lanthanum from the rare earths since the 4f shell has not started filling. Yet actinium and thorium are included by them with what we now call the actinoids even though these two metals have no f electrons. No explanation is provided for this puzzling lack of consistency with their categories.

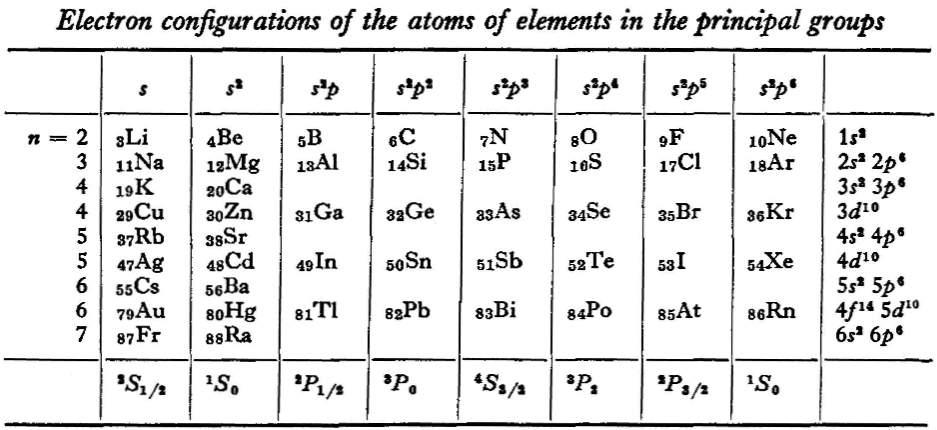
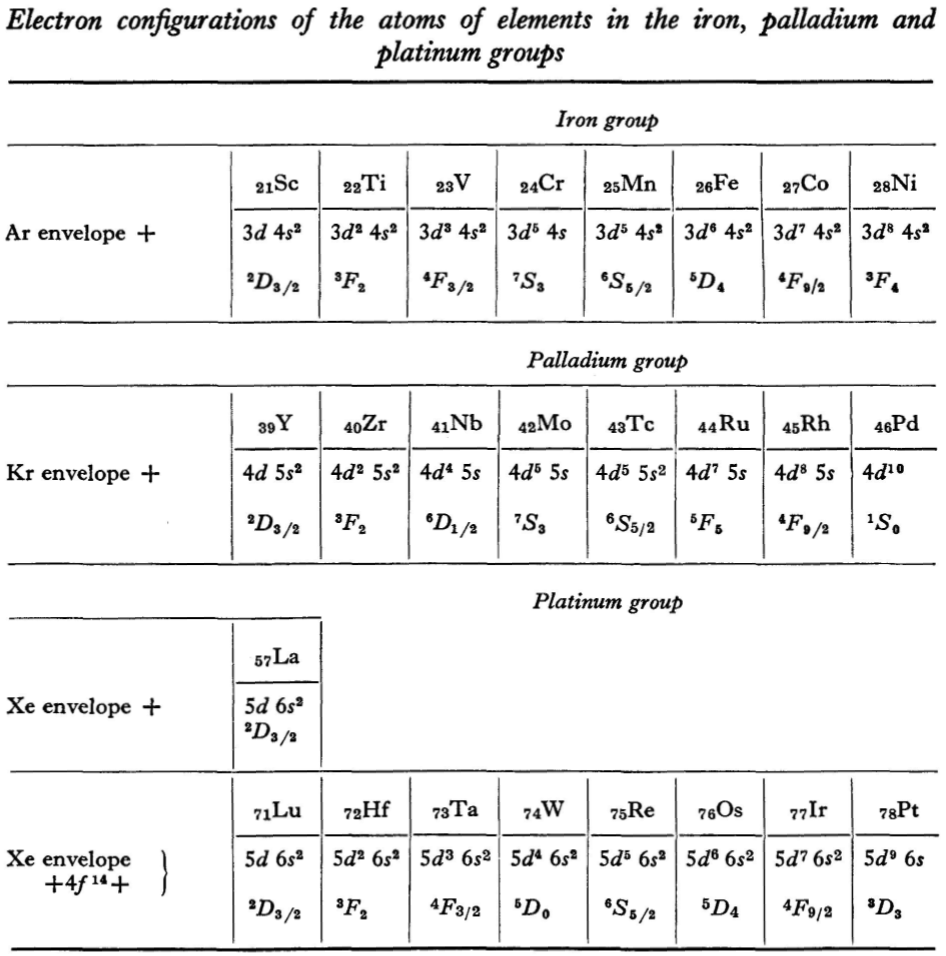
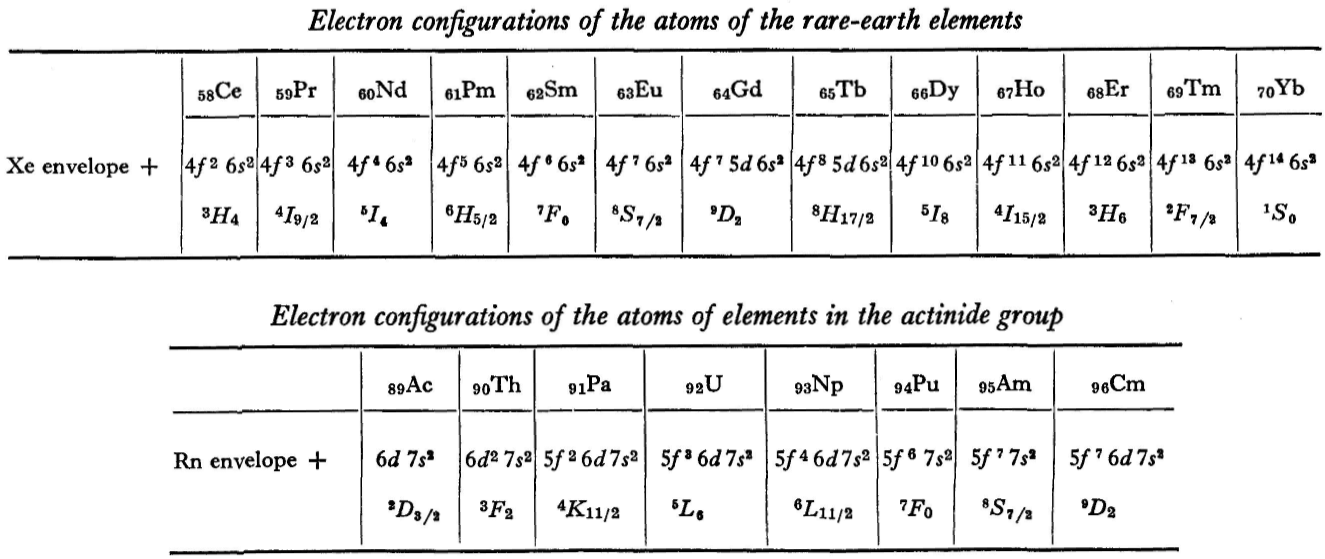
René Vernon writes: I have joined up their one note and three tables. (Curium was the last known element at their time of writing; transcurium elements are shown in parentheses.):
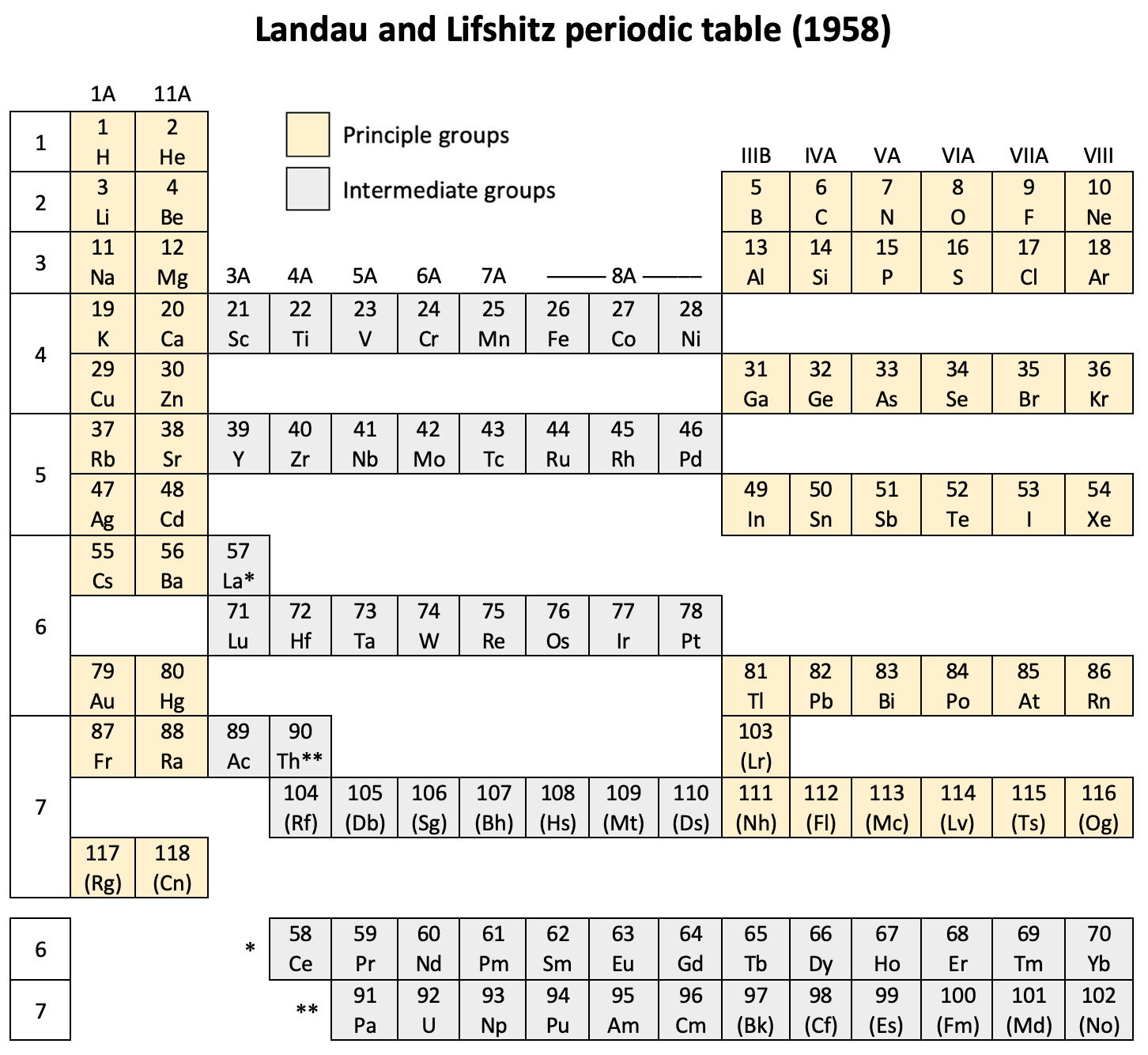
| Year: 1958 | PT id = 1263, Type = formulation |
Weaver & Foster's Laminar Chart of the Elements
Weaver EC & Foster LS 1960, Chemistry For Our Times. 3rd ed., McGraw-Hill, New York, p. 382
René Vernon writes:
An earlier version of this table appeared in JChemEd in 1949. The authors then wrote:
"It is apparently difficult to give a proper idea of electronic configuration in two dimensions without spreading out vertically or horizontally, and thereby sacrificing the order of atomic number, or compactness, or both. In three dimensions it is entirely feasible, but the first reaction is to discard three dimensions as too awkward. The laminar chart here proposed seems to the authors to possess the advantages of both the two dimensional and three dimensional charts and to have none of their disadvantages.
"A minor feature of the table, introduced for reasons of expediency, is the artificial break between the first and the second main shell. Use is made of this space to print the traditional group headings, I A, III A, IVB, etc., which are firmly entrenched in the literature, and still find active use as classifying labels. Other objects in making the artificial break were to minimize the resemblance between hydrogen and the alkali metals and to emphasize helium's character as an inert gas (completed 1s subshell), rather than, as might otherwise be supposed, a member of the alkaline earth family.
"CONTOUR LAMINAR TABLE
"By another modification, constructing the Periodic Chart in the form of contour laminae, it is possible to represent actual energy levels without the necessity of referring to auxiliary tables. This is done by proportioning the rises between each subshell to correspond to the Pauling energy diagram. Thus, although the subshells having the same principal quantum number will be on the same contour lamina, they will not be on the same planar level. The recognition of these contour laminae is facilitated by the use of a different color for each one. A table of this type will then be more physically correct than the previous laminar models, and it is a question as to which form has the most practical utility.
"We believe that the laminar periodic tables, in either the original or a modified form, will greatly facilitate systematic teaching of the properties of the chemical elements. Students indoctrinated with the new system cannot fail to obtain a clearer and more lasting conception of the fundamental principles of inorganic chemistry."
Note the 4f and 5f series have been split into dyads of seven apiece. This is consistent with Shchukarev (1974, p. 118) who wrote that the filling sequence among the 4f metals is periodic, with two periods. Thus, after the occurrence of a half-full 4f subshell at europium and gadolinium, the filling sequence repeats with the occurrence of a full subshell at ytterbium and lutetium (Rokhlin 2003, pp. 4–5). A similar, but weaker, periodicity (Wiberg 2001, pp. 1643–1645) is seen in the actinoids, with a half-full 5f subshell at americium and curium, and a full subshell at nobelium and lawrencium.
Note that Zn, Cd, Lu and Hg have no electron numbers above them since the underlying shells were filled at Cu, Cd, Yb, and Au respectively.
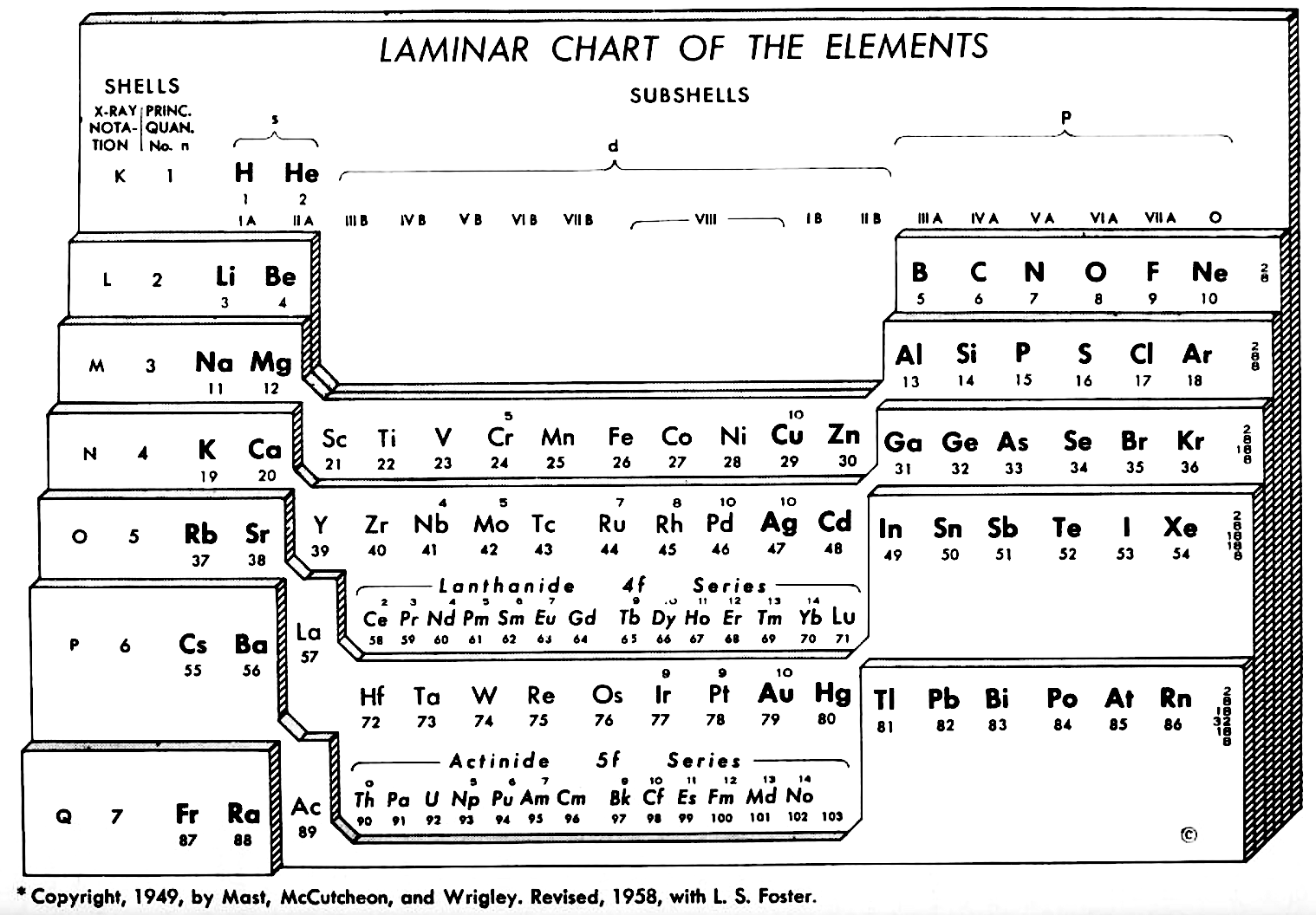
| Year: 1960 | PT id = 1012, Type = formulation |
International Rectifier Corporation Periodic Table
International Rectifier Corporation was an American power management technology company manufacturing analog and mixed-signal ICs, advanced circuit devices, integrated power systems, and high-performance integrated components for computing. It is now part of Infineon Technologies.
The periodic table below was produced in the late 1950s to early 1960s. The earliest version we can find on the web dates from 1960.
Thanks to René for the tip!
| Year: 1961 | PT id = 883, Type = element |
Discovery of Lawrencium
Lr ![]()
Lawrencium, atomic number 103, has a mass of 262 au.
Synthetic radioactive element.
Lawrencium was first observed in 1961 by A. Ghiorso, T. Sikkeland, E. Larsh and M. Latimer.
| Year: 1961 | PT id = 1251, Type = formulation spiral |
Circular Periodic Chart of The Elements
Chris R. Hagness writes:
"I own a wall chart that doesn't seem to be in your database of periodic tables. It's a circular 'periodic chart of the elements' published by Dickinson Brothers, Inc. in USA, distributed by American Seating, copyrighted 1961 by H. Edmund Matthews, Jr.
"I have never been able to find any information on this on the internet. Not on Edmund Matthews, no old catalogs from American Seating, no reference to this particular circular periodic table. I've never seen another of these anywhere, and I've been looking on and off for 10 years. I can't imagine this would have been a popular selling item. If you have any info, that would be appreciated!"
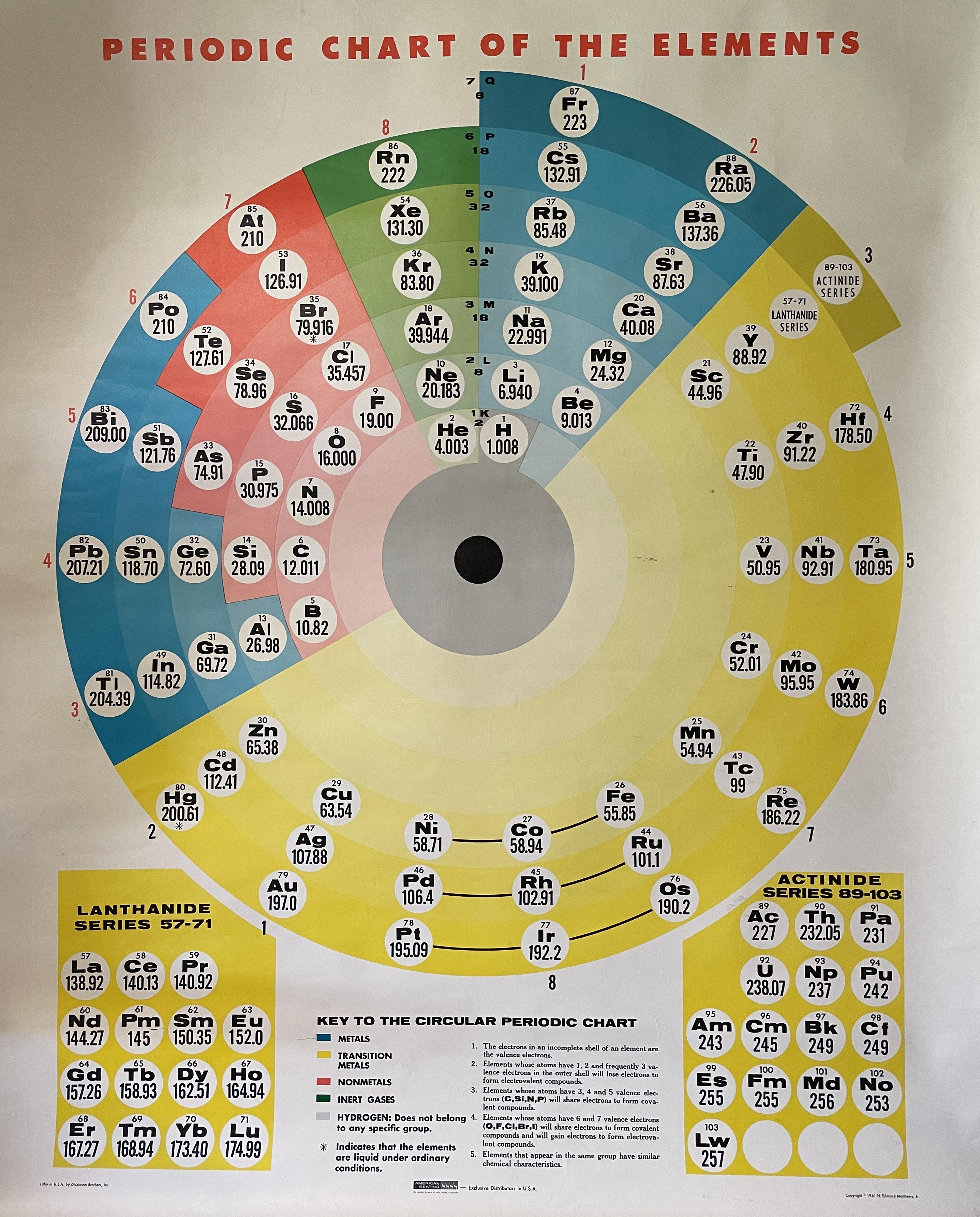
| Year: 1962 | PT id = 1177, Type = formulation |
Scott & Kendal Periodic Table
René Vernon shows an extract from Scott E.C. & Kendal F.A., The Nature of Atoms & Molecules: A General Chemistry. Harper & Row, New York, 1962 pp 385, categorising the metals.
Rather than providing a holistic treatment of the nonmetals, the authors take a group-by-group approach.
Items of interest: Al over Sc; the split between groups 3 and 3; and the inclusion of Pt with the soft metals.
On the right is my add-on for the nonmetals, plus extracts from the literature speaking to the analogies between the four metal and four nonmetal categories.
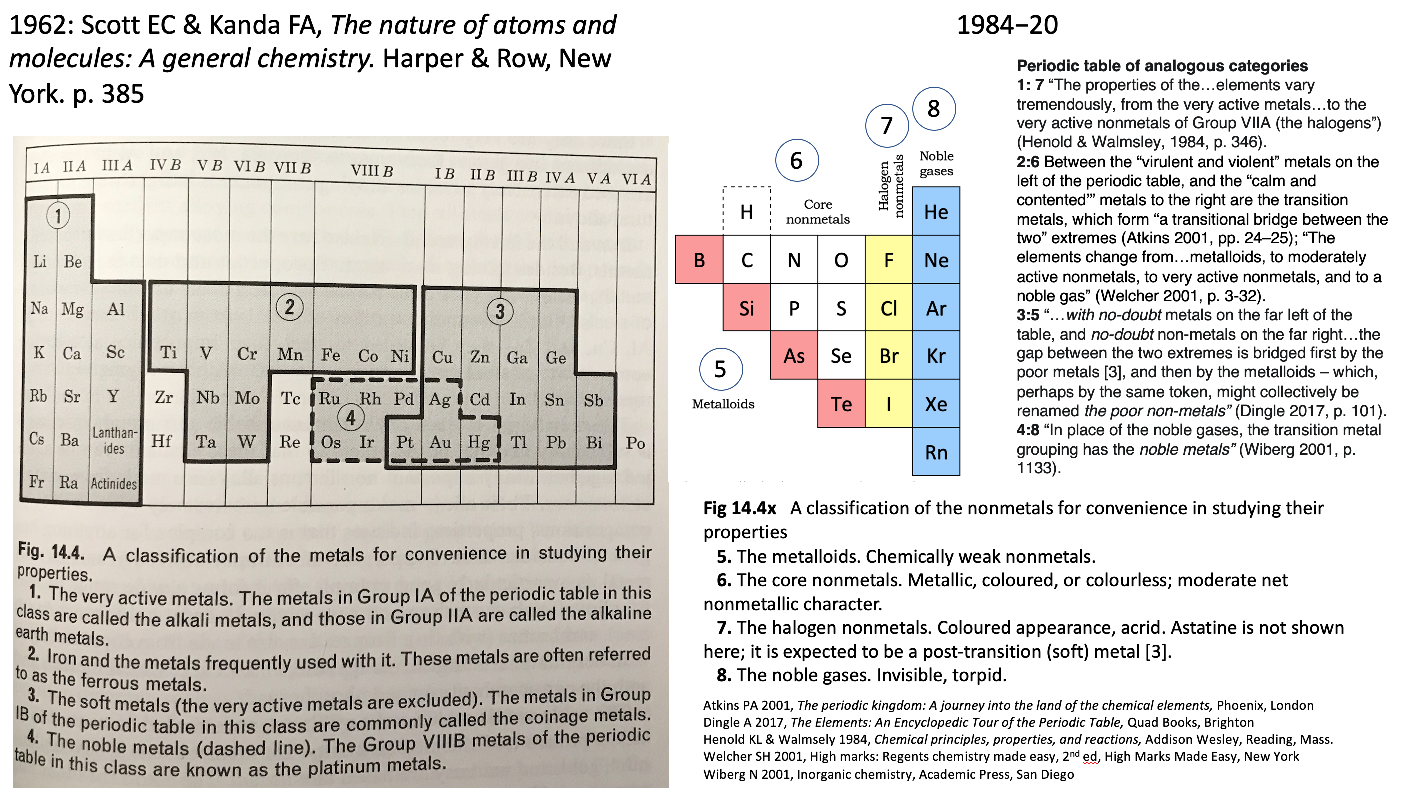
| Year: 1963 | PT id = 990, Type = formulation |
Bedreag's Système Physique Des Éléments
From Le Journal De Physique Et Le Radium, 24, pp27 (1963).
After a short historical account of the evolution of the periodic system Bedreag analyses some properties of various groups of elements: density, spectra, ionic radii, ionization potentials and so on, arguments are given in favour of the division of the transuranic elements into "uranides" and "curides".
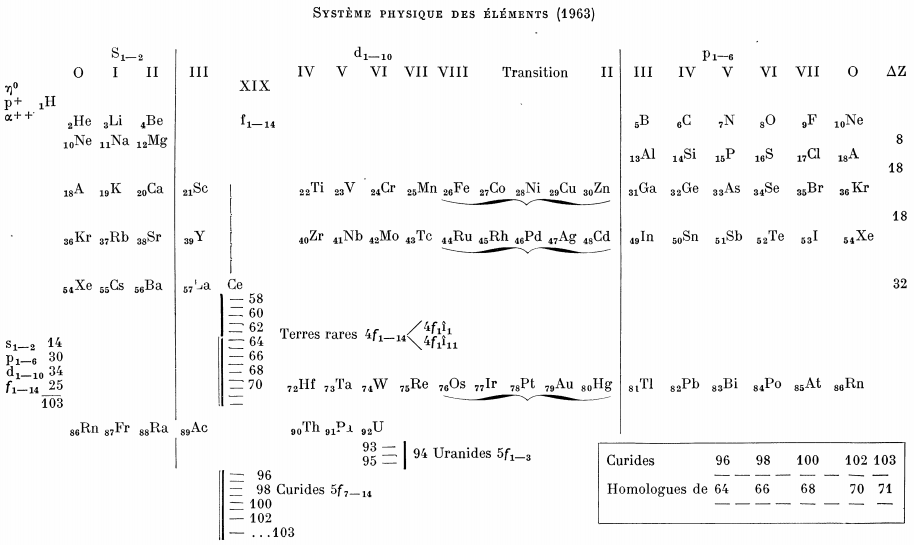
Thanks to René for the tip!
| Year: 1963 | PT id = 1249, Type = formulation |
Hutton's Periodic Table of The Elements
Hutton, K 1963, Chemistry: The Conquest of Materials, Penguin Books. Harmondsworth, Middlesex, pp. 38–39
René Vernon writes:
"Hutton shows:
Hutton refers to group 6A (Cr, Mo, W) as the "steel hardening" elements".
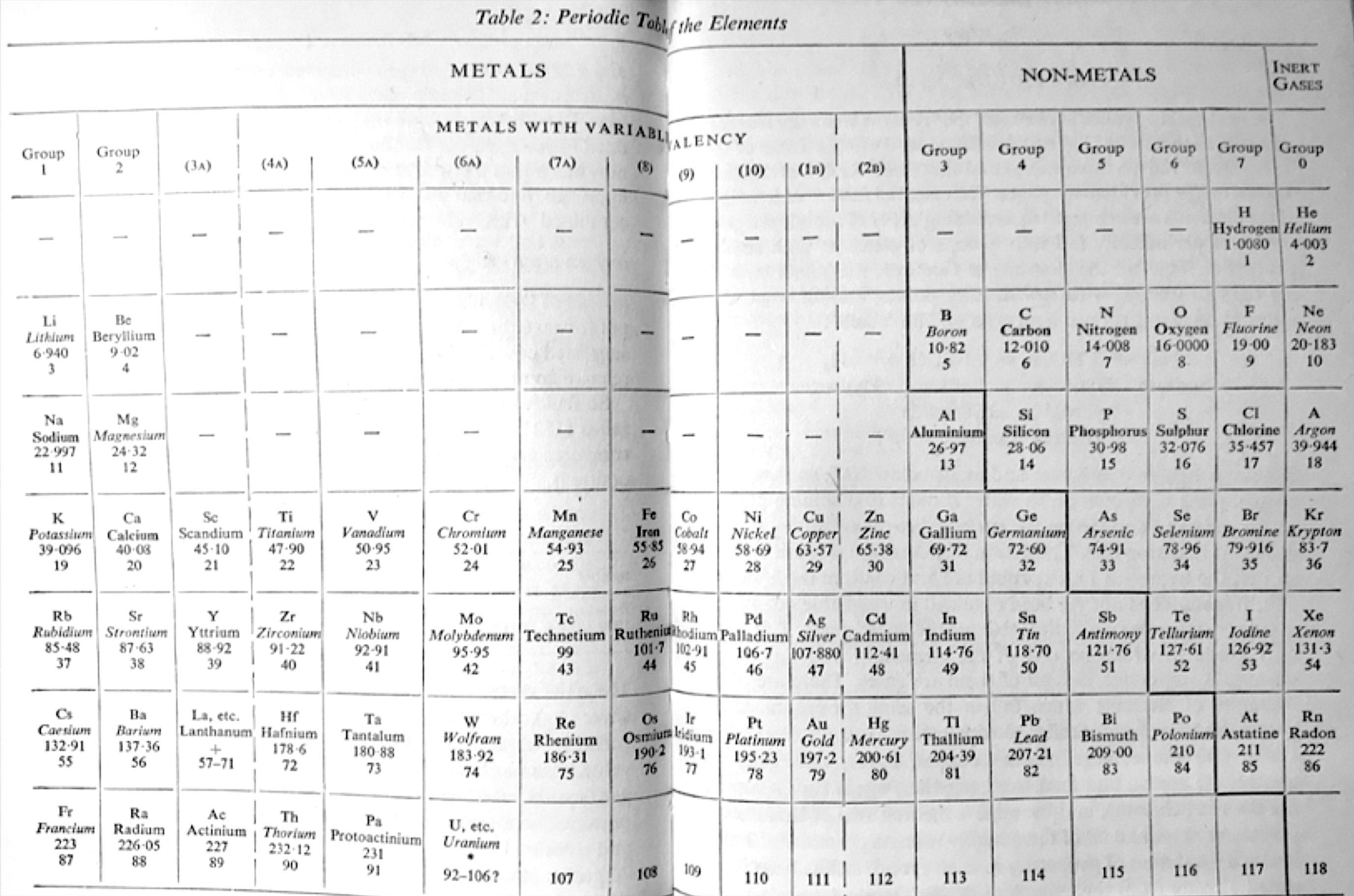
| Year: 1964 | PT id = 1006, Type = formulation |
Haward's Periodic Table
Roger Hayward created this periodic table for the book: Pauling & Hayward, p4, The Architecture of Molecules, W H Freeman and Company, San Francisco (1964).
From The Pauling Blog:
"By the end of the 1950s, Roger Hayward had retired from his professional work as an architect at the same time that his career as an illustrator was reaching its peak. Hayward signed a contract in the early 1960s that helped to solidify his position as a technical artist. The contract that Hayward signed was with W.H. Freeman & Company, a San Francisco-based publishing house that rose out of relative obscurity primarily by publishing Linus Pauling's hugely popular textbook, General Chemistry."
Thanks to René for the tip!
| Year: 1964 | PT id = 1271, Type = formulation |
Ternström's Periodic Table
Ref: A Periodic Table, Torolf Ternström, J. Chem. Educ. 1964, 41, 4, 190
René Vernon writes:
"Ternström gives us a triple-combo table drawing on the advantages of:
The outcome resembles the left step form of Janet (1928).
Some interesting features of Ternström's formulation are:
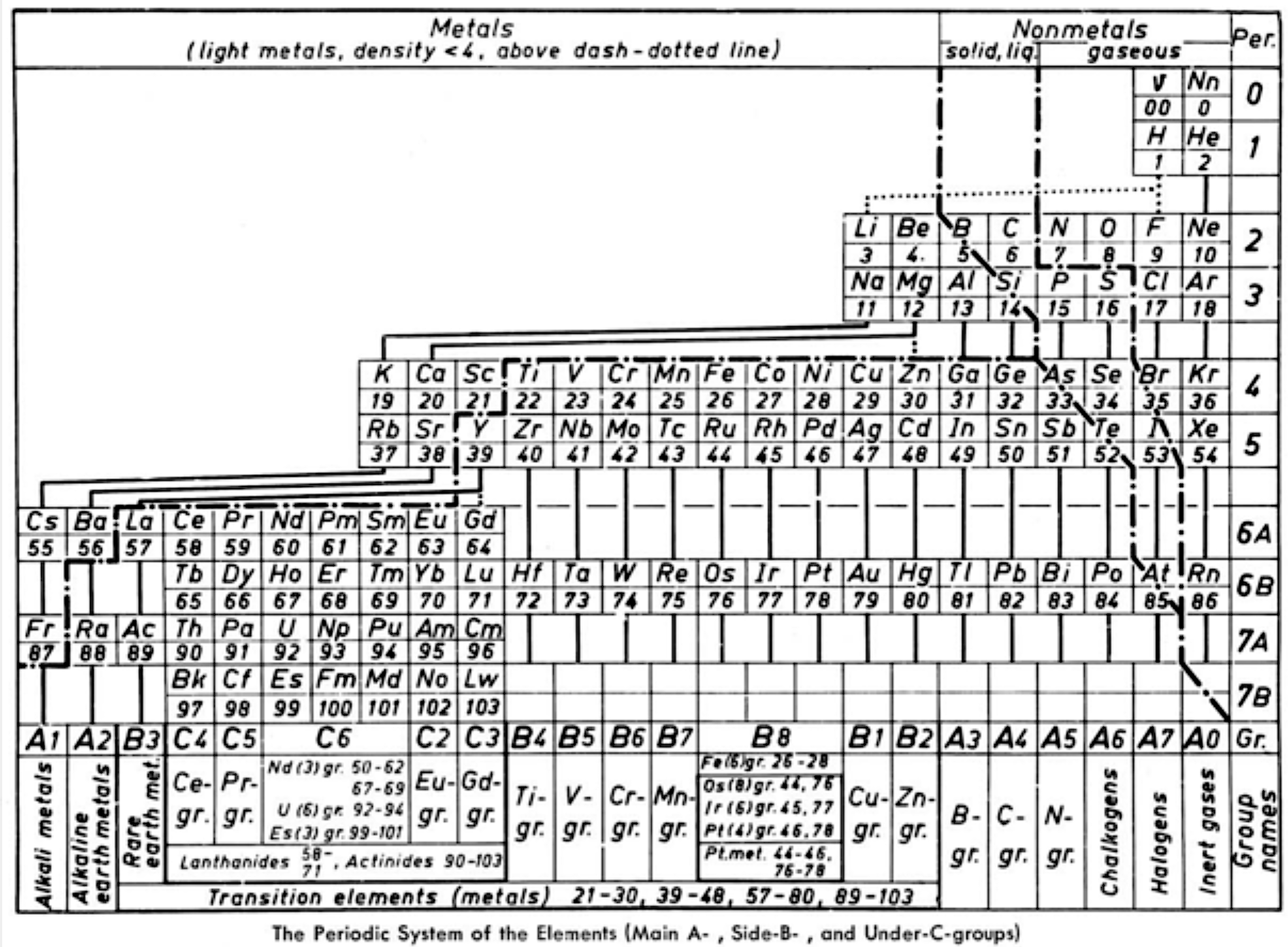
| Year: 1965 | PT id = 525, Type = formulation spiral 3D |
Giguère's Periodic Table
Paul Giguère's Periodic Table formulation, "The 'new look' for the periodic system". Chemistry in Canada vol. 18 (12): 36–39 (see p. 37). More info here: https://github.com/groverlab/giguere-3D-periodic-table.
René Vernon writes:
"I have not considered Giguère’s table at any length, so the following pros and cons are off the top of my head:
Pros:
Cons:
It’s striking that there are only two pros but several cons, perhaps a reflection of the inherent difficulties faced by three-dimensional periodic tables in improving on the conventional form?"

| Year: 1965 | PT id = 21, Type = formulation 3D spiral |
Alexander Arrangement of Elements
The Alexander Arrangement of Elements is a 3D periodic table concept based on strict adherence to the Periodic Law, and, like the first representation of elements in periods by de Chancourtois, connects every element data box in unbroken order.

Roy Alexander, a Brooklyn born science museum exhibit and teaching aid designer, has told me in a personal communication: "I came up with the idea (being ignorant of anything but the flat Sargent Welch charts) in 1965. I wasn't able to patent [the downslant in the p-block] until 1971." (U.S.Patent #3,581,409)
At the time Roy had no idea that others had employed a similar technique to build a 3D table - including the very first periodic table developer, de Chancourtois, who is often credited with being the original discoverer of the periodicity of elements and the originator of the three-dimensional method of element arrangement and representation.
These 3D forms attempt to return the Seaborg separated f-block to its proper position in the table rather than remaining exiled. This, and contemporary attitudes about Hydrogen as being in more families than one - is uniquely addressed in Roy's 3D models.
Subsequent study of the Periodic Law and the periodic table's value in education convinced Roy that the basic rationale for developing the Alexander Arrangement of Elements was only one of the many good reasons for producing it for the public to share, so he sought and was granted a U.S. patent on the p-block downslant in order to manufacture and market the AAEs as teaching/learning aids.
Roy Alexander's goal of introducing the AAE into classrooms, laboratories, chemistry textbooks, and reference material remains the same today, but rather than replacing the conventional charts, its niche in education is at the very point that a lesson on arrangement of atoms into a chart begins. Element sequencing (vs. 24 breaks/gaps) credits the chart as well as the Periodic Law, which establishes subsequent confidence in the common flat charts, much as the world globe establishes the reality, and flat printed projections - maps - are vital (and relished) for convenience.
The first commercial production of Alexander Arrangements was in 1995, when Roy pioneered by constructing a website - periodictable.com - for marketing. Three versions were printed: two versions for student entry of element symbols, the larger die-cut for easier assembly.
An even larger model was produced with basic element data printed in the boxes, also die cut. These were printed on white card stock, with black ink.
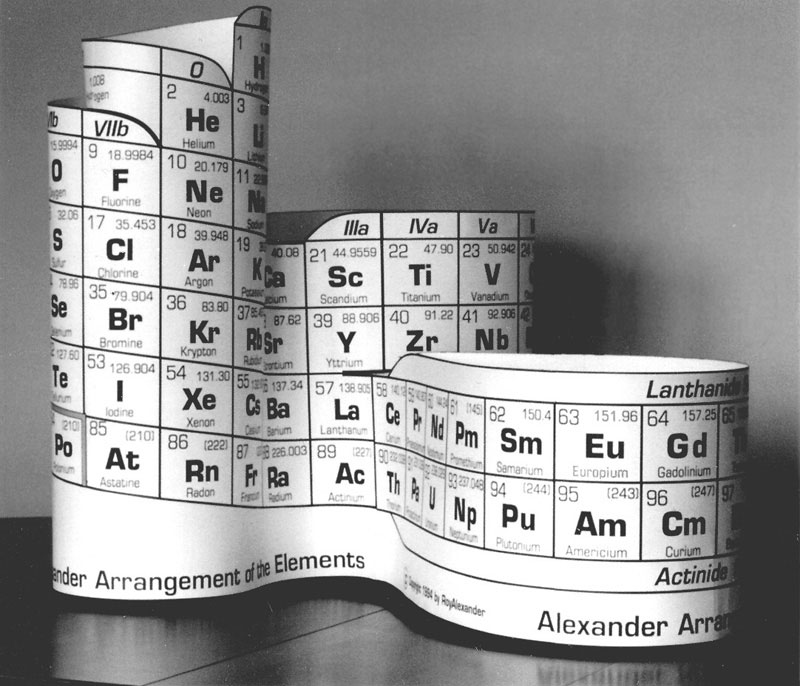
Another version (below) was produced in conjunction with ATMI's annual report in 2000. This was added to Roy's product offerings, called the DeskTopper, and is still available. They are die cut to form a 7.25" high model with the f-block position attached after La, but can be altered to put La on the f-block. (See AAE Features at the top of this page.)

Besides the hands-on educational application, the DeskTopper can be used as a pen & pencil caddy, and flattened without losing the continuity of the element data. This flattened form has suggested design of a Braille periodic table of the same format, and this is also being pursued.
Marketing the Alexander Arrangements was moved to AllPeriodicTables.com in cooperation with Theodore Gray in 2006, who purchased the PeriodicTable.com domain name and funded the production of Roy's newest model, illustrated with Theo's amazing element photos.

For the first time, the elements beyond those naturally occurring have been omitted from a modern periodic table, simplifying initiation to chemistry. This factor denies the concept of obsolescence, and this version has been called the Forever Periodic Table. Details of this new 3D periodic table model kit have been placed at 3DPeriodicTable.com.

Further AAE information and images may be found at the Alexander Arrangement website.
| Year: 1966 | PT id = 1314, Type = formulation |
Tottle's Periodic Table
Tottle CR 1974, The Science of Engineering Materials, reprint of 1966 ed., Heinemann Educational Books, London, p. 20
René Vernon writes:
I was drawn to the attached periodic table by the strange-looking arrangement of dividing lines, one "full" and one dashed, in the p-block.
Semimetals
Ge, As, Se, Sn, Sb, Te, Bi and Po are shown as semi-metals. Tottle does not explain the basis for this division.
Showing Sn as a semi-metal or metalloid is dubious. Sure, white-Sn becomes gray-Sn at a temperature of below 13.2 °C but even here it has the electronic band structure of a semi-metal.
The same can be said for Po which has electronic band structure of a true metal, unlike the situation in As, Sb and Bi, all of which have electronic band structures of semi-metals.
Metals & Nonmetals
Starting with H, note the left to right path of the full dividing line between metals and nonmetals is continuous, except for the unique break above Be, presumably to show that there is no element above Be. This is actually not well thought-out since the metallic or nonmetallic status of the IIA elements is not then clarified.
Tottle is further interesting since, as well as referring to metals and nonmetals in the periodic table sense he later includes a chapter on Metals and alloys, and a chapter on Non-metallic materials. Some examples given by him of non-metallic materials are alumina, magnesia, graphite, beryllia, titanium carbide, glass, rubber, nylon and wood. So, he here is mixing nonmetallic elements and nonmetallic materials (which is fine).
Tottle gets into trouble in his chapter on Metals and alloys, since he includes some discussion on interstitial solid solutions, such as cementite Fe3C, which is an insulator, and intermetallic compounds, which appears fine on the surface, until one realises that some intermetallic compounds are semiconductors, such as FeGa3, RuGa3, and IrGa3. I have never heard of semiconducting or insulating metals or alloys.

| Year: 1966 | PT id = 1265, Type = formulation review 3D |
Rare Earth Pop Out Periodic Table
From Rare Earths, The Fraternal Elements by Karl A. Gschneidner Jr., United States Atomic Energy Commission Division of Technical Information Library of Congress Catalog Card Number: 65-60546 1964; 1966 (Rev.)
There is an interesting point made in the text concerning the term "Rare Earths":
"The name rare earths is actually a misnomer for these elements are neither rare nor earths. They are metals, and they are quite abundant. Cerium, which is the most abundant, ranks 28th in the abundances of the naturally occurring elements and is more plentiful than beryllium, cobalt, germanium, lead, tin, or uranium. The least abundant naturally occurring rare earth, thulium, is more plentiful than cadmium, gold, iodine, mercury, platinum, or silver. Indeed, 25% of the elements are scarcer than thulium."
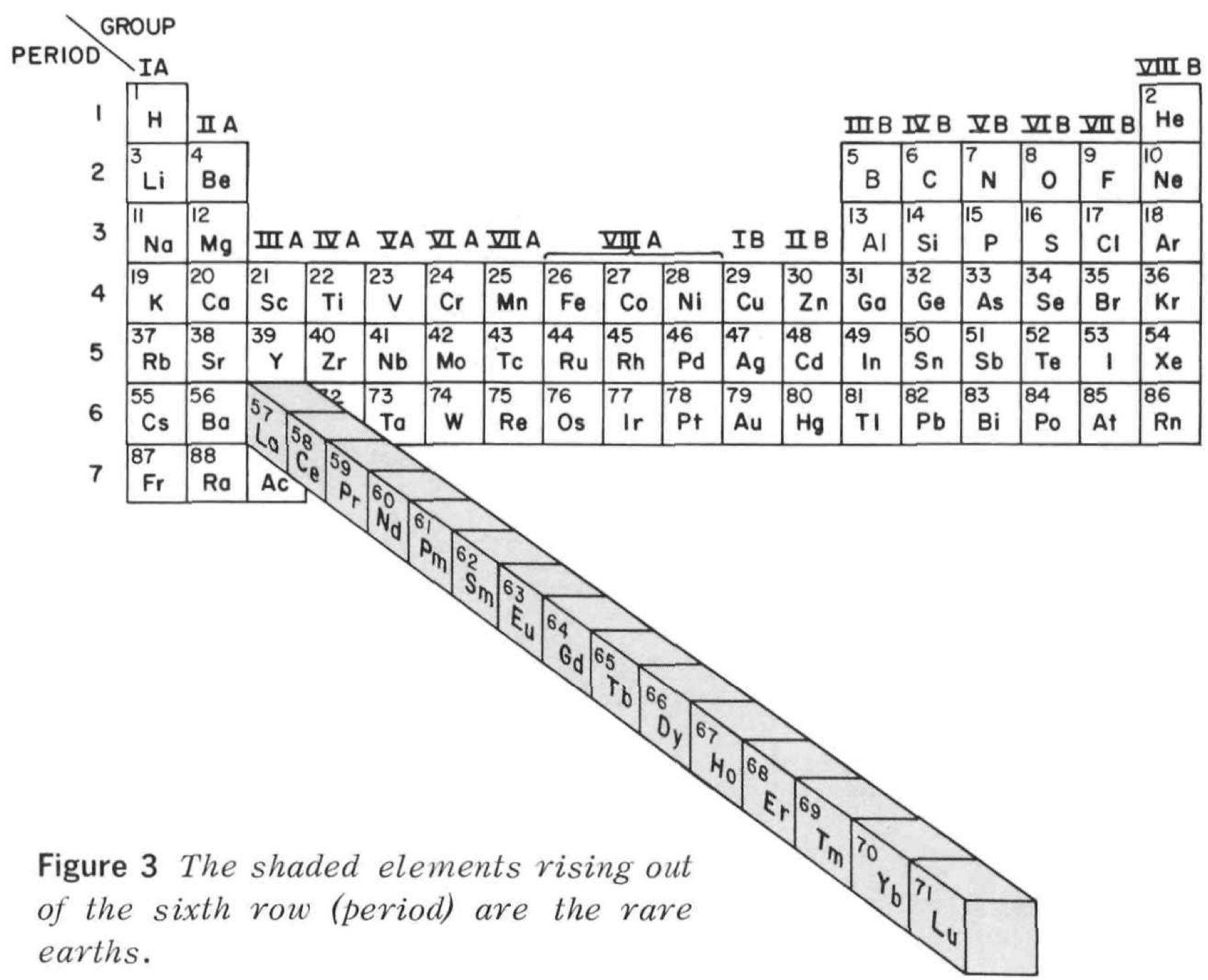
Thanks to René for the tip!
| Year: 1969 | PT id = 1308, Type = review formulation |
100 Years of the Periodic Law of Chemical Elements
A Soviet Union publication in Russian celebrating Medeleeve's seminal work of 1869: 100 Years of the Periodic Law of Chemical Elements, X Centennial (Jubilee) Mendeleev Congress. The work is the product of 23 Authors. (Thanks to Ann E. Robinson, René Vernon & Valery Tsimmerman for the info.)




| Year: 1969 | PT id = 1146, Type = review |
Mendeleevian Conference, Periodicity and Symmetries in the Elementary Structure of Matter
Atti del Convegno mendeleeviano : periodicità e simmetrie nella struttura elementare della materia : Torino-Roma, 15-21 settembre 1969 / [editor M. Verde] Torino : Accademia delle Scienze di Torino ; Roma : Accademia Nazionale dei Lincei, 1971 VIII, 460 p.
Google Translate: Proceedings of the Mendeleevian Conference: periodicity and symmetries in the elementary structure of matter: Turin-Rome, 15-21 September 1969 / [editor M. Verde] Turin: Turin Academy of Sciences; Rome: National Academy of the Lincei, 1971 VIII, 460 p.
From the Internet Archive, the scanned book. Papers are in Italian & English.
For the 100th Anniversary of Mendeleev's iconic periodic table, a conference was held to look at (review) the elementary structure of matter. The 1960s saw huge developments in particle physics, including the theory of quarks. Papers were presented by many notable scientists including John Archibald Wheeler and the Nobel laureates: Emilio Segrè & Murray Gell-Mann.Thanks to René for the tip!
| Year: 1969 | PT id = 1010, Type = formulation |
Dash's Quantum Table of the Periodic System of Elements
Harriman H. Dash, A quantum table of the periodic system of elements, International Journal of Quantum Chemistry, vol. 3, no. S3A, supplement: Proceedings of the International Symposium on Atomic, Molecular, and Solid?state Theory and Quantum Biology, 13/18 January 1969, pp. 335–340.
The abstract reads:
"The shortcomings of the long form of the periodic table of the chemical elements and the evident need for updating this format are briefly reviewed. To the question 'what format?' quantum physics provides an unequivocal answer. The foundations for the design of a quantum table are outlined. These are based on the principal quantum number as derived from the Schroedinger wave equation, the law of second order constant energy differences, and the coulomb–momentum interaction. These concepts are all combined into a single format which optimally and explicitly relates periodicity to atomic structure and the physical, chemical, and biological properties of the elements. This relationship emphasizes the unity and universality of all sciences."
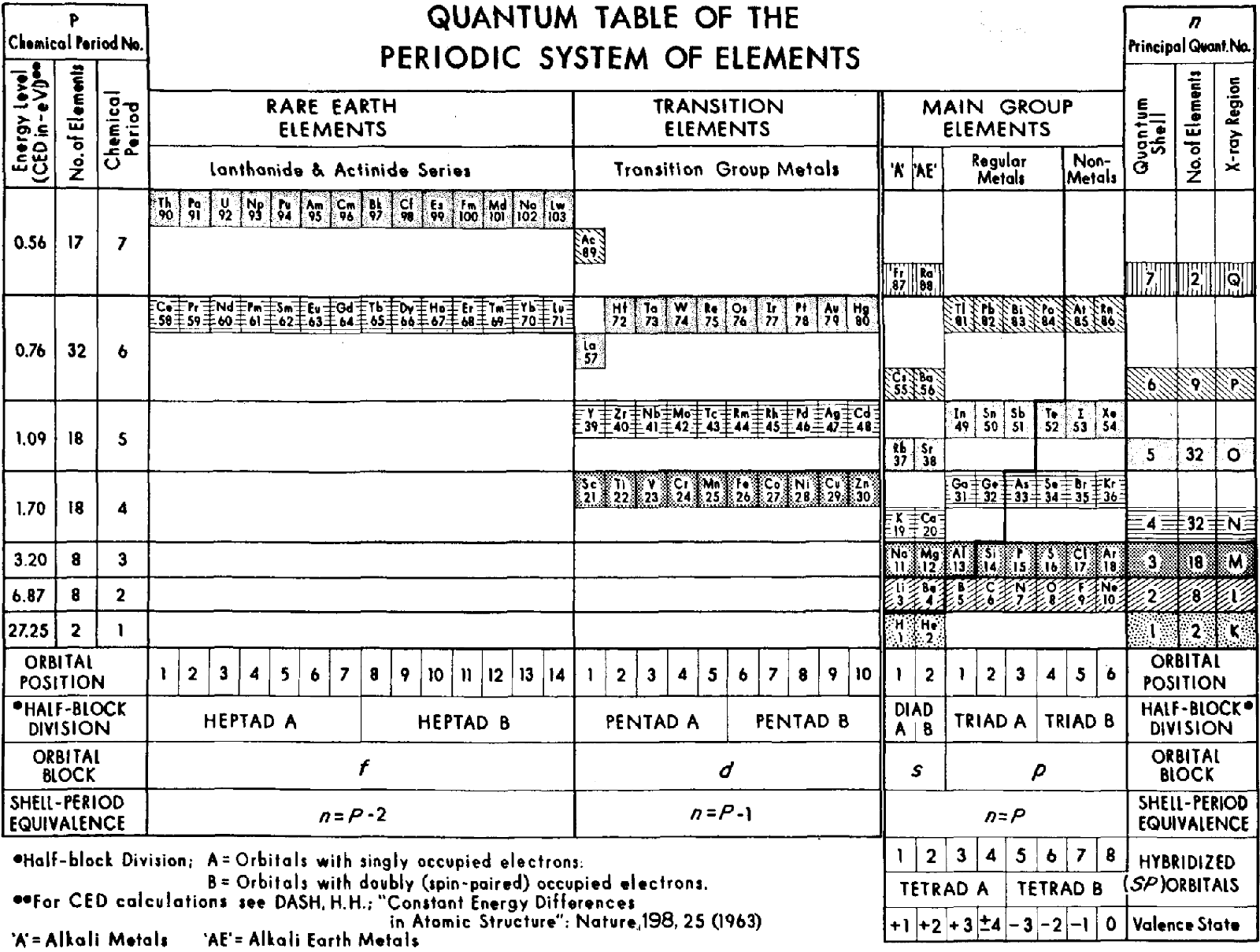
Thanks to René for the tip!
| Year: 1969 | PT id = 1270, Type = formulation data misc |
Seel-Klechkovskii Version of Madelung's Rule for Orbital Filling
Seel F., Bild der Wissenschaft, 6, 44 (1969), a monthly popular scientific journal.
Thanks to René for the tip!
| Year: 1969 | PT id = 1273, Type = formulation data |
Martin's Crystal Structure Periodic Table
Ref: Martin JW 1969, Elementary Science of Metals, Wykeham Publications, London
René Vernon writes:
Note the unusual placement of La-Ac in two places, under Y and before Ce-Th. On another aspect, Martin writes:
"The non-metals, which occupy the top right-hand corner of the Periodic Table... form about one-sixth of all elements, and they are characterized by having melting-points and boiling points below about 500°C, and by having their solid and liquid phases not conducting electricity. About two-thirds of all elements are metals, and a further one sixth have properties intermediate between those of metals and non-metals."
His approach to the question of which elements are metals and non-metals, and which are intermediate may be the most useful "rough-and-ready" rubric I've seen. It is remarkable for its use of four criteria.
Perhaps we can then parse the elements as follows
Non-metals (16) = 15.5%
Fluids: H, N, O, F, Cl, Br; He, Ne, Ar, Kr, Xe, Rn 2
Solids: P, S, Se*, IIntermediate (16) = 15.5%
Metalloids: B, Si, Ge, As, Sb, Te
Near metalloids: C, At 3
Sub-metalloids: Al, Ga, In, Tl; Sn, Pb; Bi; PoMetals (71) = 68.9%
Be,^ Zn^
All the rest^ Borderline intermediate
Dingle (2017, The Elements: An Encyclopedic Tour of the Periodic Table, Quad Books, Brighton, p. 101) puts the situation this way:
"...the gap between the two extremes [of metals and nonmetals] is bridged... by the poor metals, and... the metalloids – which, perhaps by the same token, might collectively be renamed the poor non-metals.”
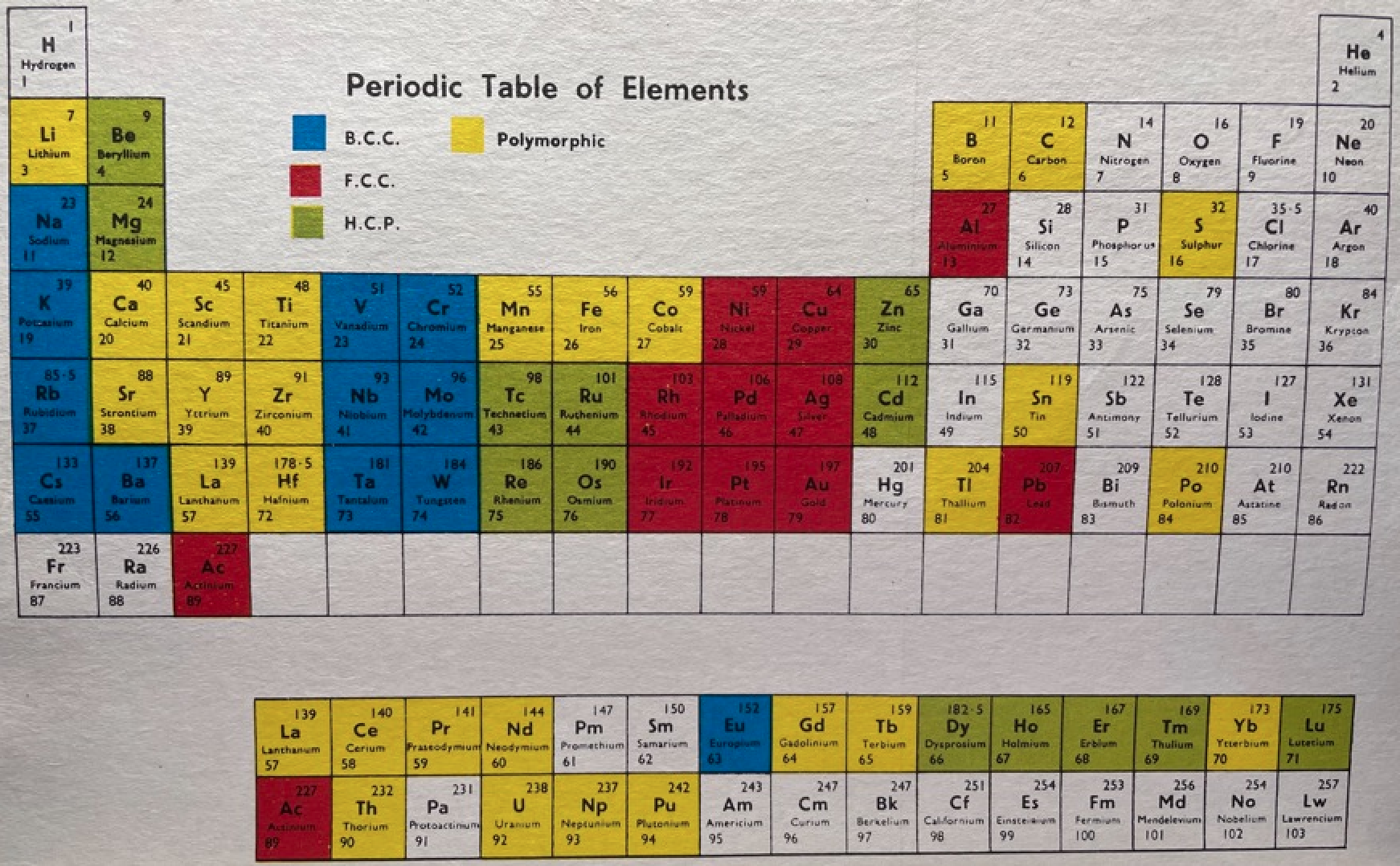
Redrawn by Vernon:
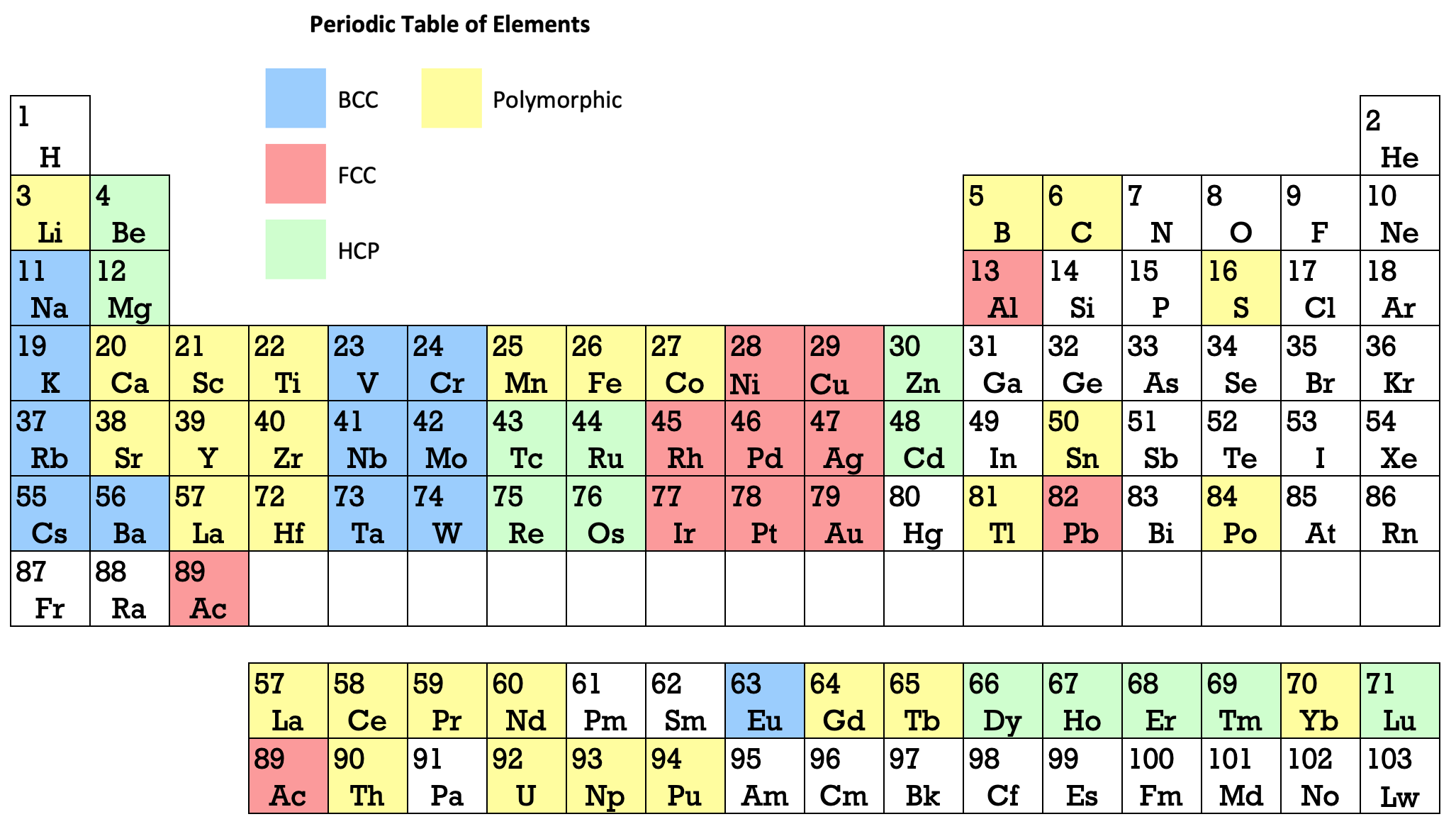
Thanks to René for the tip!
| Year: 1970 | PT id = 1007, Type = formulation |
Pauling's "General Chemistry" Periodic Table
From Linus Pauling's General Chemistry (3rd Ed.). Notice that the noble gases apear twice, at the beginning and the end of each period.
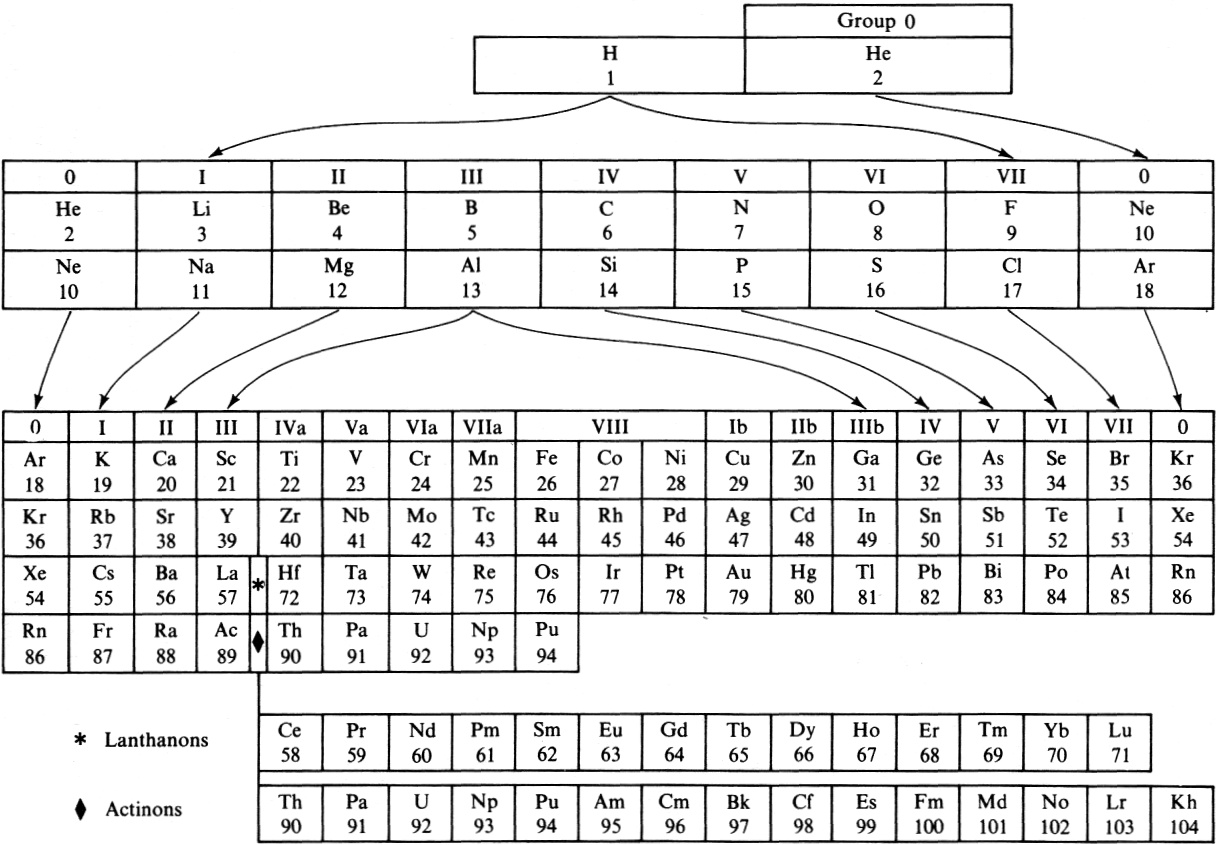
Thanks to René for the tip!
| Year: 1971 | PT id = 672, Type = formulation |
Clark, John O. E. Periodic Table
Thanks to René Vernon who found this formulation, and writes:
"Here's a strange table I found in the following book: Clark Jonh O.E. 1982, Chemistry (The Hamlyn Publishing Group, Feltham, Middlesex) ISBN 0600001245. The colour coding is exasperating. The way the table is laid out is bizarre. The copy I have is a reprint of the original 1971 edition so I have to wonder if the graphic designer was drawing inspiration from the trippy 60s."
| Year: 1971 | PT id = 1269, Type = formulation data misc |
Goldanskii's Chess Board Version of The Madelung Rule (For Orbital Filling)
Ref: Goldanskii, V I: The Periodic System of D I Mendeleev and Problems of Nuclear Chemistry pp 137-162 ex: Verde M (ed.): 1st International Conference on the Periodic Table, Vincenzo Bona, Torino 1971.
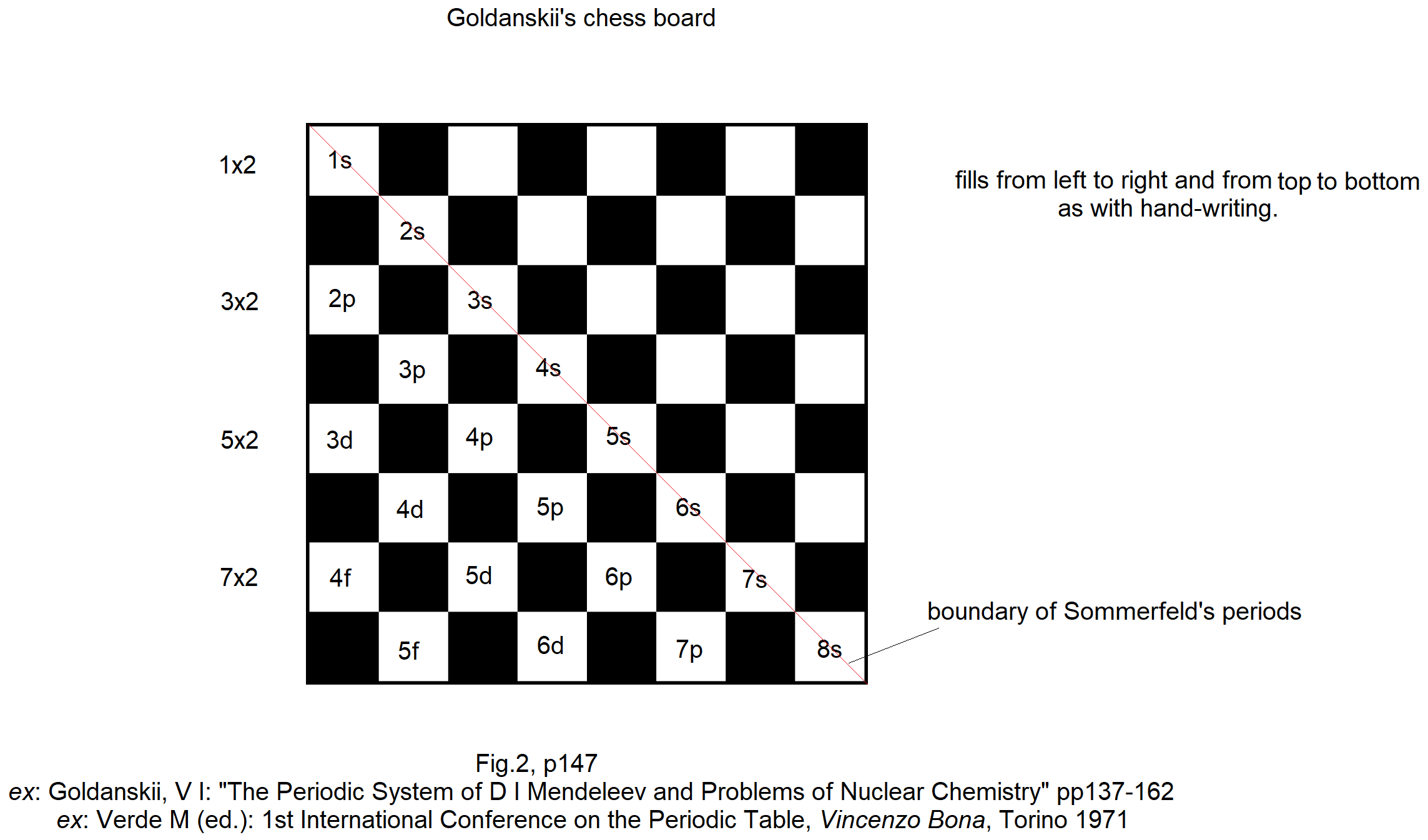
Thanks to John Marks for the tip!
| Year: 1974 | PT id = 267, Type = formulation 3D |
Mazurs Wooden Version of Mendeleev's Periodic Table
There is a posting in the The Elements Unearthed blog by David V Black concerning a view of the Marzus archive:
"My biggest discovery this week has been a collection in our archives of the notes of Edward Mazurs, who wrote the definitive work on classifying different systems of periodic tables in 1957 with a revised edition in 1974 (Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press). He collected articles and wrote extensive, detailed notes on every version of the periodic table he could find as it developed from its start in the early 1860s with the work of de Chancourtois through 1974. All of those notes have been donated to Chemical Heritage Foundation and fill up ten binders, with meticulous drawings, charts, tables, and frequent additions and changes. There are also some pieces of the original artwork prepared for the book, and a wooden model of the periodic table Mazurs built himself. "
| Year: 1974 | PT id = 1058, Type = formulation spiral |
Mazurs' Redrawing of Stedman's Formulation
An spiral formulation by Mazurs, cited as being after Janet (1928). However, it is actually, it is after Stedman (1947).
In an article Bull. Hist. Chem., VOLUME 34, Number 2 (2009) O.T. Benfey writes:
"After we had developed our own [Periodic Snail] spiral design, we found that E. G. Mazurs had published a spiral with a separate protrusion for the lanthanides which, under the image, he misleadingly ascribed to Charles Janet in 1928, the same year that Janet had published a simple circular form also shown by Mazurs. The Mazurs diagram with the lanthanide protrusion was reprinted in [the journal] Chemistry. However, [Philip] Stewart informed me that the Mazurs figure bears no resemblance to the Janet diagram he indicated nor to any other of his designs. Detailed references given a few pages later by Mazurs suggested correctly that the spiral derives from Stedman and is so identified and depicted by van Spronsen. The Mazurs diagram is a mirror image of the Stedman spiral, updated to include elements discovered since 1947." [For references, see the article.]"
Mazurs (p. 77) writes:
"Subtype IIIA3–1a Helix on a modified cone. The transition and inner transition elements have special revolutions in the form of loops. This table, originated by Stedman in 1947 is not a successful one."
Thanks to René for the tip and information!
| Year: 1976 | PT id = 945, Type = formulation |
Seaborg's Futuristic Periodic Table
A Futuristic Periodic Table Showing Predicted Locations of a Large Number of Transuranium Elements (Atomic numbers in parentheses) by Glenn Seaborg in 1976. Internal reference number: XBL 751-2036
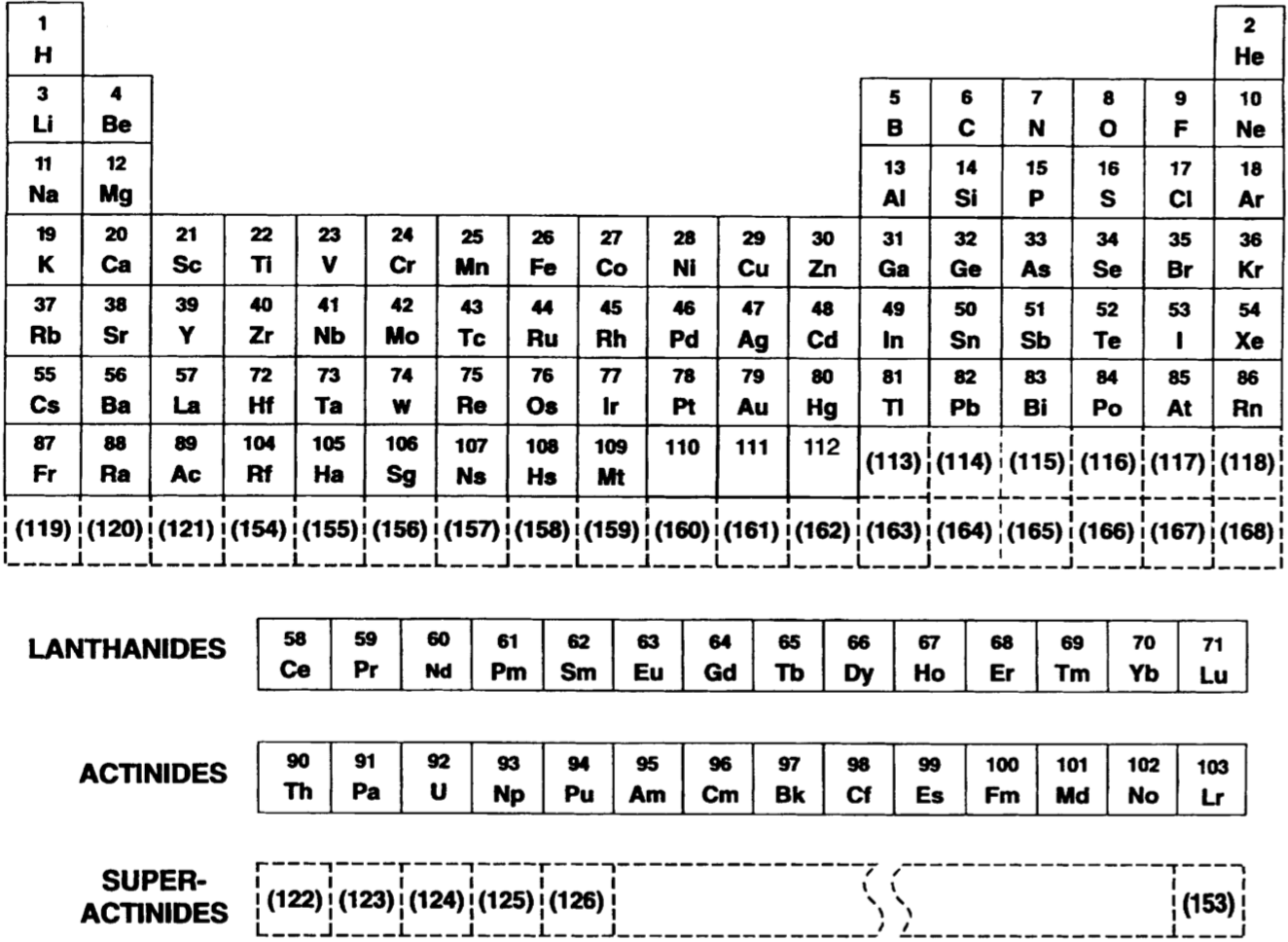
| Year: 1979 | PT id = 471, Type = non-chem formulation spiral |
Mann's Spiral Periodic Table
From AT Mann:
"I designed a spiral periodic table which was published first in my book The Divine Plot: Astrology, Reincarnation, Cosmology and History (George Allen & Unwin, London, 1986) which attempts to correlate the PT with astrological understanding of the inherent properties of the signs and planets":
| Year: 1982 | PT id = 49, Type = formulation 3D |
Cement Chemist's Periodic Cube
Periodic table designed in the style of a cube by J. Francis Young, Professor of Civil and Ceramic Engineering, University of Illinois. This table was published by Instruments for Research and Industry and includes instructions for assembly into a 3-D model.
More information, including high resolution files, at the Science History Institute.
Thanks to René Vernon for the tip!
| Year: 1983 | PT id = 50, Type = formulation 3D |
Periodic Pyramid
Periodic table designed in the style of a pyramid by Charles E. Gragg. This table was published by Instruments for Research and Industry and includes instructions for assembly into a 3-D model.
More information, including high resolution files, at the Science History Institute.

Thanks to René Vernon for the tip!
| Year: 1983 | PT id = 233, Type = data |
Seawater Periodic Table
A periodic table of references to analytical chemistry papers associated with the elements. If you want to know how much gallium in seawater, this would be a good place to start:
| Year: 1984 | PT id = 1258, Type = formulation |
Cherkesov: Two Periodic Tables
Cherkesov AI 1984, Ionization energy of 1-6 p-electrons and formation enthalpies of lutetium and lawrencium halides. Position of these elements in Periodic system, Radiokhimiya, vol. 26, no. 1, p. 53?60 (in Russian), https://inis.iaea.org/search/search.aspx?orig_q=RN:16012913
René Vernon writes:
"Two Russian offerings, the first is Mendeleev style, including He over Be and the integration of the Ln and An into the main body of the table.
"The second is the first time I have seen a genuine right step table, albeit at the expense of the numbers going backwards, and the non-intuitive group numbering scheme. Bonus marks for out-of-the box thinking."
| Year: 1987 | PT id = 1039, Type = formulation |
Step-Pyramid Form of the Periodic Chart
By Bill (William) Jensen, a Step-Pyramid form of the periodic chart.
This formulation is an updated version of the charts by Thomsen (1895) and Bohr (1922) with more elements, including placeholders up to 118, electronic configuration lables, etc. Read more on the Science History Institute website.
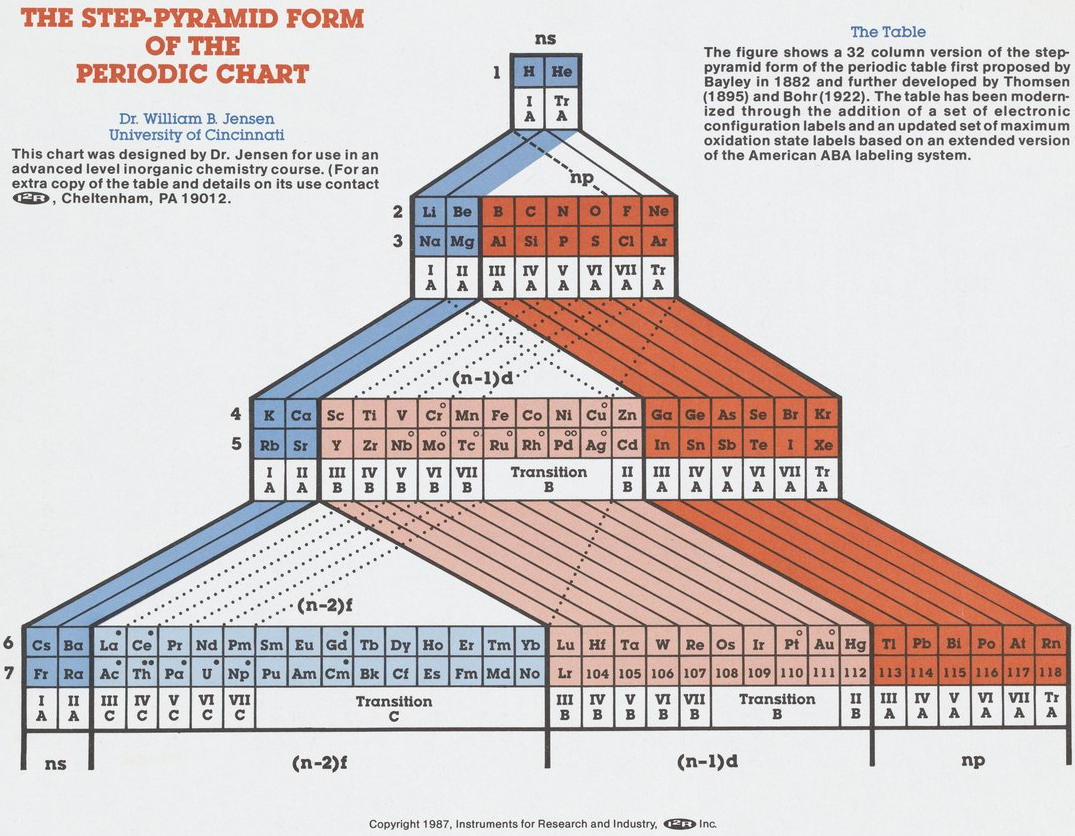
Thanks to René for the tip!
| Year: 1987 | PT id = 1115, Type = formulation data |
Variation of Orbital Radii with Atomic Number
From Jour. Fac. Sci., Hokkaido Univ., Ser. IV. vol. 22, no. 2, Aug., 1987, pp. 357-385, The Connection Between the Properties of Elements and Compounds; Mineralogical-Crystallochemical Classification of Elements by Alexander A. Godovikov & Yu Hariya.
The analyses of the variations of the orbital atomic radii values (rorb) with the increase of the atomic number (Z) allow establishment of the following recurring regularities of their change:
Click image below to enlarge:
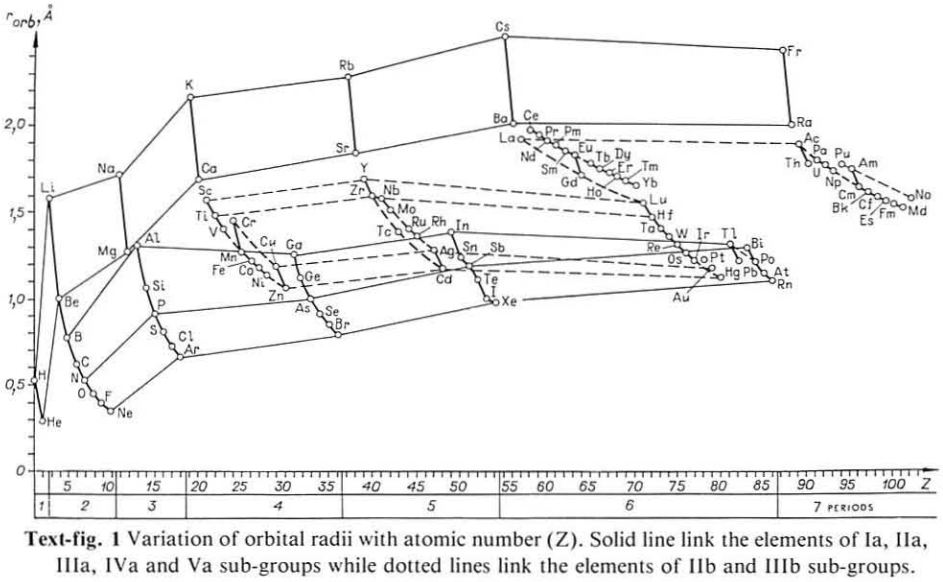
Thanks to René for the tip!
| Year: 1987 | PT id = 1116, Type = formulation data |
Mineralogical-Crystallochemical Classification of Elements
From Jour. Fac. Sci., Hokkaido Univ., Ser. IV. vol. 22, no. 2, Aug., 1987, pp. 357-385, The Connection Between the Properties of Elements and Compounds; Mineralogical-Crystallochemical Classification of Elements by Alexander A. Godovikov & Yu Hariya.
Any mineralogical-crystallochemical classification of elements must provide answers to the following queries:
Click images below to enlarge:
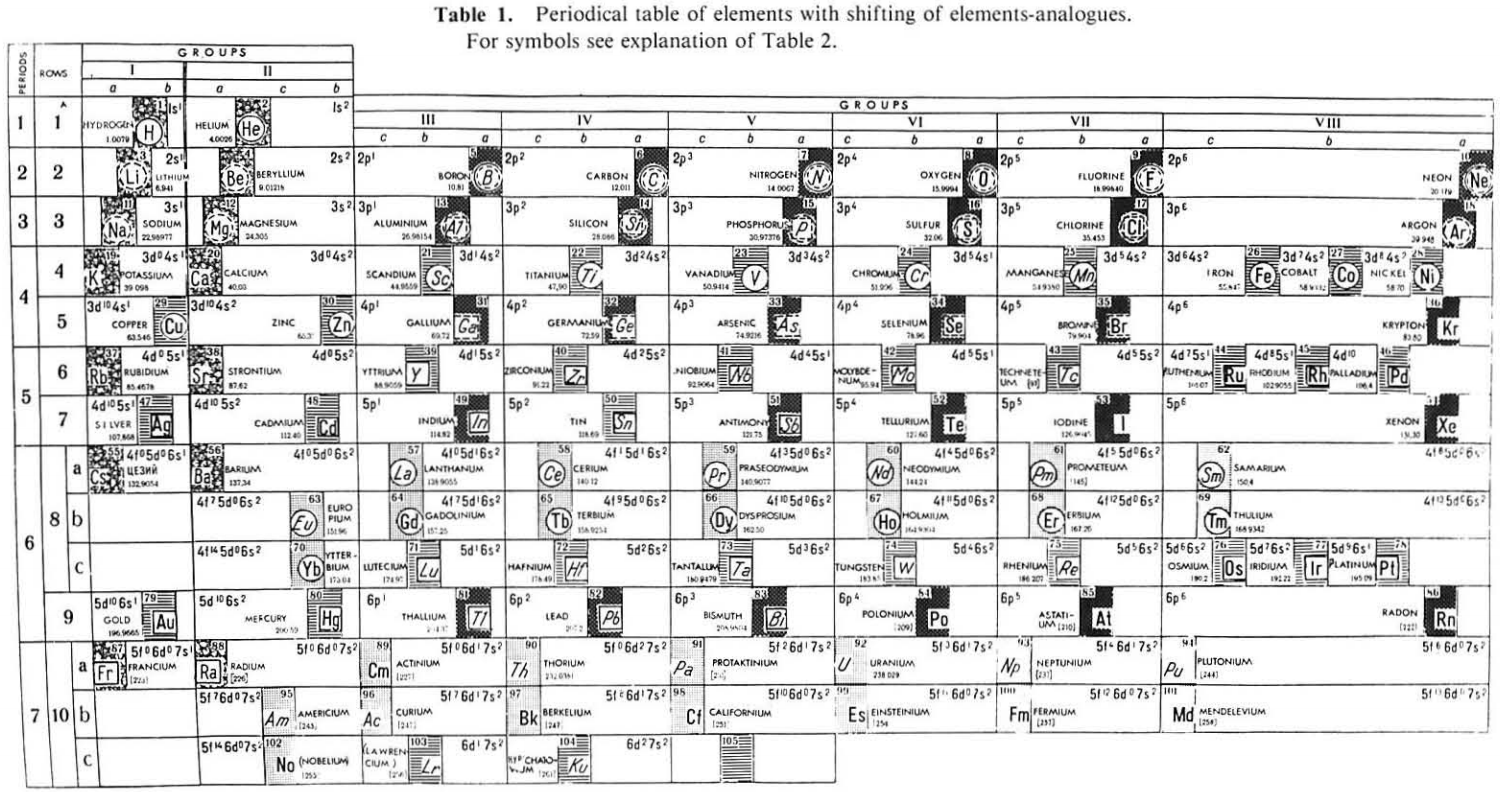
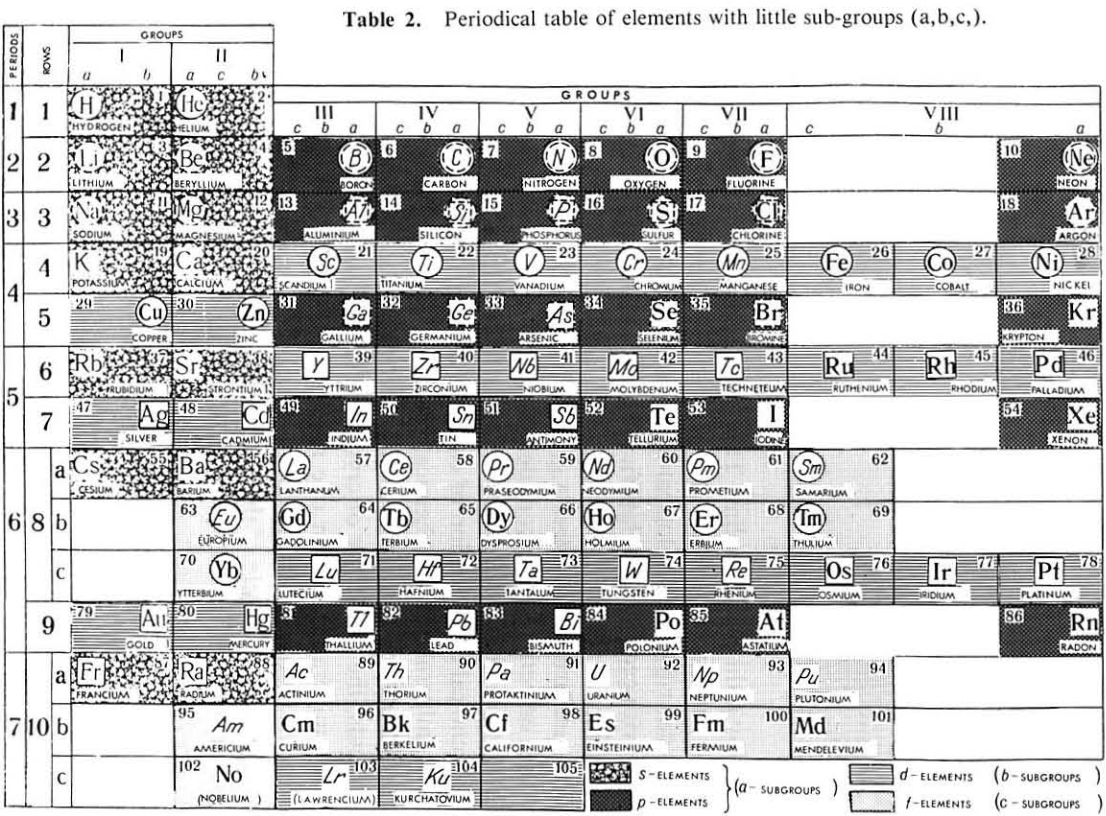
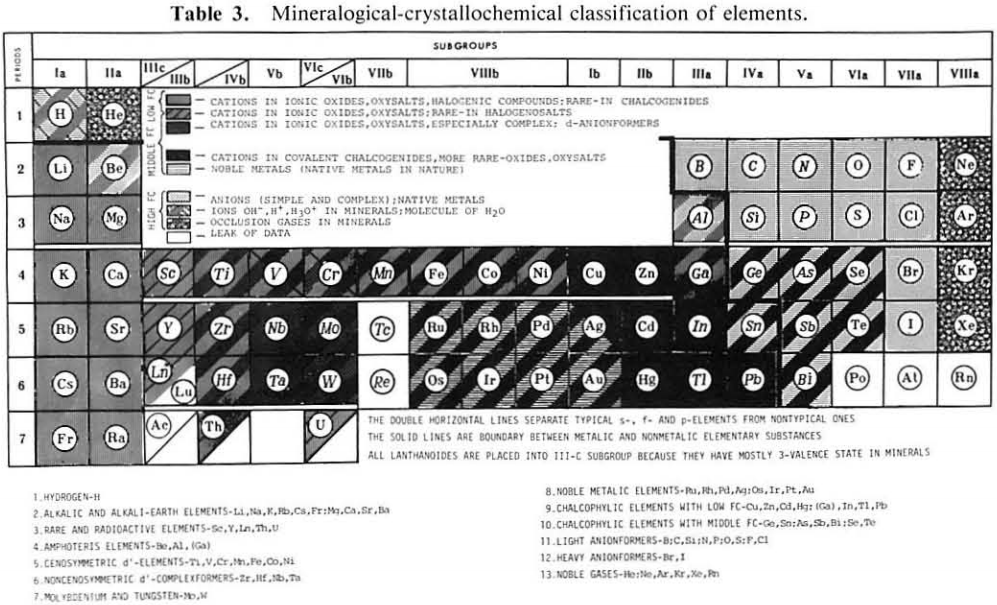
Thanks to René for the tip!
| Year: 1987 | PT id = 743, Type = data |
Elsevier's Periodic Table of the Elements
Prepared by P. Lof is Elsevier's Periodic Table of the Elements.
This educational wall chart features the periodic table of the elements supported by a wealth of chemical, physical, thermodynamical, geochemical and radiochemical data laid down in numerous colourful graphs, plots, figures and tables. The most important chemical and physical properties of the elements can be found - without turning a page.
All properties are presented in the form of tables or graphs. More than 40 properties are given, ranging from melting point and heat capacity to atomic radius, nuclear spin, electrical resistivity and abundance in the solar system. Sixteen of the most important properties are colour coded, so that they may be followed through the periodic system at a glance. Twelve properties have been selected to illustrate periodicity, while separate plots illustrate the relation between properties. In addition, there are special sections dealing with units, fundamental constants and particles, radioisotopes, the Aufbau principle, etc. All data on the chart are fully referenced, and S.I. units are used throughout.
Designed specifically for university and college undergraduates and high school students, "Elsevier's Periodic Table of the Elements" will also be of practical value to professionals in the fields of fundamental and applied physical sciences and technology. The wall chart is ideally suited for self-study and may be used as a complementary reference for textbook study and exam preparation.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1989 | PT id = 222, Type = formulation |
Electron Shell Periodic Table
A modified form of a periodic table showing known and predicted electron shells.
From G.T. Seaborg, Lawrence Berkeley National Laboratory, 1989. From the Encyclopedia Britanica website:
| Year: 1990 | PT id = 436, Type = formulation spiral |
Pawlowski Circular Periodic Table
On John Pratt's website there is an article that is both an introduction to Helen Pawlowski's model of the atom and to her Circular Periodic Table, as well as a book review of her book The Visualization of the Atom (Riverton, UT: Pawlowski Family Trust, 1990). First Helen and her work are introduced, then the model's strengths and weaknesses are summarized:
| Year: 1990 | PT id = 716, Type = formulation spiral |
Circular Model of the Atom: Opposition in the Elements
The Circular Model of the Atom is a circular periodic table that shows atomic structure in addition to periodicity. Unlike any other periodic table or model, it demonstrates that the atomic structure has an inherent dipole magnet that create positve and negative fields and elemental qualities at the atomic level.
The Circular Model of the Atom was created by Helen A. Pawlowski in the 1980s, and published in her work, Visualization of the Atom.
Her brother, Paul A. Williams extended many of Helen's ideas with his examination of the standard model using Helen's Circular Atom Model. This website contains some of Helen's ideas and Paul's writings.
| Year: 1992 | PT id = 1329, Type = formulation data |
A Chemistry Teacher's Perspective of the Periodic System
A Chemistry Teacher's Perspective of the Periodic System, from Science History Institute, Museum & Library Digital Collections.
René Vernon writes:
"It looks like, going by groups, the alkali and alkaline metals & the halogen nonmetals get the most attention. Among the rest of the nonmetals, CHONPS get a good profile with poor Se left in a hole. That leaves B-Si-Ge (in a hole)-As-Sb-Te and the noble gases."

| Year: 1992 | PT id = 1091, Type = formulation 3D |
Magarshak & Malinsky's Three Dimensional Periodic Table
Y. Magarshak & J. Malinsky's Three Dimensional Periodic Table from Nature, 360, 114-115 (1992).
M&M say:
"We believe that our three dimensional representation is a useful tool for visualizing properties of chemical elements and is in complete accord with quantum mechanics."
Thanks to René for the tip!
| Year: 1993 | PT id = 905, Type = formulation misc |
Chemistry Imagined: The Periodic Table
From Roald Hoffmann & Vivian Torrence's book, Chemistry Imagined: Reflections of Science, a picture entitled The Periodic Table:

Thanks to Marcus Lynch for the tip!
| Year: 1993 | PT id = 1268, Type = formulation misc data |
Huheey's Version of The Madelung Rule (For Orbital Filling)
Huheey, J.E., Keiter, E.A., Keiter, R.L.: Inorganic Chemistry: Principles of Structure and Reactivity. 4th edn. HarperCollins College Publishers (1993), p. 22
René Vernon comments: "A peculiar depiction of the Madelung Rule order of filling diagram."
| Year: 1994 | PT id = 1159, Type = formulation |
Treplow's Periodic Table of The Atoms
R.S. Treplow, J. Chem. Educ. 1994, 71, 12, 1007: The Periodic Table of Atoms: Arranging the Elements by a Different Set of Rules.
"Although periodic tables differ greatly in their appearance, examination shows they are all designed according to a common set of conventions. This paper reviews those conventions and asks how the table would look under a different set of rules."
Ground-state multiplicity vs. atomic number for elements 1 to 103. Subblocks are labeled S, P, D & F. Lines connecting the dots show the "ideal" pattern. Atoms not on the lines are "nonideal" (where ideal refers to Madelung's rule):
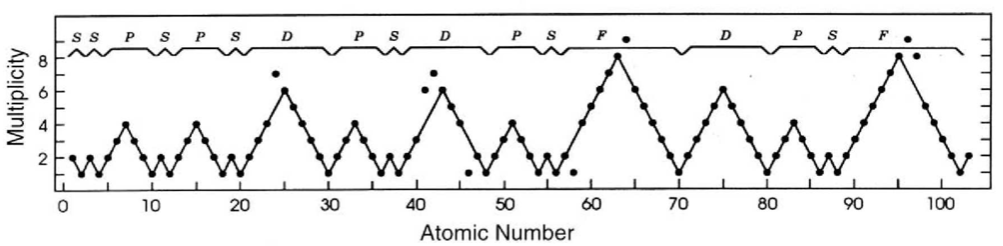
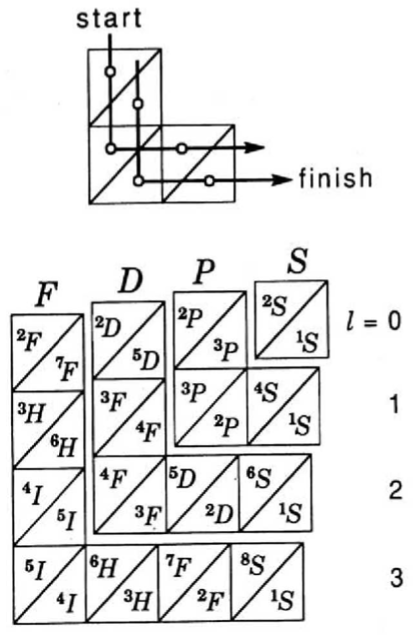
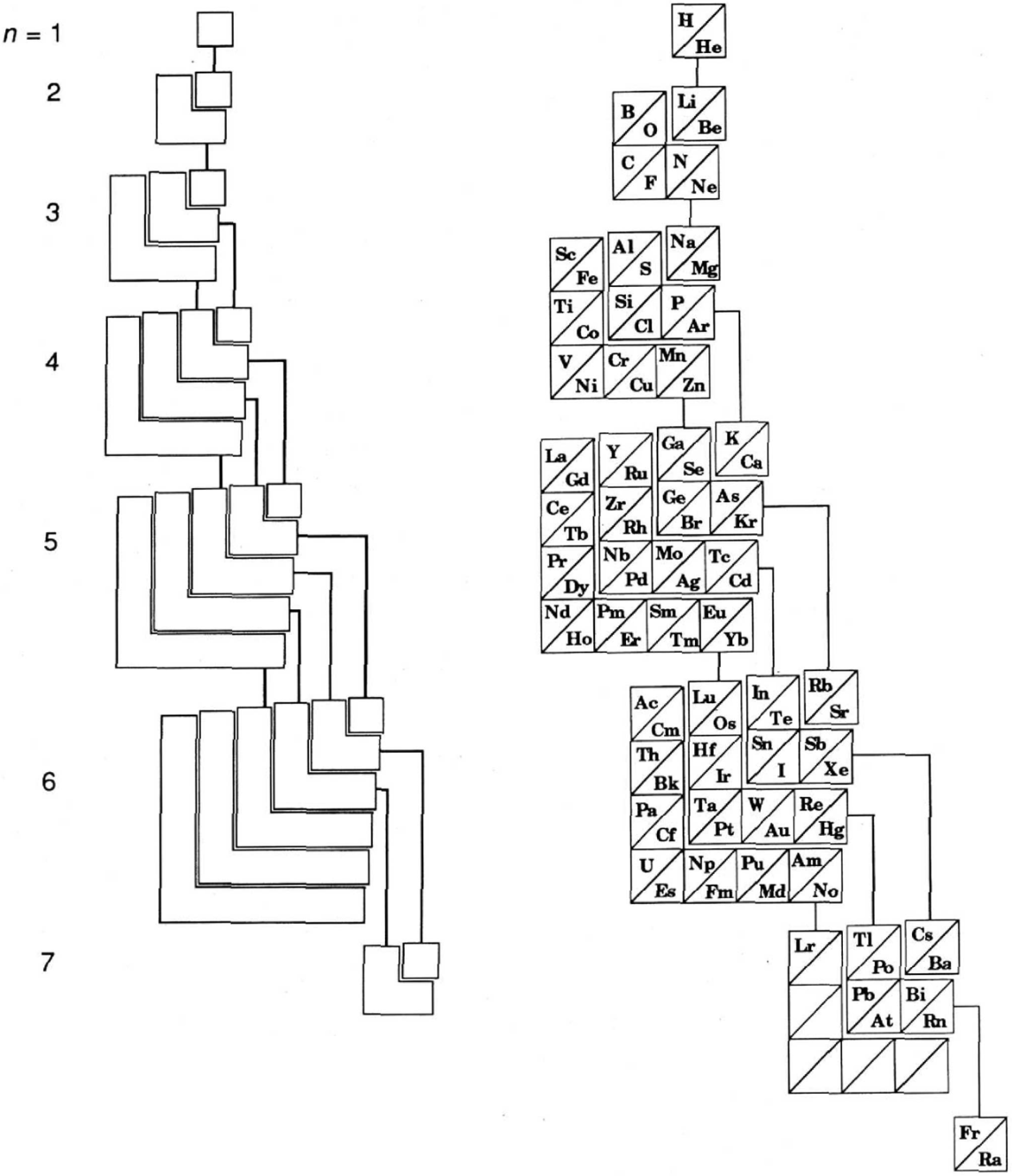
Thanks to René Vernon for his help.
| Year: 1994 | PT id = 1016, Type = formulation 3D |
f-Block Elements 3D Periodic Table
From conference in Helsinki on the f-Block Elements to commemorate the bicentennial of Johan Gadolin's 1794 analysis of Yittria.
Pekka Pykkö writes to say:
"We used [this formulation] in Helsinki in 1994 on the cover of ICFE-2 conference proceedings. Who invented it or where it was copied from, I do not know. Anyway, all the hundreds of participants received it from us":
Claude Piguet's paper, Chimia 73 (2019) 165–172, also uses this 3D version of the standard periodic table. The text says: "Periodic table highlighting the location of Rare Earths (red elements). The elements shown in blue correspond to the actinide series":
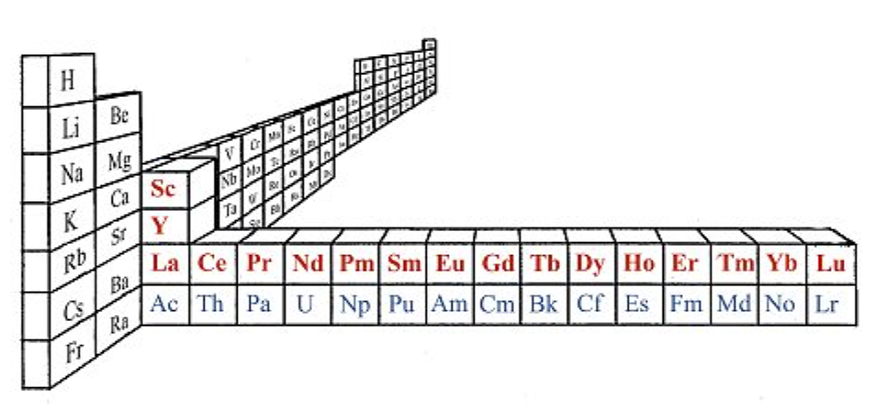
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1995 | PT id = 1292, Type = formulation spiral |
Considine's Polar Periodic Table
From: Considine DM (ed.) 1995, Van Nostrand’s Encyclopedia of Science, 8th ed. New York, p. 2376
René Vernon writes:
"A nice design but of quite limited practical utility for quick reference or detailed chemical analysis."
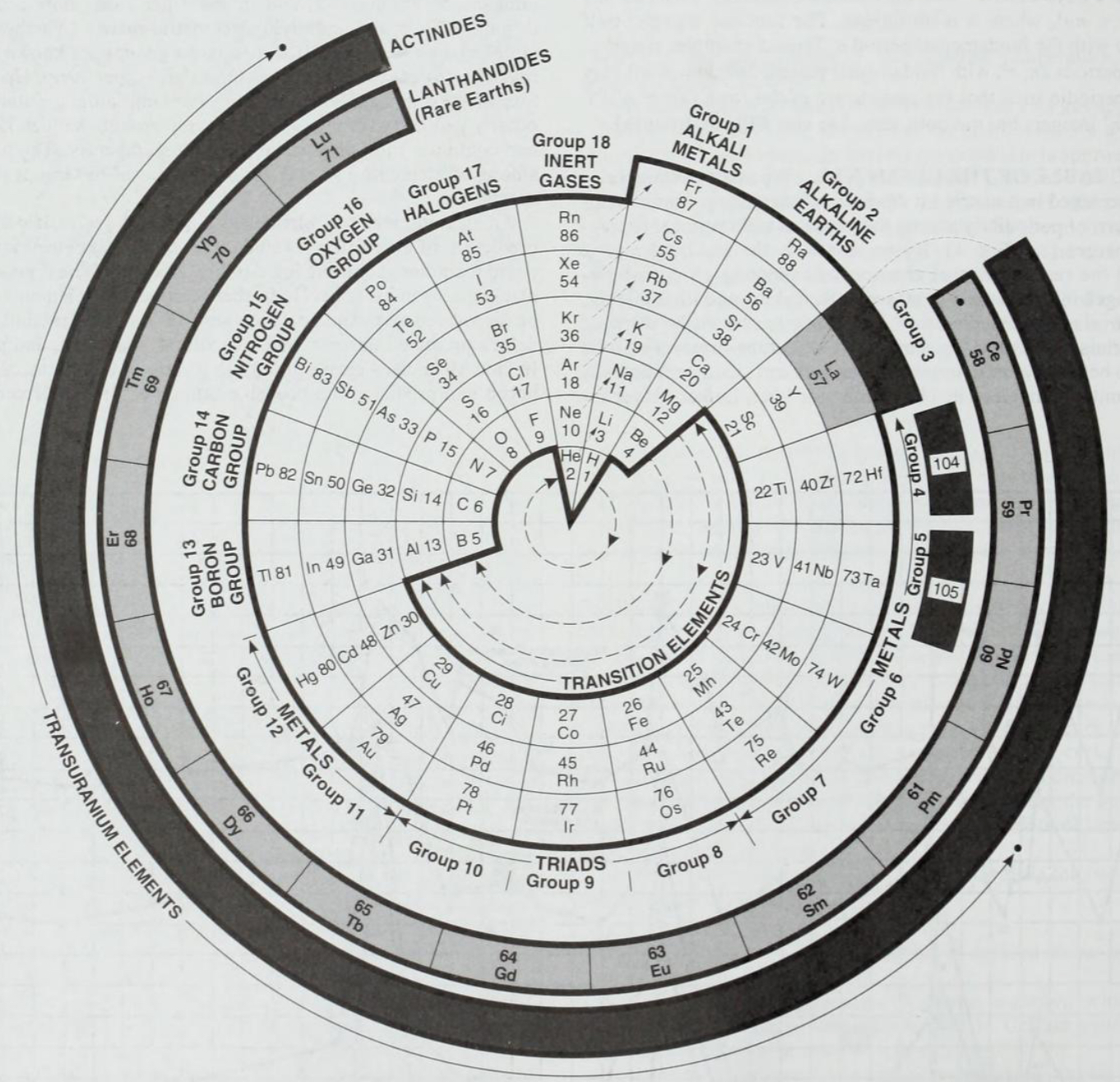
| Year: 1995 | PT id = 1130, Type = formulation |
Klein's Periodic Table of The Elements
Klein DJ, Similarity and Dissimilarity in Posets, Journal of Mathematical Chemistry, 18(2), 321–348 (342) (1995)
The relevance of partially ordered sets (or posets) in a wide diversity of contexts in chemistry is emphasized, and the utility of distance functions (or metrics) on such posets is noted. First a notion of "scale similarity" is introduced to make comparisons within certain so-called "scaled" posets, for which there is formulated natural "comparators", which in turn lead to associated distance functions. Beyond taking note of several chemically relevant examples of these "scaled" posets and their consequent associated similarity measures, a second chemically relevant class of so-called "shifted" posets is similarly developed, with examples. Even further extension of some aspects of the current approach is indicated, and finally the multi-posetic character of chemical periodic law is suggested.
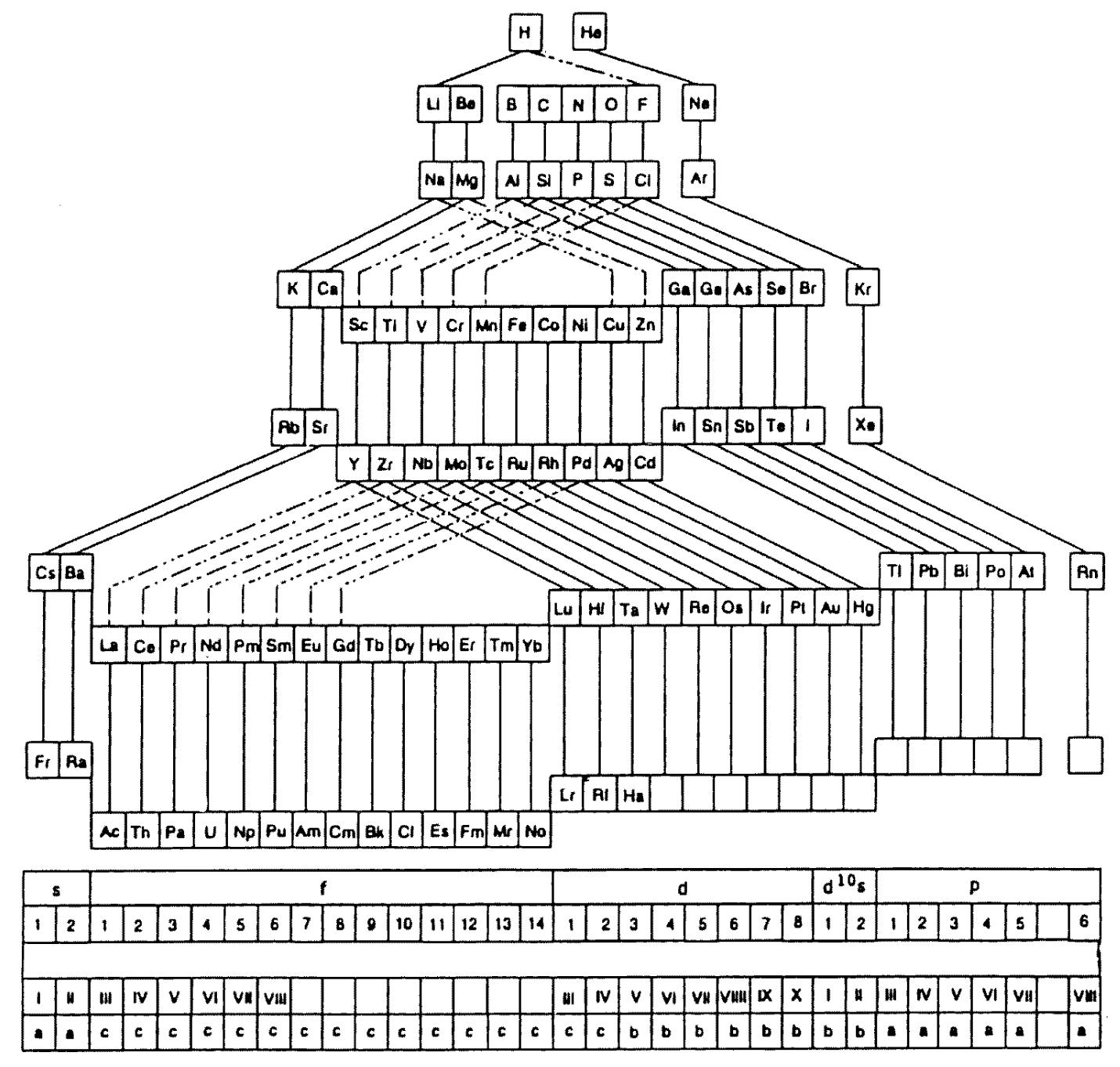
Thanks to René for the tip!
| Year: 1996 | PT id = 238, Type = formulation misc |
First Ionisation Energy of The Elements
Periodic trend for ionization energy, for example Mg → Mg+ + e–
Each period begins at a minimum for the alkali metals, and ends at a maximum for the noble gases. From Wikipedia:
Based on data from: Martin, W. C.; Wiese, W. L. (1996). Atomic, Molecular, & Optical Physics Handbook. American Institute of Physics. ISBN 156396242X.
| Year: 2000 | PT id = 449, Type = formulation 3D |
Chemical Elements Pyramidal Diagram
A Chemical Elements Pyramidal Diagram by Thomas Zerkov.
"The present work introduces a new arrangement of the chemical elements. Unlike the most popular existing arrangements, which are two-dimensional, this new arrangement is three-dimensional. It organizes the elements in a pyramidal structure of four levels, giving a clear spatial expression of different relations between the chemical elements. Since the three-dimensional structures are harder to perceive than the two-dimensional ones, the present work also suggests a two-dimensional table representation of the three-dimensional pyramidal diagram, where the four levels are all placed in a single plane, instead of one above the other."
| Year: 2000 | PT id = 1240, Type = formulation data |
Sneath's Dendtogram
Sneath, P., Numerical Classification of the Chemical Elements and Its Relation to the Periodic System. Foundations of Chemistry 2, 237–263 (2000).
Abstract: "A numerical classification was performed on 69 elements with 54 chemical and physicochemical properties... Only 15 properties were scorable for the noble gases, but despite the paucity of properties reflecting chemical reactivity, analysis of the 69 elements on these properties still showed the major features seen from the full set."
Sneath writes:
"The UPGMA tree shows three major clusters at the 70% similarity level:
1. Hydrogen, noble gases, reactive nonmetals and halogens.
2. A large cluster of less reactive metals and metalloids, with a few nonmetals.
3. A cluster of highly reactive metals.These major clusters are almost consistent with the blocks based on electron shells. The first cluster belongs to the p-block plus hydrogen. The second belongs to the d-block together with a few p-block elements. The third consists of the s-block plus aluminum from the p-block.
Cluster 1: There are three subclusters. 1a. This contains hydrogen and the noble gases."
Thanks to René for the tip!
| Year: 2000 | PT id = 757, Type = misc |
MIT Periodic Table Characters
Eric Scerri writes:
"This apparently hangs on a wall of Building 6 at MIT. I have identified the people around the old-school periodic table, they are (from left to right): Zosimos, Ko Hung, Jabir, Boyle, Lomonosov, Lavoisier, Berzelius, Wohler, Cannizzaro, Berthelot & Mendeleev":

Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 2001 | PT id = 1025, Type = data formulation review |
Wikipedia Periodic Table
The Wikipedia Periodic Table pages are astonishing, giving hyper-linked data about:
| Year: 2001 | PT id = 1062, Type = formulation |
Gorbunov and Filippov's Doubled Periodic Table
Gorbunov, A. I., Filippov, G. G.: Fine Structure of D. I. Mendeleev Periodic Table: secondary periodicity, early and late elements. Khim-ya Tekhnol. 11, 43–45 (2001). (in Russian)
Naum S. Imyanitov (Foundations of Chemistry) writes:
"The two-table design is of particular interest. Atoms with odd n+l are located in the upper table, and the ones with even n+l are placed in the bottom table (Tables 5). The elements are divided by a vertical line of symmetry to the early and late ones both in the upper and bottom tables. The advantage of Tables 5 is a clear demarcation into subsets, with each subset having its own separate place in the table. The drawback is directly related to this advantage: this table does not reflect the similarity between members of different subsets."
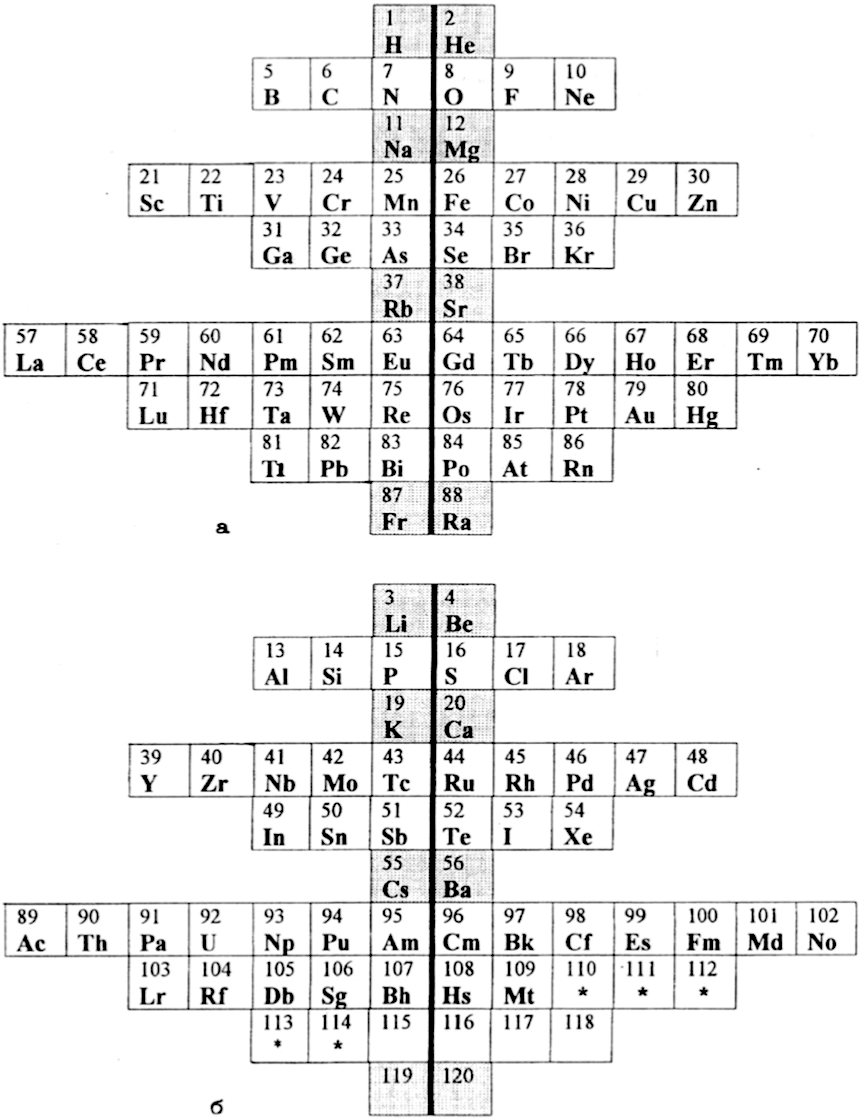
| Year: 2001 | PT id = 1322, Type = review misc formulation |
Oliver Sacks, Uncle Tungsten: Memories of Tungsten of a Chemical Beyond
René Vernon writes:
On the paperback cover of Oliver Sack's Uncle Tungsten (below) the periodic table shows a 16–wide set of elements at its base. This is quite unusual since this set is normally shown as being 15— or 14— elements wide. See, for example, the table found on the site of the International Union of Pure & Applied Chemistry which shows a 15–wide set of elements at its base.
It looks like the second pair are La and Ac, but what then are two immediately preceding elements?
I suspect they are probably the alkaline earth metals, Ba and Ra. This may be an homage to Mr Rare Earth^ aka Karl A. Gschneidner Jr (1930–2016), who wrote that:
...since Ba has a 4f06s2 configuration, these three elements are the first (Ba), mid (Eu), and end (Yb) members of the divalent 4f transition series.
The notion of 4f0 is not unprecedented; the IUPAC periodic table, with its 15-wide f-block presumably implies La as 4f0 5d1 6s2.
There is some good chemistry going on here, given the pronounced similarities between Ba and the lanthanides, and the alkaline earth metals generally with about 20 properties involved:
Kudos to Oliver.
^Pecharsky 2016
Sources

| Year: 2001 | PT id = 555, Type = misc |
Funny Periodic Table
By Eric J Stone a Funny Periodic Table of chemical reactivity.
"This periodic table is unique -- it is informational, educational, and humorous at the same time. Arranged in the standard Mendeleev layout, this table depicts the elements interacting with each other in many interesting ways. The jokes are designed to impart useful information within the context of humor. Ideal for science buffs of all ages -- this is truly the periodic table for the masses. It can be appreciated by children and professionals alike. Children especially like the table, which draws them in with its funny vignettes. This poster is based on the original art of Slavomir Koys. The poster makes a great promotional item. Use it to promote your schools chemistry club or as science fair prizes":
| Year: 2002 | PT id = 531, Type = misc |
Protein Structure Periodic Tables
From a paper by W. R. Taylor, A 'Periodic Table' for Protein Structures, Nature, 2002 Apr 11;416(6881):657-60
Abstract:
Current structural genomics programs aim systematically to determine the structures of all proteins coded in both human and other genomes, providing a complete picture of the number and variety of protein structures that exist. In the past, estimates have been made on the basis of the incomplete sample of structures currently known. These estimates have varied greatly (between 1,000 and 10,000; see for example refs 1 and 2), partly because of limited sample size but also owing to the difficulties of distinguishing one structure from another. This distinction is usually topological, based on the fold of the protein; however, in strict topological terms (neglecting to consider intra-chain cross-links), protein chains are open strings and hence are all identical. To avoid this trivial result, topologies are determined by considering secondary links in the form of intra-chain hydrogen bonds (secondary structure) and tertiary links formed by the packing of secondary structures. However, small additions to or loss of structure can make large changes to these perceived topologies and such subjective solutions are neither robust nor amenable to automation. Here I formalize both secondary and tertiary links to allow the rigorous and automatic definition of protein topology.
This work has been developed by Efrosini Moutevelis and Derek N. Woolfson in their paper A Periodic Table of Coiled-Coil Protein Structures, J. Mol. Biol. (2009) 385, 726–732.
Abstract:
Coiled coils are protein structure domains with two or more ?-helices packed together via interlacing of side chains known as knob-into-hole packing. We analysed and classified a large set of coiled-coil structures using a combination of automated and manual methods. This led to a systematic classification that we termed a "periodic table of coiled coils", which we have made available here. In this table, coiled-coil assemblies are arranged in columns with increasing numbers of α-helices and in rows of increased complexity. The table provides a framework for understanding possibilities in and limits on coiled-coil structures and a basis for future prediction, engineering and design studies.
| Year: 2002 | PT id = 714, Type = formulation |
Tetrahedral Twist: Chemistry Puzzle and Teaching Device
A twisting three dimensional puzzle apparatus for the study of chemistry and its history and based upon the Zmaczynski equilateral triangular model of the periodic table of the chemical elements. Each face of the pyramid has a series of equilateral shaped portions bearing portions of the periodic table of elements. The different segments can be rotated around in order to scramble the puzzle. Such portions can be constructed using same or similar technology that was used to design the Meffert PYRAMINX PUZZLE that is similar to the RUBIK'S CUBE design.
From a US Patent.
| Year: 2003 | PT id = 54, Type = formulation 3D misc |
Elephant Periodic Table
The periodic table does not map to an elephant very well:

Click on the poster below to go to a large version:
| Year: 2003 | PT id = 1082, Type = formulation 3D |
Two-Amphitheater Pyramid Periodic Table
From Chemical Education Journal (CEJ), Vol. 7, No. 2
A Novel Way of Visualization of the Periodic Table of the Elements by Alaa El-Deen Ali Mohamed, Alexandria University, Egypt.
The author writes:
"New form of the periodic table of the elements is given in this paper. This form can be seen as two amphitheater pyramids facing each other. The cubes that meet are s-elements (interior) then the p-elements then d-elements and the f-elements at last (exterior). The table can be represented by X-, Y- and Z-axes, where the Z-axis gives the number of the period that the element occupies. The table can be modeled by colored cubes helping in introducing the periodic table to the pupils early in the primary education."
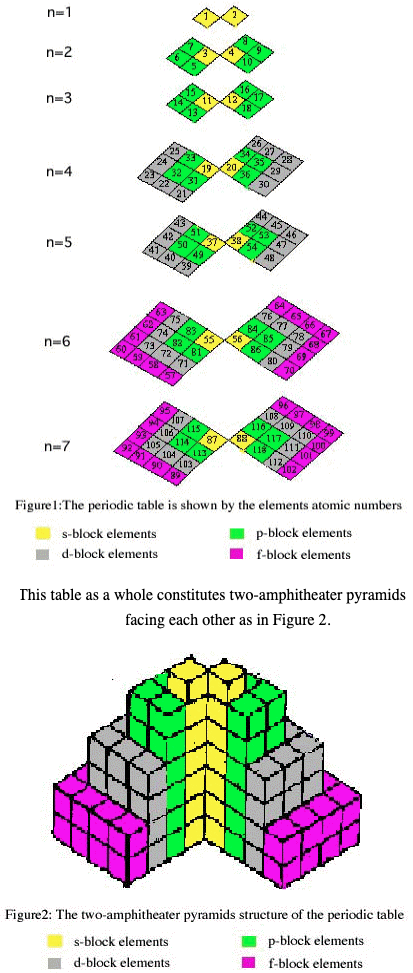
Thanks to René for the tip!
| Year: 2003 | PT id = 1150, Type = formulation data |
Stable Isotopes, Periodic Table of
From Boeyens, JCA 2003, J. Radioanal. Nucl. Chem., 257, 33 a periodic table of the 264 stable isotopes arranged as an 11 x 24 matrix.
Click the image to enlarge:
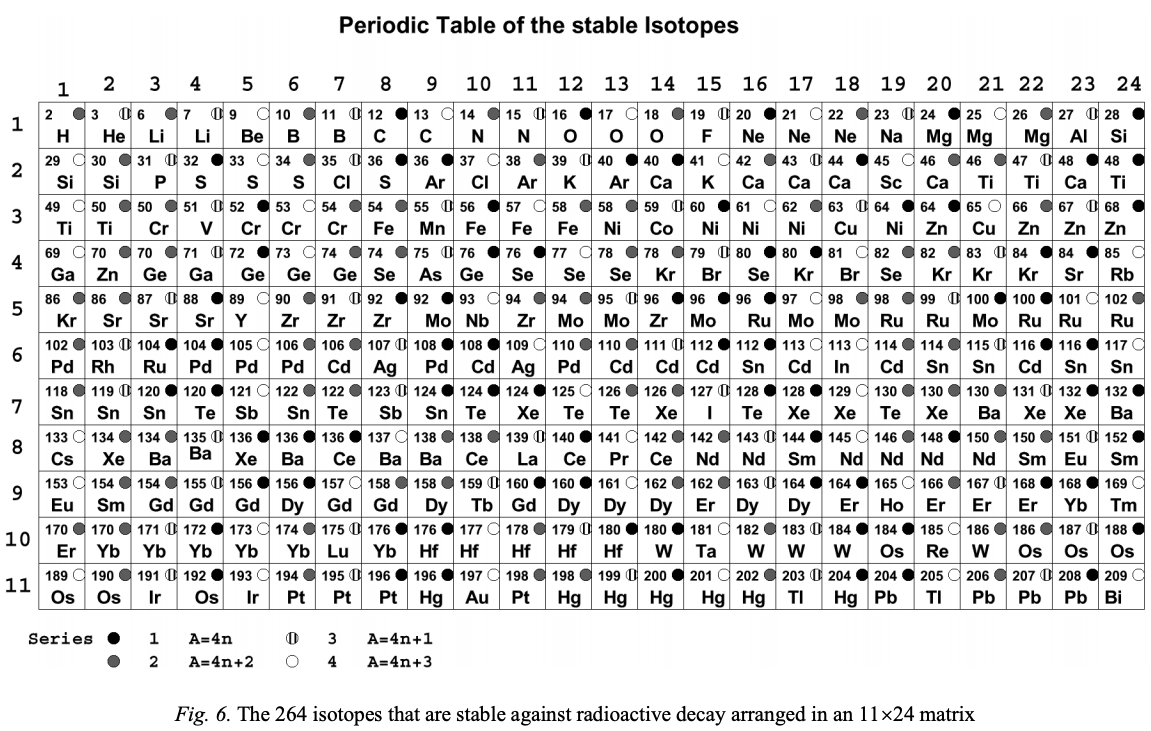
Thanks to René for the tip!
| Year: 2003 | PT id = 142, Type = data |
Earth Scientist's Periodic Table of The Elements and Their Ions
by Bruce Railsback.
Click to enlargeThe Earth Scientist's Periodic Table of the Elements and Their Ions is a new periodic table designed to contextualize trends in geochemistry, mineralogy, aqueous chemistry, and other natural sciences. It is fundamentally different from the conventional periodic table in organizing entities by charge and consequently in showing many elements multiple times because of the multiple charges or valence states taken by those elements. These differences make the new table much more effective in showing trends and patterns in geochemistry, mineralogy, aqueous chemistry, and other natural sciences.
Version 4.6 of this table was published in September 2003 as an article in the Geological Society of America's journal Geology and subsequently featured in several news outlets. Version 4.7 was published in May 2004 in the Geological Society of America's Map and Chart Series. Version 4.8 was released in May 2007.
| Year: 2004 | PT id = 1096, Type = misc |
Classroom Kids Periodic Table
From a paper by René Vernon, a drawing of the elements as classroom personality kids, drawing by Richard Thompson 1957-2016.
From a National Geographic coffee table book: Curt Suplee, The New Everyday Science Explained, National Geographic Society, Washington DC, p. 130 (2004). The undated credit is given to Richard Thompson.
| Year: 2004 | PT id = 124, Type = data |
Material Type Periodic Table
All of the the main group elements are common laboratory reagents or chemical in bottles. They appear as metals, metalloid (semi-metals) and non-metals. Most of the non-metals are molecular materials while most of the metalloids have an extended network-covalent structure.
Elsewhere in the chemogenesis web book, material type is discussed in terms of the Laing Tetrahedron, an analysis that classifies binary materials in terms of four extreme types: metallic, ionic, molecular and network. However, none the chemical elements present as ionic materials, only as metals, molecular (van er Waals) and network materials:
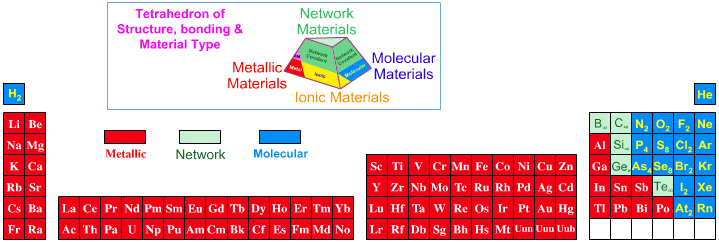
The elements B, C, Si, P, S, Ge, As, Se, Sn, Sb and Te can form allotropes: pure elemental substances that can exist with different crystalline structures from the Wikipedia. Allotropes may be metallic, network or molecular.
By Mark Leach
| Year: 2005 | PT id = 28, Type = formulation |
Laing's Revised Periodic Table with the Lanthanides Repositioned
Michael Laing's "Revised Periodic Table with the Lanthanides Repositioned", from Foundations of Chemistry 7:203-233.
Philip Stewart's modification of the Laing formulation:
Philip Stewart says (in a personal communication):
"It seems wrong to suggest an analogy between Pr to Sm and Dy to Tm with the V, Cr, Mn, Fe groups. I have pushed them to the right to suggest that those lanthanides are like the old group VIII (including the coinage metals); like them they cannot use all their outer electrons in bonding (with the exception of Ru viii and Os viii. I have treated the actinides differently to take account of Pa v and U vi. It's ability to lose the juxtaposition of Tc and Pm, but it is physical rather than chemical anyway."
| Year: 2005 | PT id = 657, Type = data |
Chemical Thesaurus Reaction Chemistry Database Periodic Table
A periodic table front end to the Chemical Thesaurus Reaction Chemistry Database Periodic Table. Clicking on an element gives access to database searches of chemical species and their interactions.
A quote neatly sums up what the ChemThes reaction chemistry database project is trying to achieve:
"The Chemical Thesaurus is a reaction chemistry information system that extends traditional references by providing hyperlinks between related information. The program goes a long way toward meeting its ambitious goal of creating a nonlinear reference for reaction information. With its built-in connections, organizing themes, and multiple ways to sort and view data, The Chemical Thesaurus is much greater than the sum of the data in its database.
"The program does an excellent job of removing the artificial barriers between different subdisciplinary areas of chemistry by presenting a unified vision of inorganic and organic reaction chemistry."
By Mark Leach
| Year: 2005 | PT id = 1242, Type = formulation |
Rich's Periodic Chart Exposing Diagonal Relationships
Rich, R. L. (2005). Are Some Elements More Equal Than Others? Journal of Chemical Education, 82(12), 1761. doi:10.1021/ed082p1761.
Thanks to René for the tip!
| Year: 2006 | PT id = 30, Type = non-chem |
Homeopathic
A Homeopathic Periodic Table by Jan Scholten.
From his book Geheime Lanthanide (Secret Lanthanides) 2006. The basic idea is that successive elements in each series ( = row) are like the stages in a heroic story like the labours of Hercules or the voyages of Odysseus, each one appropriate to meet a different challenge.
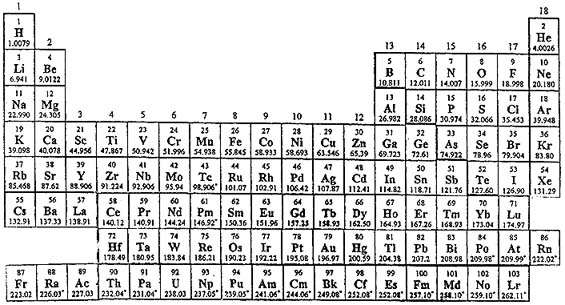
| Year: 2006 | PT id = 56, Type = formulation |
Reaction Chemists' Periodic Table
OK, so which Is The Best formulation of The Periodic Table?
Personally as a reaction chemist, my preferred periodic table is the 'long' form shown below, with hydrogen above and between boron and carbon, although clearly other scientists have other ideas.
All periodic tables show the increase in mass and atomic number, Z, but only the long form unambiguously shows the general top-right-to-bottom-left trends in electronegativity, atomic radius, metallic properties and first ionisation energy.
Electronegativity is absolutely crucial to the understanding of structure, bonding, material type (van Arkel-Ketelaar triangle and Laing tetrahedron) and chemical reactivity, and it underpins much of the chemogenesis analysis.
| Year: 2007 | PT id = 1282, Type = formulation |
Seeger-Quadbeck Periodic Table
Seeger-Quadbeck H-J 2007, World of the Elements Elements of the World, Wiley-VCH, Wienheim, inside cover.
René Vernon, who provided the graphic, writes:
"An example of a rarely seen 32-column table. The categorisation scheme is interesting.

| Year: 2007 | PT id = 778, Type = formulation data |
Mechanical Engineer's Periodic Table
Avallone EA, Baumeister T & Sadegh AM (eds) 2007, Marks' Standard Handbook for Mechanical Engineers, 11th ed., McGraw-Hill, New York, p. 6-6. Click here for a larger version.
This mech eng PT has a couple of odd features: hydrogen is in Group 17 above fluorine and the lanthanides are split:
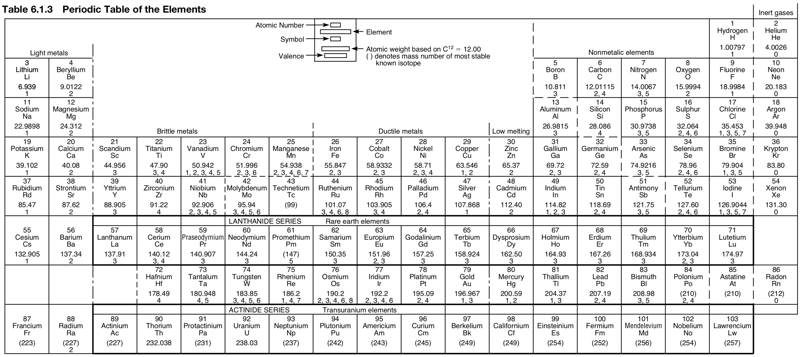
Thanks to René for the tip!
| Year: 2007 | PT id = 404, Type = non-chem |
Freaky Trigger Periodic Table
The Freaky Trigger Periodic Table. "What the FreakyTrigger periodic table is not: science - not even in the loosest limbed definition that FT uses (ie thought experiments and puns). It is not a way of ordering the world, the makers of the desert periodic table put up last week would be quite disappointed by the general lack of periodicity. Whilst there may be some serendipity in the periodic placement of some of the "elements", this is more by accident than by design. Indeed the design merely fits that of the periodic table because that was the idea in the first place. And as the methodology shows, any over-arching idea to create a consistent cosmology out of this project was soon scuppered by the organic scourge of many bright ideas. Alcohol.":
| Year: 2007 | PT id = 218, Type = non-chem |
Canadian Periodic Table
This Canadian PT has lots of subtle Canadian references (apparently) from here.
| Year: 2007 | PT id = 1021, Type = formulation 3D spiral |
Bent & Weinhold's 2D/3D Periodic Tables
From a paper by Henry Bent & Frank Weinhold, J. Chem. Educ., 2007, 84, 7, 1145 and here. The authors write in the abstract:
"The periodic table epitomizes chemistry, and evolving representations of chemical periodicity should reflect the ongoing advances in chemical understanding. In this respect, the traditional Mendeleev-style table appears sub-optimal for describing a variety of important higher-order periodicity patterns that have become apparent in the post-Mendeleevian quantal era. In this paper we analyze the rigorous mathematical origins of chemical periodicity in terms of the quantal nodal features of atomic valence orbitals, and we propose a variety of alternative 2D/3D display symbols, tables, and models.":
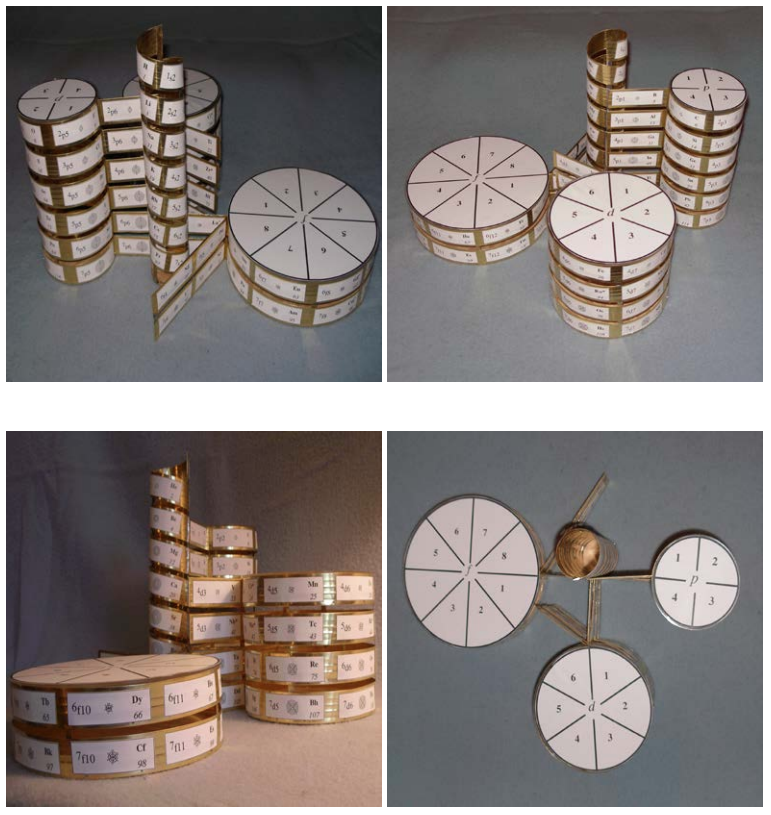
Thanks to René for the tip!
| Year: 2008 | PT id = 1302, Type = formulation |
Franklyn's Periodic Table
Franklyn writes on sciencemaddness.org: Electronic Orbital Periodicity Mendelevian grouping is only one possible organizational scheme, regardless of the schematic choice. A table is useful only to the extent that it provides easy reference to data and comparison. Most everyone who has considered arranging elements in tabular form has pondered what layout best serves the purpose. Below is a table I once made to determine the electronic shell and orbital structure of any element at a glance. Everything to the left and above the elements position indicates the complete full orbitals for those shells. Actually you can see the goup memebers run diagonally from upper left to lower right This arrangement shows that the progression of successive electrons is not straight forward with regard to placement within the atoms. The Mendelevian sequence begining with period 6 through the Lanthanides back to period 6 transition metals until Radon, continuing with period 7 ending with the first member of the Actinides, is as follows:

Thanks to René Vernon for the tip!
| Year: 2008 | PT id = 333, Type = misc review |
Braille Guidebook Interactive Periodic Table Study Set
Azer's Interactive Periodic Table Study Set is designed to make learning about the Periodic Table of the Elements accessible to students with visual impairments or blindness.
The tangible materials included with this study set complement APH's Periodic Table of the Elements Reference Chart and allow students to enhance their understanding of concepts consistent with the National Science Standards.
Inspired by Samir Azer, a science teacher at the Kentucky School for the Blind, this set can assist in the instruction and demonstration of concepts related to the arrangement of the periodic table, atomic structure, ionic and covalent bonding, and balancing of chemical equations to students who benefit from a hands-on, interactive model.
Special attention was given to make the materials tactually discriminable and visually appealing to the target population, yet appropriate for all students regardless of visual acuity:
| Year: 2008 | PT id = 659, Type = misc |
f--l--A--r--k's Fractal Periodic Table
A fractal periodic table by f--l--A--r--k:
After nearly a year of work and research, the Periodic Table is complete.
I have endeavored to the best of my ability to accurately represent each element as a fractal. The table itself is up to date with current findings and research as of 2008.
Each element has been individually rendered at a resolution of 3200 x 2400, and is available for a full-view in my gallery. Every fractal was designed, composed, and rendered using Apophysis and then the final assembly done with Photoshop.
Many thanks go to Tony (~atd85) for his assistance in rendering quite a few of these elements, and to my wife for her inspiration and encouragement:
| Year: 2008 | PT id = 155, Type = formulation spiral 3D |
Tomás A. Carroll's Spherical & Russian Doll Formulations
Tomás A. Carroll has devised a spherical formulation of the Periodic Table, and from this a nested Russian Doll formulation.
Tomás writes: "I accept your veiled challenge that it is not possible to formulate a spherical periodic table and propose two solutions for your consideration. The EXCEL spreadsheet shows exactly how I transformed the quantum numbers from the standard 4D Cartesian coordinates to spherical coordinates in 3D, using two different centers. I included cylindrical coordinates too, just for fun."

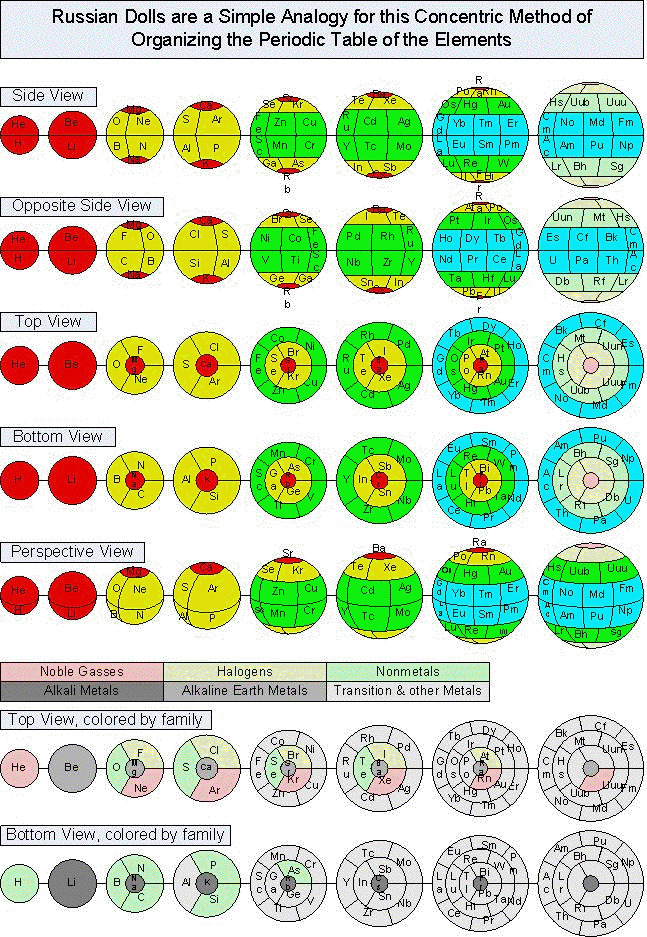
| Year: 2008 | PT id = 156, Type = formulation 3D |
Pyramid (Stack) Periodic Table
The Janet Periodic Table of Elements (1928) may be re-arranged as a series of square matrices.
The matrices are of different sizes and each matrix organizes the atomic orbitals into square concentric rings. Each cell may be assigned an atomic number which also identifies a “most significant electron”. The matrices may be stacked vertically to form a periodic Pyramid Stack of Elements as shown below.
The sub-atomic particles may also be arranged as square matrices. These matrices may be stacked. Read more here.
Please send your comments to: rick_kingstone777@hotmail.com
| Year: 2008 | PT id = 468, Type = formulation spiral 3D |
Teluric Helix from Gutierrez Samanez
The Teluric Helix from Gutierrez Samanez is inspired by the telluric helix Chancortois (1864) with the difference that the sequence of the elements are rolled into a cone shape rather than a cylinder:
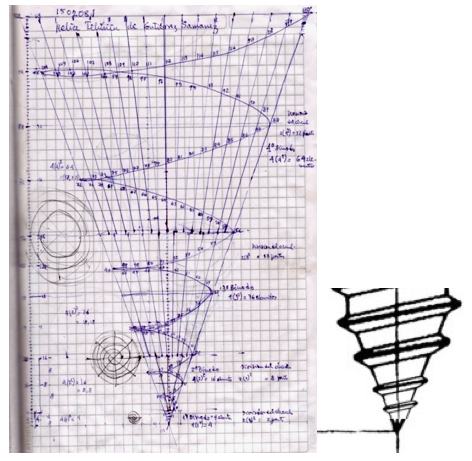
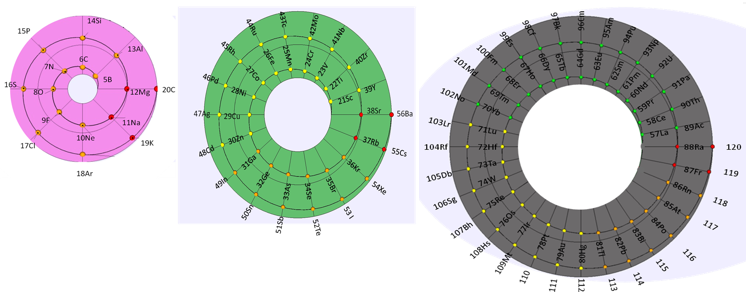
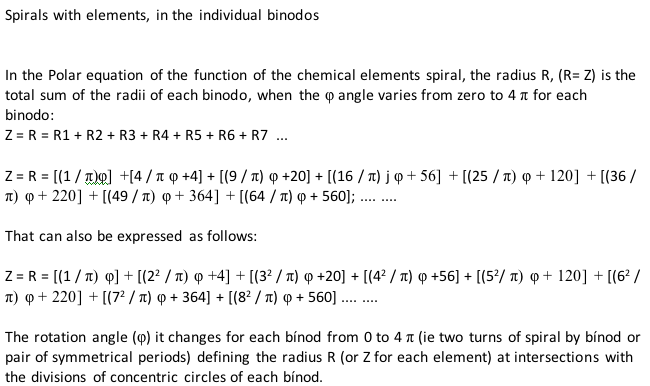
| Year: 2008 | PT id = 221, Type = misc non-chem |
Snelson Atom
"Kenneth Snelson's Portrait of an Atom is a multi-media artwork that [attempts to] describe the atom's electronic architecture. If you happen to have a rapid prototype printer this STL file can be downloaded free for creating a desktop model at any preferred size of the Snelson atom."

| Year: 2008 | PT id = 243, Type = formulation |
Bernard Schaeffer's Quantum Mechanics Consistent Periodic Table
My graphic representation of the orbitals needed for the periodic table is without brilliant colors, but much simpler. It shows the nodes of vibration of the spherical resonator (a spherical musical instrument) also called spherical harmonics appearing in the spherical solution of the Schrödinger equation. It may be noticed that the atom is also a spherical resonator, not of sound but of the de Broglie waves.
The spherical harmonics (feminine word in french!) have been discovered by Legendre two centuries ago, see my book. Only the plane nodes of vibration are shown. The nodes of the orbitals are a 3D equivalent of the Chladni figures (also discovered two centuries ago) on a vibrating plate: "Aufbau" with spherical harmonics.
The random electronic exceptions in the subshells don't appear. The spherical nodes of the orbitals are represented only for the s subshells. This is a much simpler representation than the usual 3D representations. It can be used to represent the entire periodic table as I have shown earlier. The elements are in regularly increasing atomic numbers.
Bernard Schaeffer's Quantum Mechanics Consistent periodic table from here:
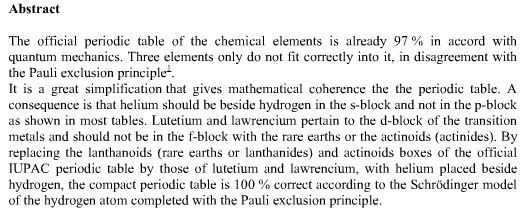
| Year: 2009 | PT id = 552, Type = non-chem |
Canadian Periodic Table of The Elements
From Uncyclopedia: Contrary to popular belief, Canadian science organizations and the Canadian education systems are quite sophisticated programs. These programs rely on up to date information, and a wide plethora of information. Most notably, the Canadian Periodic Table of the Elements.
Established 3 days after the discovery of Canada by Humans, and consisting of only 3 elements (Me, Wa, Ro) it provided the foundation for today's table which sports 18 different Elements, with more being added every decade or so:
| Year: 2009 | PT id = 339, Type = non-chem |
Political Interference in Science, Periodic Table of
In recent years, scientists who work for and advise the federal government have seen their work manipulated, suppressed, distorted, while agencies have systematically limited public and policy maker access to critical scientific information.
To document this abuse, the Union of Concerned Scientists has created the A to Z Guide to Political Interference in Science:
| Year: 2009 | PT id = 200, Type = review |
Scerri's Selected Papers on The Periodic Table
Edited by Eric Scerri (University of California, Los Angeles, USA)
Published by: Imperial College Press in London
The book contains key articles by Eric Scerri, the leading authority on the history and philosophy of the periodic table of the elements. These articles explore a range of topics such as the historical evolution of the periodic system as well as its philosophical status and its relationship to modern quantum physics. In this present volume, many of the more in-depth research papers, which formed the basis for this publication, are presented in their entirety; they have also been published in highly accessible science magazines (such as American Scientist), and journals in history and philosophy of science, as well as quantum chemistry. This must-have publication is completely unique as there is nothing of this form currently available on the market.
Contents:
Readership: Academic readers: philosophers and science historians, science educators, chemists and physicists. 200pp (approx.) Pub. date: Scheduled Fall 2009
200pp (approx.) Pub. date: Scheduled Fall 2009
ISBN 978-1-84816-425-3
1-84816-425-4 US$88 / £66
| Year: 2009 | PT id = 204, Type = formulation |
Silberstein Periodic Table
The organization of the periodic table that follows is based on the principle that, as the
position of Lanthanum, Actinium, Lutetium, and Lawrencium is debated with regard to the
elements in Group III, all four of these elements can be placed in an “extended” Group III and
still have the correct arrangement on the periodic table. Although Scandium and Yttrium appear
to be separated from the rest of the transition metal elements, they in fact should be considered to
retain their original positioning as in a short-form table; that is, they are immediately to the right
of Calcium and Strontium and immediately to the left of Titanium and Zirconium. The curving of
the rare earth elements is merely a tool to denote the position of Lanthanum, Actinium, Lutetium,
and Lawrencium in Group 3, with the remainder of the rare earth elements placed outside of an
existing group, or rather creating their own group. View larger pdf file.
David Silberstein, August 2009
| Year: 2009 | PT id = 249, Type = formulation misc non-chem spiral 3D |
Steve Jensen's "In-Finite Form"
"I'm a figurative sculptor, living in Minneapolis MN. A few years ago, while looking at a two dimensional version of the periodic table, I too wondered if it would be possible to create a Periodic Table without any visual breaks in its numerical sequence. Although I had never seen anything other than the rectangular flat table, I thought I might be able to solve this spatial continuity problem three dimensionally. I also wanted to limit myself to using a 3-D "line" that had no sudden changes in direction. After coming up with what I thought was a new and unique sculptural resolution, I put the project aside. Only recently (after re-building my paper model out of a translucent material) did I do some research on the web, and immediately recognized the strong likeness between my version and the Alexander Arrangement. Even more surprising was my models' visual similarity to Crookes' figure eight design from some 111 years ago.
"Although there are obviously many inventive and well thought out responses to this design challenge, I believe that my solution is a unique one, and an improvement over some of the previous three dimensional forms. The "line" of my model allows for contiguous numerical placement of all the symbols (while maintaining group continuity along its vertical axis), even as the shape of its plan view makes visual reference to the well-known symbol for infinity. What's more, in my version, the Lanthanide & Actinide series do not occupy a separate field but are fully integrated into the continuous linear flow. This piece, which I've entitled "In-Finite Form" speaks to the mystery of the endless flow of space, even as it folds back onto itself within the confines of a finite system."
| Year: 2010 | PT id = 277, Type = formulation misc non-chem |
Periodic Arch of The Elements
Cynthia K. Whitney of Galilean Electrodynamics writes: "In his paper Explaining the periodic table, and the role of chemical triad, Eric Scerri mentioned the existence of at least four different candidate places for Hydrogen: Group 1 (alkali metals - Lithium, etc.), Group 17 (halogens - Fluorine, etc.), Group 14 (Carbon, etc.), or off the Periodic Table entirely, because it is so odd! The four-fold multiplicity (and maybe more) of candidate places for Hydrogen triggered in me the following thought: the excessive multiplicity of candidate places may have to do with the rectangular nature of the Periodic Tables under consideration there." Read more in this pdf file.
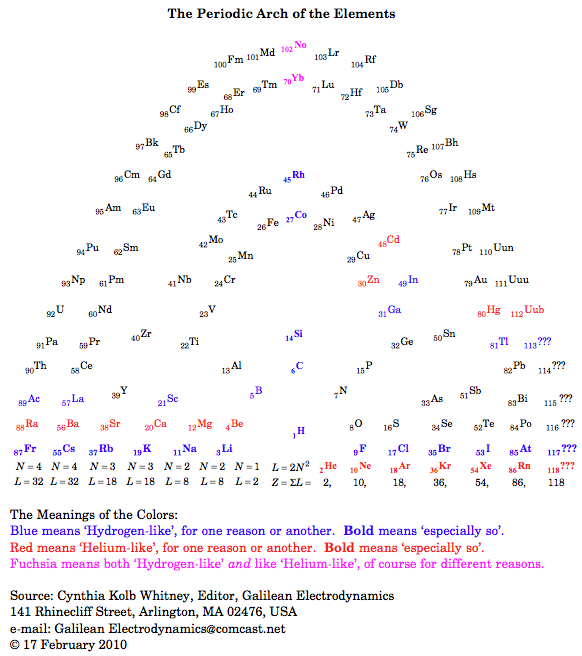
| Year: 2010 | PT id = 306, Type = non-chem |
Wisdom Table
Periodic Table of the World's Religions & Philosophical Traditions - by Dr. Thomas C. Daffern, Director, IIPSGP - www.educationaid.net www.lulu.com/iipsgp (Copyright 2009).
The Wisdom Pages are hosting a Periodic Table of the Worlds Religious and Philosophical Traditions or Wisdom Table for short. It can be viewed by following the website link below. If you click in any box on the table it takes you to a database behind giving more information. We are still currently adding to this database however it is nearly complete.:
| Year: 2010 | PT id = 320, Type = non-chem |
Meat Periodic Table
From the Pleated-Jeans blog:
"Scientists have long referred to meat as 'the building blocks of delicious meals'. In an effort to catalog the world's most popular (and unpopular) types of meat into an informative and easy-to-reference tabular form, I give you the The Periodic Table of Meat":
| Year: 2010 | PT id = 326, Type = non-chem |
Brand Evolution Terms
By Kolbrener, a Periodic Table of Brand Evolution Terms:
| Year: 2010 | PT id = 334, Type = data |
NIST Atomic Physical Reference Data
Access the NIST (National Institute of Standards and Technology) physical reference data:
| Year: 2010 | PT id = 340, Type = non-chem |
HTML 5 Elements, Periodic Table of
This table, from the Josh Duck blog, shows the 104 elements currently in the HTML5 working draft and two proposed elements (marked with an asterisk).
| Year: 2010 | PT id = 351, Type = formulation |
Vajra Periodic Table
The Vajra Periodic Table, which can be found at APM Periodic Tables, lays out according to electron orbitals and thus gives insights into the electron structure surrounding the nucleus. The nucleus organizes with different rules and thus a different periodic table is needed to visualize the nuclear bindings:
| Year: 2010 | PT id = 355, Type = formulation |
Pyykkö's Extended Elements
From an RSC new page: Pekka Pyykkö at the University of Helsinki has used a highly accurate computational model to predict electronic structures and therefore the periodic table positions of elements up to proton number 172 - far beyond the limit of elements that scientists can currently synthesise.
From the paper, A suggested periodic table up to Z = 172, based on Dirac-Fock calculations on atoms and ions:
| Year: 2010 | PT id = 356, Type = non-chem |
Baseball Hall of Famers
From Wired: When it comes to central repositories of awesomeness, science has its Periodic Table of Elements. Baseball has its Hall of Fame. And now, an unlikely marriage between the two has been fashioned.
Larry Granillo, who runs the über-awesome Wezen Ball, took it upon himself to essentially mash up the Periodic Table (which currently boasts 118 known elements) with those who've been formally voted into baseball's most elite circle (109 members, to date). With a little categorizing and a whole lot of inventiveness, Granillo came up with the definitive classification system of baseball legends.
Click to embiggen:
| Year: 2010 | PT id = 375, Type = formulation data |
Upper Limit in Mendeleev's Periodic Table - Element No.155
This book (PDF), by Albert Khazan, represents a result of many-year theoretical research, which manifested hyperbolic law in Mendeleev's Periodic Table.
According to [Khazan's] law, an upper limit (heaviest element) exists in Mendeleev's Table, whose atomic mass is 411.66 and No.155. It is shown that the heaviest element No.155 can be a reference point in nuclear reactions. Due to symmetry of the hyperbolic law, the necessity of the Table of Anti-Elements, consisting of anti-substance, has been predicted. This manifests that the found hyperbolic law is universal, and the Periodic Table is common for elements and anti-elements.
| Year: 2010 | PT id = 386, Type = data |
Chemical Elements as a Collection of Images
Using Google Translate (German -> English):
"The periodic table of chemical elements as a collection of images [click to zoom in]. A collection of images of materials constitute the basic components of the whole universe. This is a periodic table of chemical elements (also called short PSE) with a difference! Visible in pure form, as it really looks like. Not only naked dry boring data. There are the alkali metals, alkaline earth metals, boron group, carbon group, nitrogen group, chalcogens, halogens, noble gases, hard metals, ferrous metals, precious metals, lanthanides..." from the website, here:
| Year: 2010 | PT id = 1195, Type = formulation data |
Schwarz & Rich's Periodic Table
W. H. Eugen Schwarz & Ronald L. Rich, Theoretical Basis and Correct Explanation of the Periodic System: Review and Update, J. Chem. Educ. 2010, 87, 4, 435-443. DOI: https://doi.org/10.1021/ed800124m
Periodic table, representing some aspects of the periodic system of chemical elements (mainly to support the discussions in [the attached] article, perhaps not for the classroom):
Note that the richness of chemistry sometimes prevents clear-cut classifications and assignments.
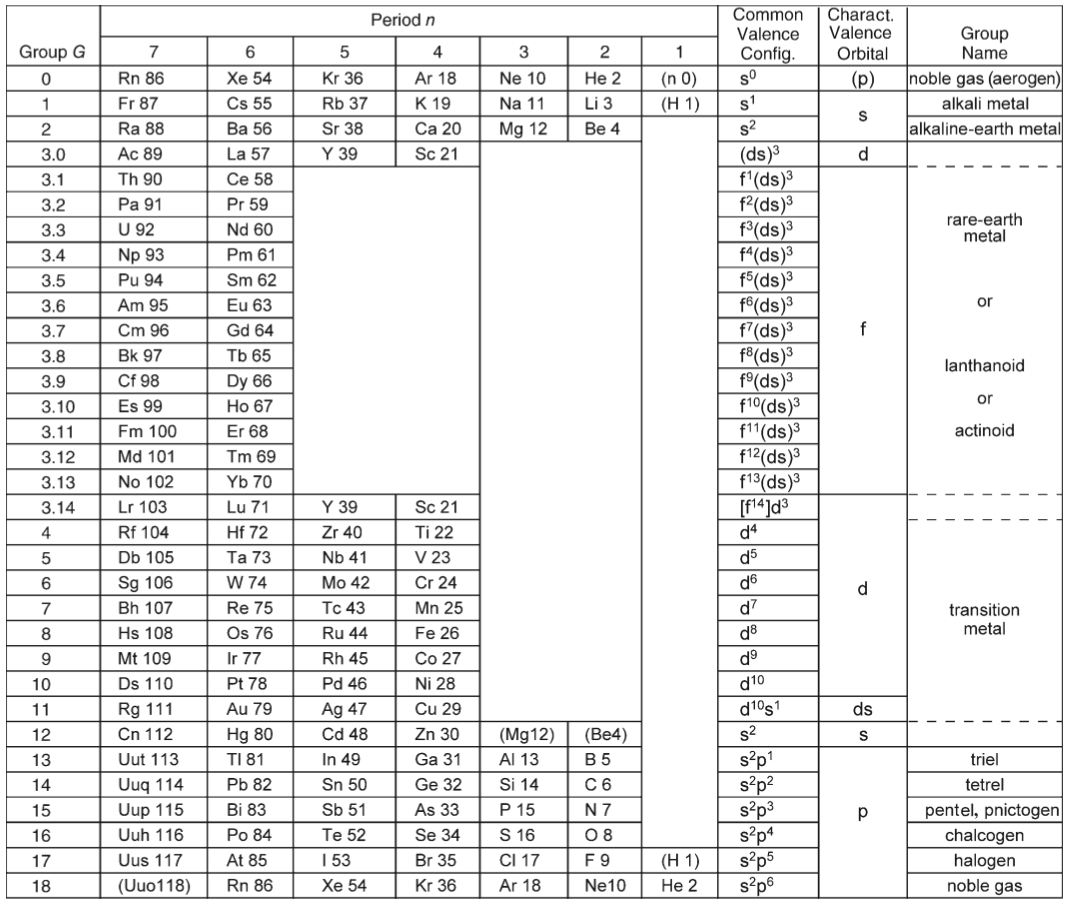
| Year: 2011 | PT id = 775, Type = formulation 3D |
Weise's Tetrahedron
Dmitry Weise shows how it is possible to go from the Janet [left-step] periodic table formulation, to a tetrahedral formulation.
Dmitry writes:
"Three-dimensional table of the periodic law can be constructed in the form of a tetrahedron having an inner order. A comparison of the tetrahedron shells and the table of elements shows, that one tetrahedron shell corresponds to 4 periods of the 2D table."
Jess Tauber adds:
"The spheres here also aren't labeled, but I explain how they get labeled in the text accompanying the pic. Each such period (except for s-only, which are obviously simpler) we have a 'switchback' configuration. Like a road going up a mountain back and forth to minimize verticality, or a parachute folded into a pack. There are 8 different ways to do this (4 basic types in 2 chirally opposite mappings). And the original Weise-style non-continuous tetrahedron is just another way to organize half tetrahedra."
| Year: 2011 | PT id = 1038, Type = formulation |
Tresvyatskii's Periodic Table
Powder Metallurgy and Metal Ceramics, Vol. 49, Nos. 9-10, 2011:
The paper published below represents Tresvyatskii's fundamental study. It establishes the interrelation between the ionization potential and place of an element in the periodic table. Oxides with a certain composition may form only when an element is ionized to the needed degree. Hence, the ionization potential of elements is an important parameter that governs the formation of an oxide. In this regard, the dependence of the ionization potential on the place of an element in the periodic table is of paramount importance. The role of the ionization potential in the hightemperature chemistry of oxide compounds, which underlies modern oxide materials science, is especially significant. The paper is published in Tresvyatskii's original version.
René Vernon adds:
A depiction of the short-form table, showing some clever thinking:
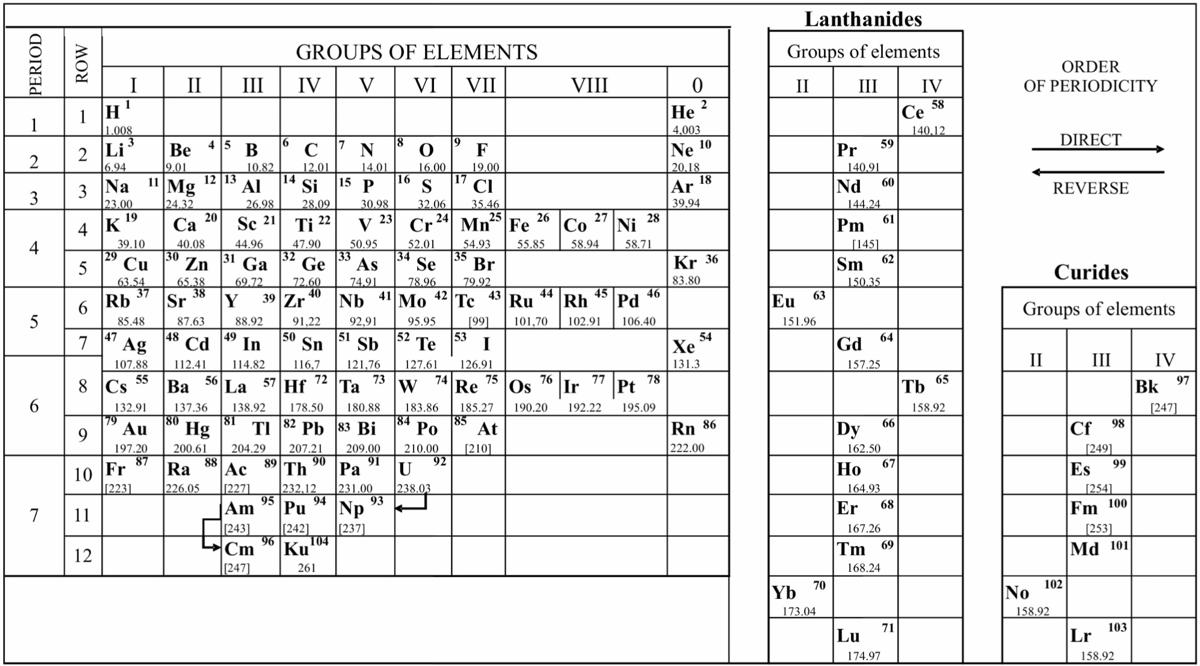
| Year: 2011 | PT id = 623, Type = review |
Scerri's Very Short Introduction To The Periodic Table
A book by Eric Scerri, The Periodic Table: A Very Short Introduction.
| Year: 2011 | PT id = 660, Type = data |
Periodicity Periodic Table
From Wikipedia, a PT showing the main periodic trends:
| Year: 2011 | PT id = 418, Type = formulation 3D |
Pacholek's Multipipe 3D Periodic Table
"I've recently invented a new type of periodic table. My table is 3-dimensional and is similar to the ADOMAH Periodic Table, but it's also very different from the ADOMAH Tetrahedron. Its main advantage is being fully geometric in the plane spanned by n, l and n+l quantum numbers."
Take a look at the Picasa images here and here:

| Year: 2011 | PT id = 422, Type = data |
Chem 13 News Periodic Table Project
The Chem 13 News Periodic Table Project celebrates the International Year of Chemistry in 2011.
This collaborative periodic table is designed by chemistry students from all Canadian provinces and territories, 20 US states and 14 different countries. Chem 13 News readers registered their chemistry students to artistically interpret one element. Combined these tiles form one innovative and unique periodic table. A poster of the table and a traveling display are currently being constructed.
| Year: 2011 | PT id = 425, Type = non-chem |
Nicholas Armstrong's Periodic Table of University Courses
Nicholas Armstrong is a graduate of Computer Engineering at the University of Waterloo, currently pursuing a Master's degree in and at the same. On his blog there is a periodic table showing the courses he took at university. Click here for a large version:
| Year: 2011 | PT id = 428, Type = misc |
The Elements Song by Tom Lehrer Periodic Table
Started by David Bradley of Sciencebase, a selection of songs about the Periodic Table including the classic Tom Lehrer track.
"An unusual periodic table in which each element represents a rendition of the classic Tom Lehrer song, The Elements, which has to be every chemist's favourite song, really. There are also a few ringers, see if you can spot them. But, more to the point there are major gaps...so what's you're favourite Elements rendition? Let me know via Twitter or Facebook. I'd be particularly interested to see personal recordings and renditions done for your own site, lab or special event. You can find the original lyrics here; the tune is that of G&S's "Major General" from The Pirates of Penzance.":
| Year: 2011 | PT id = 448, Type = misc |
BASF Periodic Table
A BASF advert showing a periodic table of school children:
| Year: 2011 | PT id = 455, Type = non-chem |
Uptime Elements
From reliabilityweb.com a periodic table graphic showing Uptime Elements:
| Year: 2012 | PT id = 524, Type = formulation |
Compact Mendeleev-Moseley-Seaborg Periodic Table (CMMSPT)
A Compact Mendeleev-Moseley-Seaborg Periodic Table (CMMSPT).
This table can be found by two different ways:
These 2 transformations lead to the same table, with 7 rows and 32 columns. Blocks p (green), d (light grey), and f (light orange) are preserved.
The 14 terms of the s block (dark orange/red) are splited in "cascads".
This table can be seen in the A173592 sequence in the On-line Encyclopedia of Integer Sequences (OEIS). Row differences are 8, 8, 18, 18, 32, 32.
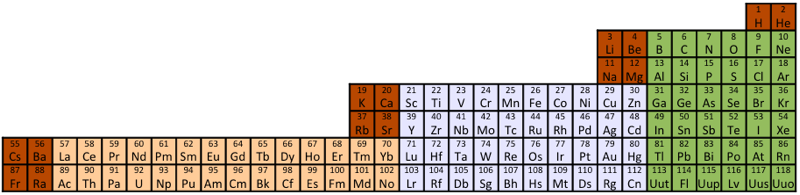
| Year: 2012 | PT id = 529, Type = formulation |
Srivaths–Labarca Periodic Table
This is an improved version of the Zigzag Periodic Table (2012). In this new arrangement the main criteria proposed to settle the placement of the elements hydrogen and helium has been taken into account: electronic configurations, the number of electrons needed to fill the outer-shell, chemical behavior, and triads of atomic number.
This is a new categorial criterion recently proposed by Eric Scerri, according to which hydrogen and helium form part of the triads H(1), F(9), Cl(17) and He(2), Ne(10), Ar(18), respectively. Thus, hydrogen preserves its place between alkali metals and halogen while helium is now in between noble gases and alkaline earth elements.
This periodic table allows visualizing easily the relationships of hydrogen and of helium with the different criteria, avoiding drawing lines to see them in contrast to other similar periodic systems.
Akash Srivaths, Chennai, India
Martín Labarca, CONICET & National University of Quilmes, Argentina
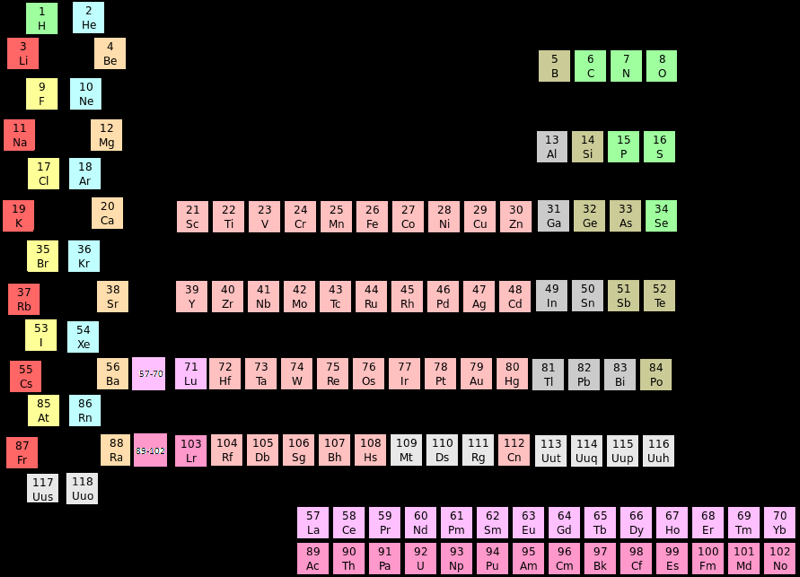
| Year: 2012 | PT id = 532, Type = formulation spiral 3D |
Alexander Arrangement of Elements, 3D Illustrated
The design of the 2012 Alexander Arrangement of Elements (AAE) follows the principles of a three-dimensional model developed by Roy Alexander in 1965: a printed representation of element information based on strict adherence to the Periodic Law, with every element data box physically and visually contiguous and continuous within the sequence of atomic numbers in generally accepted element property related columns - "...the periodic table the way it's supposed to be".
This is made possible by wrapping, folding, and joining the printed material and employing the patented p-block downslant of the element data boxes to allow the end element of a period to be adjacent to the first element of the next period.

Several unique features separate it from the previous four versions of the AAE



Designed by Roy Alexander, a science museum exhibit and teaching aid designer, the Adobe Illustrator art for the model was started by Ann Grafelman, and continued by Roy from mid 2011 through November of 2012.
Photos were provided by Theodore Gray, and Element Collection funded the printing and die cutting performed by Strine Printing in York, Pennsylvania. The model kit was first offered at Theo's PeriodicTable.com, then at Roy's AllPeriodicTables.com and the new 3dPeriodicTable.com, which site is dedicated to the 3D Forever Periodic Table only, with add-ons, application suggestions, and descriptions and commentary of all sorts.
Assembly instructions and step photos, as well as a number or completed model color photographs are included with the kit. These were developed with prototype models, and while functional, have been upgraded and accompanied by an assembly video at AlexanderArrangementOfElements.com/3D
Addendum:
Text relating to the abbreviation of the ever increasing number of elements is explained at two places on the 3D AAE illustrated periodic table model kit. One will remain with the model and one is removed at the time of assembly.
That which remains runs under the Actinoids and the d-block elements, where the lab created elements might ordinarily be expected to be found, says:
The lab created elements ordinarily found in this part of a periodic table are not to be found in nature, there can be no photographs of them, so nothing needs to be added to this element photo periodic table - ever - so it will never be obsolete, a Forever Periodic Table.
That which is removed says:
Naturally-occurring elements have been numbered variously, generally between 80 and 96, all for cogent scientific reasons.
For easier teaching and learning, we have included on this periodic table only the 92 elements actually currently existing on Earth and in the remainder of the Universe, and adding Technetium and Promethium, which, although they may have no stable forms, serve to fill what would otherwise be gaps in the sequence.
Not added for practical and educational reasons are 'elements' consisting only of pages and pages of computer data from smashing atoms in particle accelerators. Another reason is that there can be no photographs of them to show, and as a result, your arrangement is complete and never be obsolete - your Forever Periodic Table.
Included with the art of the periodic table on the die cut substrate that makes up the model is some background information about the the history of three dimensional periodic tables.
The first of these is about the discoverer of the concept of arranging the elements in periods suggested by the properties of the elements, de Chancourtois.

The second 3D periodic table information piece (on the rear of the de Chancourtois removable card) are sketches of a number of the 3D periodic tables found on the Chemogenesis website.
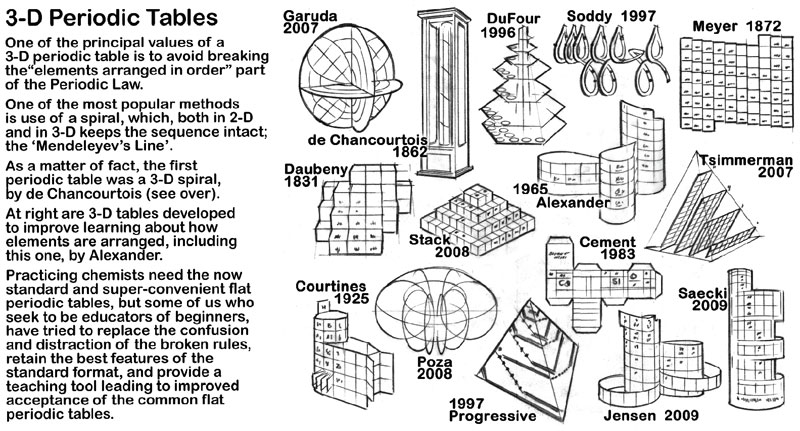
| Year: 2012 | PT id = 536, Type = formulation |
Makeyev's Verticle Form Periodic Table
A new version of the periodic table of elements on the vertical table form. Alexander K. Makeyev, a member of the Moscow Society of Naturalists, section of planetonautics; freelance interdisciplinary researcher and inventor, knowall@list.ru.
1. Makeyev A.K. Normal and pathological anatomy and physiology of the human person and society. Fundamental knowledge about the qualities of the human person, human society and the software company, produces and acts of people, based on the universal algorithm of holographic structure and function at all levels and forms of matter. / / Scientific and Technical Library. July 25, 2012. 364 p, here
2. Makeyev A.K. Particles of electrostatic and magnetic fields in the system of matter photons move faster than a photon moves himself. / / The scientific debate: Proceedings IV International Correspondence scientific conference. Part I. (20 August 2012) - Moscow:. "International Centre for Science and Education", 2012. 142., S. 47-65. ISBN 978-5-905945-37-3 UDC 08. BBK 94. H 34, here:
| Year: 2012 | PT id = 538, Type = formulation spiral |
Wheelshaped Table of Elements
From Facebook, a Wheelshaped table of elements.
Please note the symmetry of this representation.
As a result, it is possible that element 118 is the very last one in the periodic table. We have the sequence:
2 x 14 (blue)
4 x 10 (brown)
6 x 6 (violet)
8 x 2 (green)
and, logically, neither first nor last factor can be 0 or -2 (they differ in two columns above respectively by 2 and 4).
On the other hand, the coherence of the structure requires the existence of two additional elements at the beginning!
| Year: 2012 | PT id = 133, Type = data |
Dates of Discovery of the Elements
The Elements and their dates of discovery, taken from this Wikipedia page:
Two charts showing the dates of discovery of the elements, one from the 'time of the ancients' (10,000 BC) to the present day, and the second from 1700 to the present day.
These show that there were two distinct phases for the discovery of the 118 known elements:
Data from: this Wikipedia page.
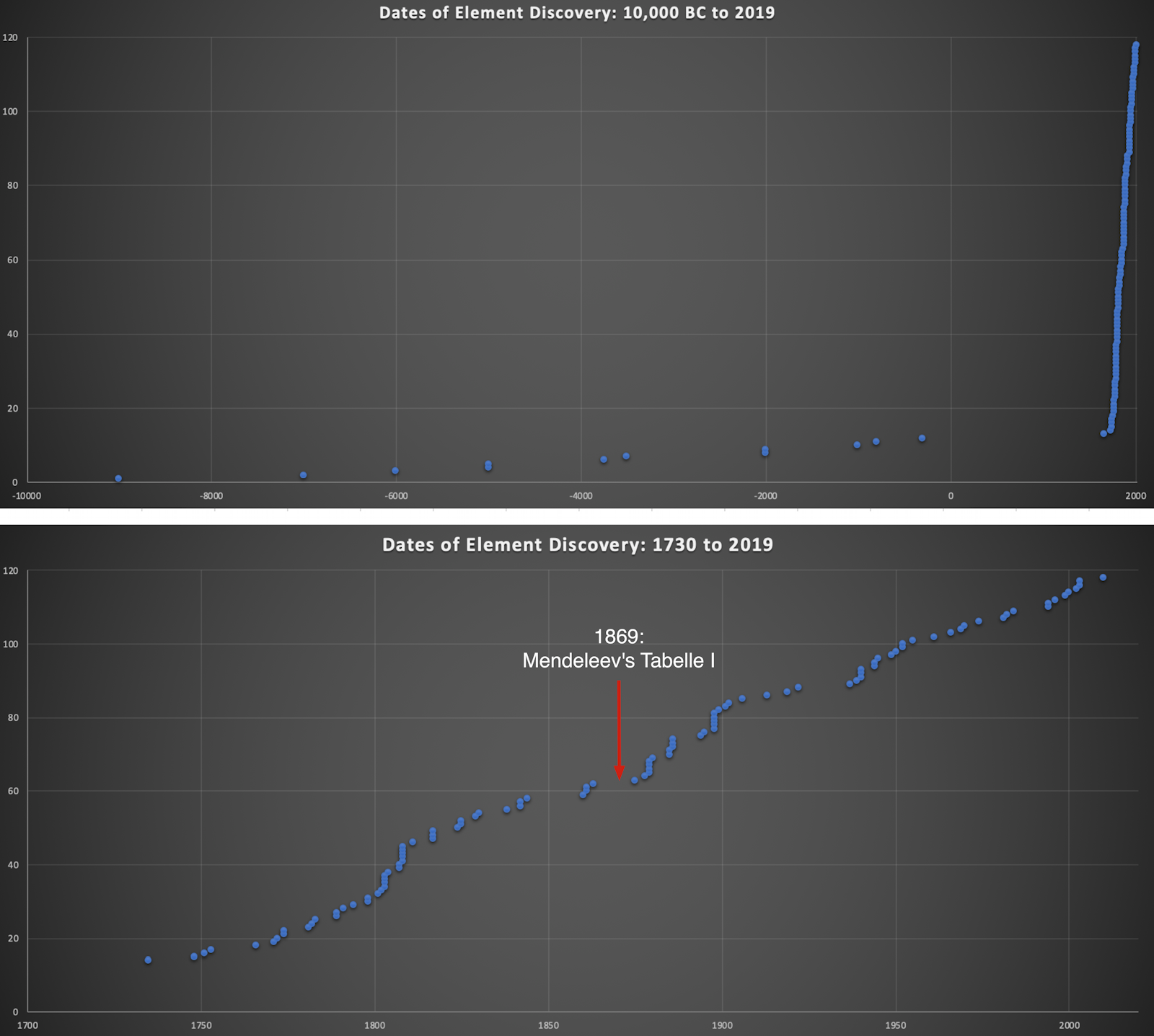
| Discovery of Copper | -9000 |
| Discovery of Lead | -7000 |
| Discovery of Gold | -6000 |
| Discovery of Iron | -5000 |
| Discovery of Silver | -5000 |
| Discovery of Carbon | -3750 |
| Discovery of Tin | -3500 |
| Discovery of Sulfur (Sulphur) | -2000 |
| Discovery of Mercury | -2000 |
| Discovery of Zinc | -1000 |
| Discovery of Antimony | -800 |
| Discovery of Arsenic | -300 |
| Discovery of Phosphorus | 1669 |
| Discovery of Cobalt | 1735 |
| Discovery of Platinum | 1748 |
| Discovery of Nickel | 1751 |
| Discovery of Bismuth | 1753 |
| Discovery of Hydrogen | 1766 |
| Discovery of Oxygen | 1771 |
| Discovery of Nitrogen | 1772 |
| Discovery of Chlorine | 1774 |
| Discovery of Manganese | 1774 |
| Discovery of Molybdenum | 1781 |
| Discovery of Tellurium | 1782 |
| Discovery of Tungsten | 1783 |
| Discovery of Zirconium | 1789 |
| Discovery of Uranium | 1789 |
| Discovery of Titanium | 1791 |
| Discovery of Yttrium | 1794 |
| Discovery of Beryllium | 1798 |
| Discovery of Chromium | 1798 |
| Discovery of Niobium | 1801 |
| Discovery of Tantalum | 1802 |
| Discovery of Palladium | 1803 |
| Discovery of Cerium | 1803 |
| Discovery of Osmium | 1803 |
| Discovery of Iridium | 1803 |
| Discovery of Rhodium | 1804 |
| Discovery of Sodium | 1807 |
| Discovery of Potassium | 1807 |
| Discovery of Boron | 1808 |
| Discovery of Magnesium | 1808 |
| Discovery of Calcium | 1808 |
| Discovery of Strontium | 1808 |
| Discovery of Barium | 1808 |
| Discovery of Iodine | 1811 |
| Discovery of Lithium | 1817 |
| Discovery of Selenium | 1817 |
| Discovery of Cadmium | 1817 |
| Discovery of Silicon | 1824 |
| Discovery of Aluminium (Aluminum) | 1825 |
| Discovery of Bromine | 1825 |
| Discovery of Thorium | 1829 |
| Discovery of Vanadium | 1830 |
| Discovery of Lanthanum | 1838 |
| Discovery of Terbium | 1842 |
| Discovery of Erbium | 1842 |
| Discovery of Ruthenium | 1844 |
| Discovery of Cesium | 1860 |
| Discovery of Rubidium | 1861 |
| Discovery of Thallium | 1861 |
| Discovery of Indium | 1863 |
| Discovery of Gallium | 1875 |
| Discovery of Ytterbium | 1878 |
| Discovery of Scandium | 1879 |
| Discovery of Samarium | 1879 |
| Discovery of Holmium | 1879 |
| Discovery of Thulium | 1879 |
| Discovery of Gadolinium | 1880 |
| Discovery of Praseodymium | 1885 |
| Discovery of Neodymium | 1885 |
| Discovery of Fluorine | 1886 |
| Discovery of Germanium | 1886 |
| Discovery of Dysprosium | 1886 |
| Discovery of Argon | 1894 |
| Discovery of Helium | 1895 |
| Discovery of Neon | 1898 |
| Discovery of Krypton | 1898 |
| Discovery of Xenon | 1898 |
| Discovery of Polonium | 1898 |
| Discovery of Radium | 1898 |
| Discovery of Radon | 1899 |
| Discovery of Europium | 1901 |
| Discovery of Actinium | 1902 |
| Discovery of Lutetium | 1906 |
| Discovery of Protactinium | 1913 |
| Discovery of Rhenium | 1919 |
| Discovery of Hafnium | 1922 |
| Discovery of Technetium | 1937 |
| Discovery of Francium | 1939 |
| Discovery of Astatine | 1940 |
| Discovery of Neptunium | 1940 |
| Discovery of Plutonium | 1940 |
| Discovery of Americium | 1944 |
| Discovery of Curium | 1944 |
| Discovery of Promethium | 1945 |
| Discovery of Berkelium | 1949 |
| Discovery of Californium | 1950 |
| Discovery of Einsteinium | 1952 |
| Discovery of Fermium | 1952 |
| Discovery of Mendelevium | 1955 |
| Discovery of Lawrencium | 1961 |
| Discovery of Nobelium | 1966 |
| Discovery of Rutherfordium | 1969 |
| Discovery of Dubnium | 1970 |
| Discovery of Seaborgium | 1974 |
| Discovery of Bohrium | 1981 |
| Discovery of Meitnerium | 1982 |
| Discovery of Hassium | 1984 |
| Discovery of Darmstadtium | 1994 |
| Discovery of Roentgenium | 1994 |
| Discovery of Copernicium | 1996 |
| Discovery of Flerovium | 1999 |
| Discovery of Livermorium | 2000 |
| Discovery of Oganesson | 2002 |
| Discovery of Nihonium | 2003 |
| Discovery of Moscovium | 2003 |
| Discovery of Tennessine | 2010 |
By Mark Leach
A nice graphic from Compound Interest: (click image to enlarge)
| Year: 2012 | PT id = 1163, Type = misc |
Itch: A Book & TV Drama About a Boy Who Collects The Chemical Elements
Meet Itch – an accidental, accident-prone hero. Science is his weapon. Elements are his gadgets.
All-action adventure perfect for fans of Alex Rider and Young Bond.
Itchingham Lofte – known as Itch – is fourteen, and loves science, especially chemistry. He's also an element-hunter: he's collecting all the elements in the periodic table. Which has some interesting and rather destructive results in his bedroom.
Then, Itch makes a discovery. A new element, never seen before. At first no one believes him – but soon someone hears about the strange new rock and wants it for himself. And Itch and his family are catapulted into a breathless adventure with terrifyingly high stakes...
The debut novel from BBC radio presenter Simon Mayo.
Children's book Itch by Simon Mayo is available from Amazon and all good bookshops, ISBN: 9780552565509. The book has been made into a TV Series, filmed in Western Australia, and is available on the BBC iPlayer.

| Year: 2012 | PT id = 483, Type = formulation |
Zigzag Periodic Table
In this periodic table we can see that the elements are arranged in a different way. Hydrogen is placed in between (and above) fluorine and lithium. This is because there is an issue on the placement of hydrogen as it has the properties of both alkali metals and halogens.
How to read the Zigzag periodic table
For periods (1), (2B), (3B) etc. read from right to left.
For periods (2A), (3A), (4A) etc. read from left to rightThe arrows will guide you through the periodic table:
By Akash Srivaths, High School Student, Chennai, India
| Year: 2012 | PT id = 493, Type = formulation data misc |
JR's Chemistry Set
For the iPhone and iPad, JR's Chemistry Set makes chemistry interesting and fun to learn. Based upon the innovative Rota Period, it is a handy and powerful reference tool for chemistry enthusiasts and practitioners at all ages and all levels.
| Year: 2012 | PT id = 501, Type = review |
Books on the Chemical Elements and the Periodic Table/System
From Eric Scerri's forthcoming book A Tale of Seven Elements (Oxford University Press, 2013) and used by permission of the author, is the most complete and up-to-date list of Books on the Chemical Elements and the Periodic Table/System, including some titles in foreign languages.
Additional books in other languages can be found listed in Mazurs, 1974
Works by D. I. Mendeleev
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2012 | PT id = 504, Type = formulation |
Three Different Long-Form, or 32-Column, Periodic Tables
From an article by Eric Scerri in the IUPAC magazine, Chemistry International, in which three different long-form, or 32-column, periodic tables with differences highlighted.
| Year: 2013 | PT id = 570, Type = non-chem |
London, Periodic Table of
Like the Tube Map, the Periodic Table is an endlessly fascinating thing. Over the years, the format has been adapted to all kinds of schemes. A few years ago, we tried to make sense of London in this way, by arranging important facets of the capital into rows and columns. It's been a while, so we've now updated it, with a few changed entries and a general tidy up. Can you work out the identity of each London 'element'? Can you spot hidden patterns and trends? Can you suggest improvements?
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2013 | PT id = 598, Type = formulation 3D spiral |
Bernard Periodic Spiral
The Bernard Periodic Spiral of the Elements (BPSE), depicts a novel rendition of the Periodic Table that replaces the flat rectangular format with a continuous unidirectional spiral that maintains all the properties of Group and Period formation.
Comparisons may be made with similar models spanning the last three decades of the 20th century (Alexander, 1971; Mazurs, 1974; & Kaufman, 1999).
In the chart form, this new rendition is referred to as the Elliptical Periodic Chart of the Elements. In the three-dimensional form, the model resembles a Christmas tree in shape with the 7 Periods represented as circular platforms situated at various levels with the elements placed appropriately at the outer edges of each of these platforms as a Period builds up. The elements may be represented as spherical objects or flat discs with radii proportionate to atomic radii (or reasonable approximations). Color schemes accentuate the four different Blocks of elements: the s-Block (green), the p-Block (blue, with the exception that the last Group is red signifying the end of a Period), d-Block (orange), and the f-Block (yellow). The grey section, called the Group-Period Interchange, is where the end of a particular Period connects to the beginning of the next Period, and, at the same time, transitions from Group 18 to Group 1.
Watch the video here:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2013 | PT id = 610, Type = review |
Top 10 Periodic Tables
There are more than 1000 periodic tables hosted by the Chemogenesis Webbook Periodic Table database, so it can be a little difficult to find the exceptional ones.
Here we present – in our humble opinion – The ten most significant periodic tables in the database.
We present the best:
Three Excellent, Data Rich Periodic Tables
The first three of our top 10 periodic tables are classic element data repositories.
They all work in the same way: click on the element symbol to get data/information about the selected element. The three are Mark Winter's WebElements, Theo Gray's Photographic Periodic Table & Michael Dayah's Ptable.
Five Formulations Showing The History & Development
The next five examples deal with history and development Periodic Table. The first is Dalton's 1808 list of elements, next is Mendeleev's 1869 Tabelle I, then Werner's remarkably modern looking 1905 formulation. This is followed by Janet's Left Step formulation and then a discussion of how and why the commonly used medium form PT formulation, is constructed.



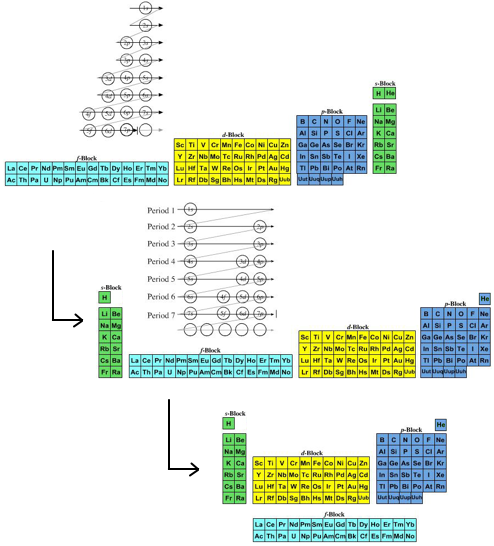
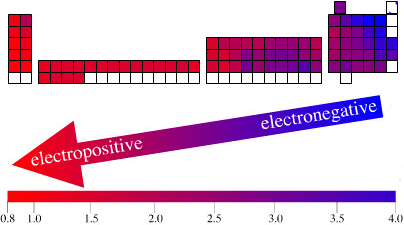
An Alternative Formulation
The internet database contains many, many alternative formulations, and these are often spiral and/or three dimensional. These exemplified by the 1965 Alexander DeskTopper Arrangement. To see the variety of formulations available, check out the Spiral & Helical and 3-Dimensional formulations in the database:

Non-Chemistry PTs
The periodic table as a motif is a useful and commonly used infographic template for arranging many types of object with, from 50 to 150 members.
There are numerous examples in the Non-Chemistry section where dozens of completely random representations can be found:
| Year: 2013 | PT id = 620, Type = formulation 3D |
Model Wooden Periodic Table
From here, and translated from Spanish:
Among the events commemorating the 75th anniversary of the creation of the School of Treball, the author of this site, B. Navarro, along with J. Semis and J. Gràcia have built a model wooden periodic table.
The table has been divided into 5 areas: representative elements, noble gases, transition elements, rare earths and finally the groups I and II of alkali and alkaline earth together. Each of these areas of the table is made with a different type of wood. The block transition elements is made with oak, ash noble gases, representative elements in cherry, sapele the rare earth and alkali/alcalinoterros beechwood.
The central idea of the model is that each element is represented by a cube of 3 cm edge so that you can see on all sides, from left to right or right to left without losing the order of increasing atomic number or the relative position of the elements:
| Year: 2013 | PT id = 629, Type = formulation 3D |
Atomic Periodic Town
Three related formulations by Baha Tangour (Tangour Bahoueddine), the Atomic Town and two Boomerang periodic tables.
Baha says: "The propositions are different representation of a 3D dimensions that depend on three properties (spectral term multiplicity, lone-pairs and period number)":
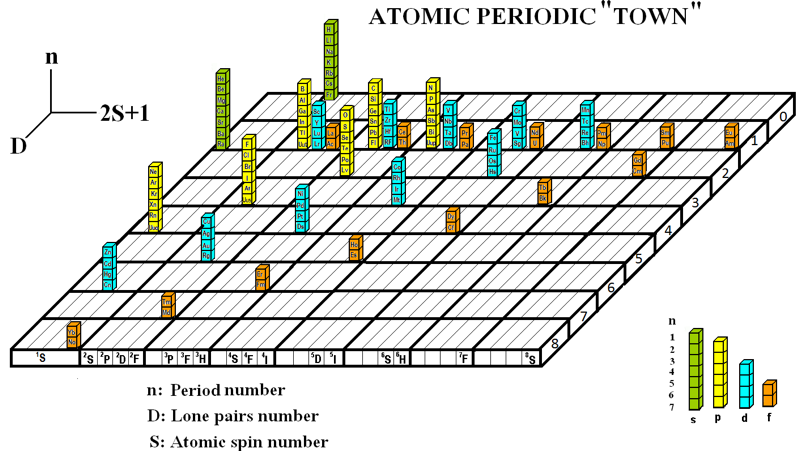
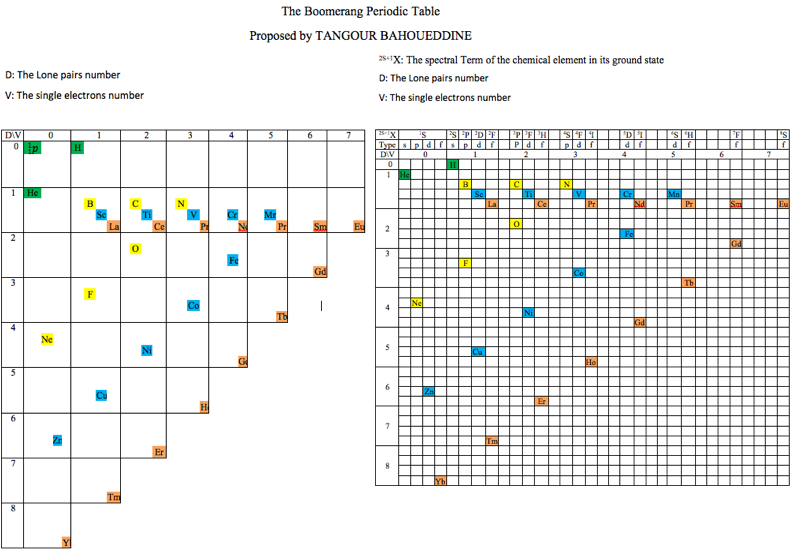
| Year: 2014 | PT id = 642, Type = formulation 3D |
ADOMAH Periodic Table Glass Cube
Valery Tsimmerman, of the ADOMAH Periodic Table and the ADOMAH Tetrahedron, has now used these ideas to produce a beautiful glass cube:

This amazing object is available for sale from Grand Illusions:
A Note by Philip Stewart stewart.phi@gmail.com
The cube represents 120 chemical elements etched into a cube of Optical Crystal glass. The s, p, d, and f blocks of the Janet periodic table form four rectangles, which are slices of a regular tetrahedron, parallel with two of its edges and with two faces of the circumscribed cube. All four quantum numbers are made visible in this arrangement. You can see a 2-D version on the Perfect Periodic Table website, click on the "skyscraper" version on the right to see the tetrahedron, and go to Regular Tetrahedron at foot of page for details.
The regular tetrahedron is the only form in which slices are rectangles of different shape and identical perimeter. When each orbital is represented by a square of unit edge, the rectangles representing the blocks all have the same perimeter, which is twice the length of the edges of the tetrahedron (which are of course √2 times the edges of the cube): 18 units = 2(values of n + values of ml).
Block |
values of n |
values of ml |
s |
8 |
1 |
p |
6 |
3 |
d |
4 |
5 |
f |
2 |
7 |
Valery Tsimmerman, orahct@gmail.com, creator of the design, has written to me as follows:
"I just had some thoughts about the Perimeter Rule that is at the basis of the tetrahedral arrangement. Dimensions of the blocks are dictated by number of values of ml and number of values of n. We know that n governs quantization of energy. Recently I learned that quantization of the possible orientations of L with respect to an external magnetic field is often referred to as space quantization. (Serway, Jewett: Physics for Scientists and Engineers. 6th edition. p.1369).
"That is, ml stands for space quantization. Therefore, the Perimeter Rule reflects a direct relationship between energy and space. I think that this could have some significance. The beautiful thing about the Universe is that each type of symmetry is related to some conservation law. Symmetry in time is related to energy. Therefore, n is related to time also, so, in the Perimeter Rule we have relationship between time and space on quantum numerical level. The interesting thing is that ml can be positive and negative, while n can only be positive. Similarly, things can move in space in positive and negative directions, but time has only one direction. There is no negative time, just as there are no negative values of quantum number n."
Adomah is a variant of Adamah, Hebrew for 'dust of the earth', from which Adam was made (Genesis 2:7).
| Year: 2014 | PT id = 654, Type = data |
IQS Periodic Tables
By Jordi Cuadros, a set of three pairs of periodic tables in Catalan, English & Spanish pointing out the differences between PT representations of atoms and PT representations of the material substances:
| Year: 2014 | PT id = 661, Type = formulation spiral |
Chandra's Polar Plot Periodic Table
MONOGRAPH ON ATOMS, BY Dr. N. Naveen Chandra, 543 Bellamy Road North Scarborough, On, M1H1G5, 416 439 6630, chandraalex@hotmail.com >© N.Naveen Chandra, 2014.
Abstract
A new way of graphical representation of atoms is developed and presented here. Atoms are recognized as functions of two variables A(r,Θ), where r =2,10,18,36,54,86,118 (given arbitrarily r=1,2,3,4,5,6,7) represents period and Θ representing group, is actually the angle between the groups. A mathematical solution is obtained for Θ having three distinct values of (π /9) radians, (π/18) radians and (π/27) radians which define three super groups satisfying the equation 15(π/27) +10(π/18) +8 (π /9) =2π. 15 groups of two Atoms with a transition zone of (π/27) radians is nominally called Grey Super Group (GSG). 10 groups of which 9 have four Atoms and 1 has two Atoms, also including a transition zone of (π/18) radians, is nominally called Blue Super Group (BSG). 8 groups of which 7 have 6 Atoms and one has 7 Atoms, including a transition zone of (π/9) radians is called Yellow Super Group (YSG). The group with 7 atoms is the so called reference group of Atoms 2, 10, 18,36,54,86 and 118. The GSG has 30 Atoms, the BSG has 38 Atoms and the YSG has 49 Atoms. The Atom 1 is at the centre of the Hub and does not belong to any group or period and has coordinates of (0, 0). Atom 1 having no neutrons is unique.
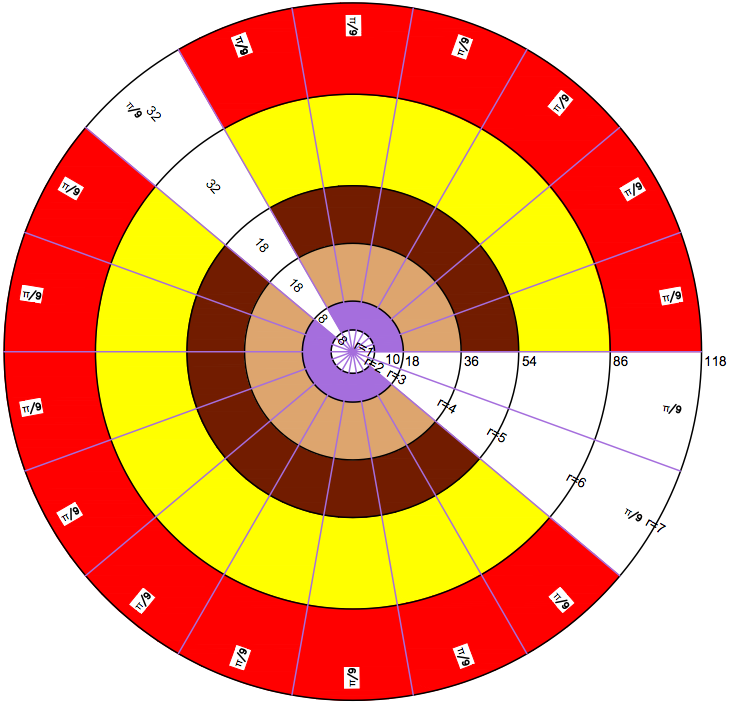
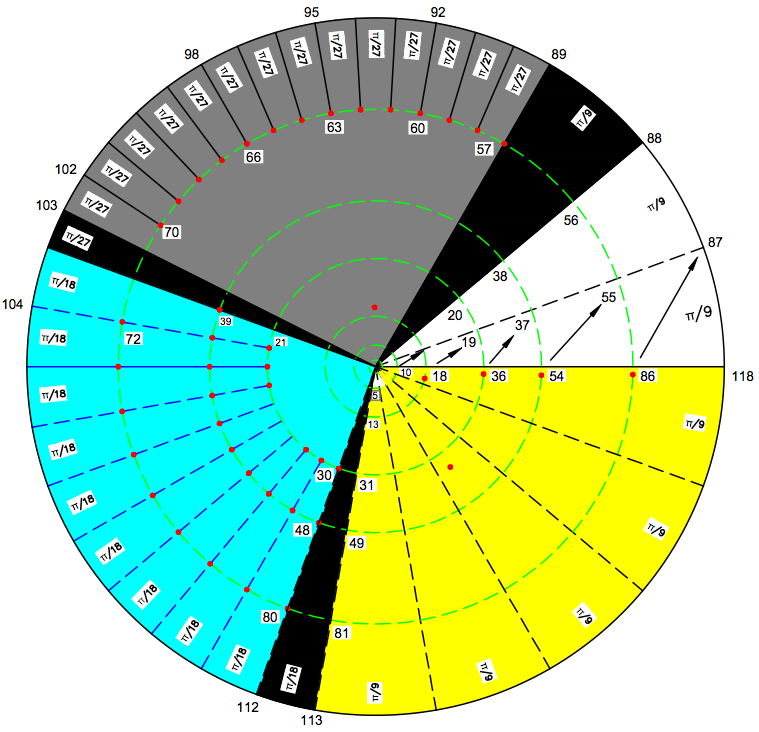
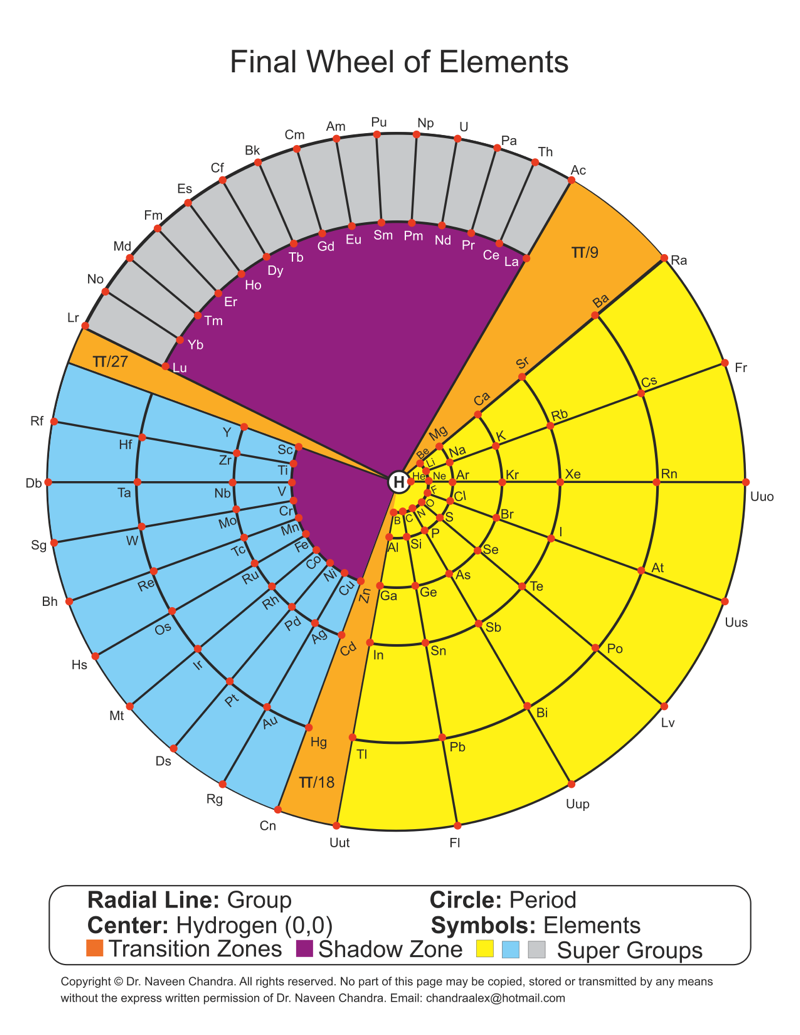
| Year: 2014 | PT id = 662, Type = formulation spiral |
Metallic Character Table
"I would like to submit you an hexagonal periodic table. It's structured in different rings. The elements are ordered on their metallic characters so in the inner rings there are noble gases and nonmetals while in the outer rings there are alkali and alkaline earth metals. I based the order on the typical metallic characteristics: low ionization energy, electron affinity, etc... "
Marco Piazzalunga <marco.piazzalunga@live.com>

| Year: 2014 | PT id = 680, Type = formulation |
Belikov's Modular Periodic Table of Chemical Elements
"I call this version of the Modular Periodic Table of Chemical Elements. I got the idea for it some time between 2005 and 2007, during the chemistry course at my university, in attempt to rationalize the clumsy common version I was being taught. I showed it to my chemistry teacher, but he didn't seem to be impressed much, so it went into the drawer. Recently I decided to resurrect it and publish somewhere. So I had a look on the web and found your excellent database, with hundreds of versions. After the first shock, I realized that only few are actually similar to my version. These are well known Janet's table and ADOMAH table. So, it appeared to me that the idea to group elements strictly according to filling of their atomic shells is not new. However, the way I have done it is slightly different from the mentioned tables. For example, s,p,d and f blocks of elements are completely autonomous and can be placed wherever desired (hence the name 'Modular'). This reflects the notion that there is little in common in chemical behavior between the elements in different blocks. Also, outer subshell type, energy level and electron count are clearly labeled, so that these parameters can be quickly determined for each element.
"Overall, I think that this version of periodic table allows easier understanding and transition from IUPAC table and could be implemented in school and university textbooks."
Aleksey Belikov
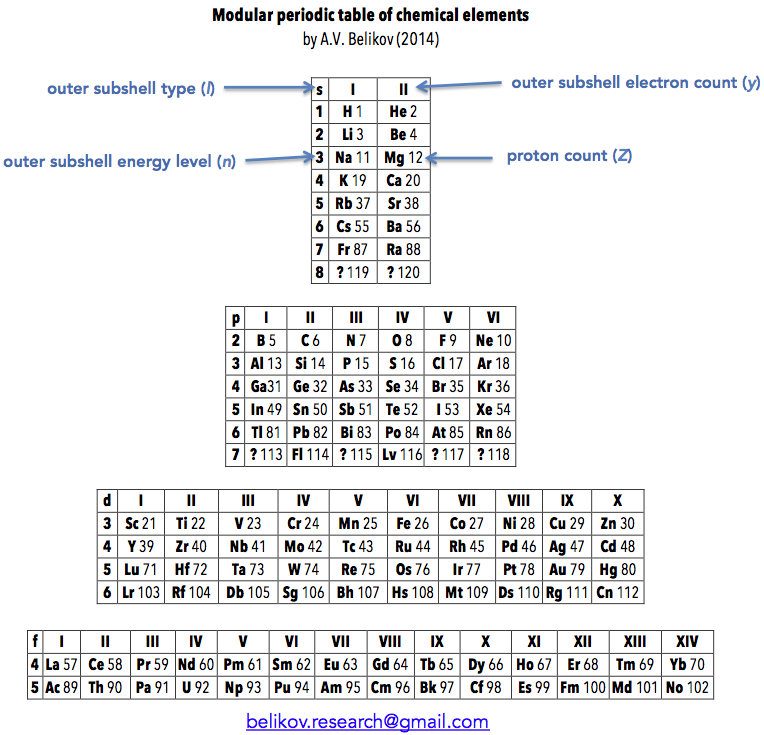
| Year: 2014 | PT id = 697, Type = formulation |
Aco Muradjan's New Notation Scheme
Aco writes:
On 08.11.2014 I added an article to the General Science Journal:
"Necessity of urgent revising and changing the present IUPAC notation scheme in the Periodic Table"
The current and present modern notation scheme for the groups in the Periodic Table exist from 1985, proposed from the IUPAC Commission on the Nomenclature of Inorganic Chemistry, as recommendation. This proposal was also verified in 2005.
Because the IUPAC Commission encourages further discussions, improvements and proposals on this subject I made this new article which article investigates the possibilities for the new notation scheme in the Periodic Table. Links:
This article has picture of the Periodic Table with new notation scheme:
| Year: 2014 | PT id = 705, Type = formulation 3d helix |
Arrangement of Elements 7th Order & Element Sequences
An exploration of some mathematics underlying the periodic table, read the PDF here, by Olivier Joseph.
Oliver says:
"May I propose you the following pattern, as the result of a personal study concerning the arrangement of the Elements, including sequences. Based on some hypothesis and as depicted in the enclosed illustrations, the elements are positioned according to a spiral function of atomic number and atomic mass, representation in 2D in a spiral form pattern, or in 3D conical helix model.
"The elements are numbered and placed consecutively along this spiral according to a specific angle, appropriately established between each element, forming a seven arm spiral pattern. With such an angle, specifically defined, a link is established between the various elements of a same group (corresponding to chemical elements with similar properties) and different layers. These latter becoming distributed among each arm of the spiral in a notable arranged way."
| Year: 2014 | PT id = 723, Type = formulation data |
Schaeffer's IUPAC Periodic Table Quantum Mechanics Consistent
IUPAC Periodic Table Quantum Mechanics Consistent, Bernard Schaeffer, Journal of Modern Physics, Vol. 5, No. 3, February 24, 2014
DOI: 10.4236/jmp.2014.53020
Abstract: Most periodic tables of the chemical elements are between 96% and 100% in accord with quantum mechanics. Three elements only do not fit correctly into the official tables, in disagreement with the spherical harmonics and the Pauli exclusion principle. Helium, belonging to the s-block, should be placed beside hydrogen in the s-block instead of the p-block. Lutetium and lawrencium belonging to the d-block of the transition metals should not be in the f-block of the lanthanides or the actinoids. With these slight modifications, the IUPAC table becomes quantum mechanics consistent.
| Year: 2014 | PT id = 728, Type = data |
URENCO Periodic Table
A periodic table by URENCO showing which non-radioactive (stable) elements are suitable for isotopic enrichment using gas centrifuge technology:
| Year: 2015 | PT id = 674, Type = formulation 3D |
UVS Periodic Table Model of a Klein Bottle Topology
This configuration can topologically suggest the g-block cycle in the 8th period for extended periodic table.
In the Klein bottle topology as illustrated, it is plausible that after the s-block cycle in the 8th periodical cycle, the topological path continues to spiral around the outer f-block cycle to harmonically form 14 elements.
And then subjected to the spiral Möbius strip topological twist, it could resonate to form 4 more elements in the anti-cyclonic path around 17th, 18th, 1st, and 2nd angular phases of the anti-cyclonic core; this would render the 18 elemental positions for the hypothetical g-block cycle in the entire half-integral anti-cyclonic cycle of the Klein bottle topology.
Hypothetically, the topological path then moves into the cyclonic cycle, and harmonically forms its d-block and p-block cycles with 16 elemental positions to complete the 8th periodical cycle with a total of 36 elements.
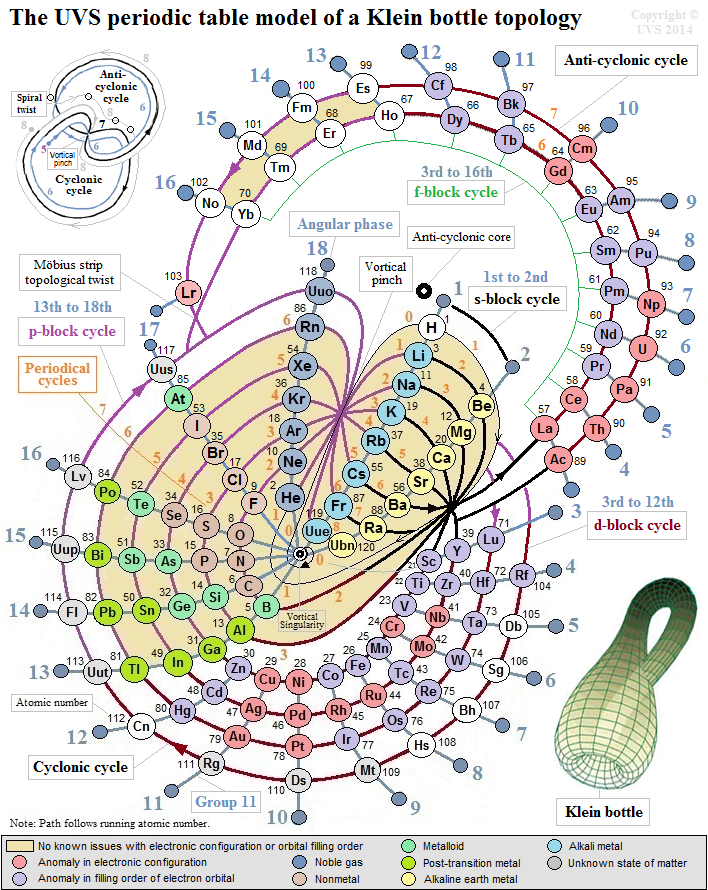
| Year: 2015 | PT id = 706, Type = data |
STEM Sheets Printable (& Customizable) Periodic Table of Elements
From STEM Sheets – where "STEM" stands for Science, Technology Engineering & Maths – a customizable and printable periodic table.
Printable Features
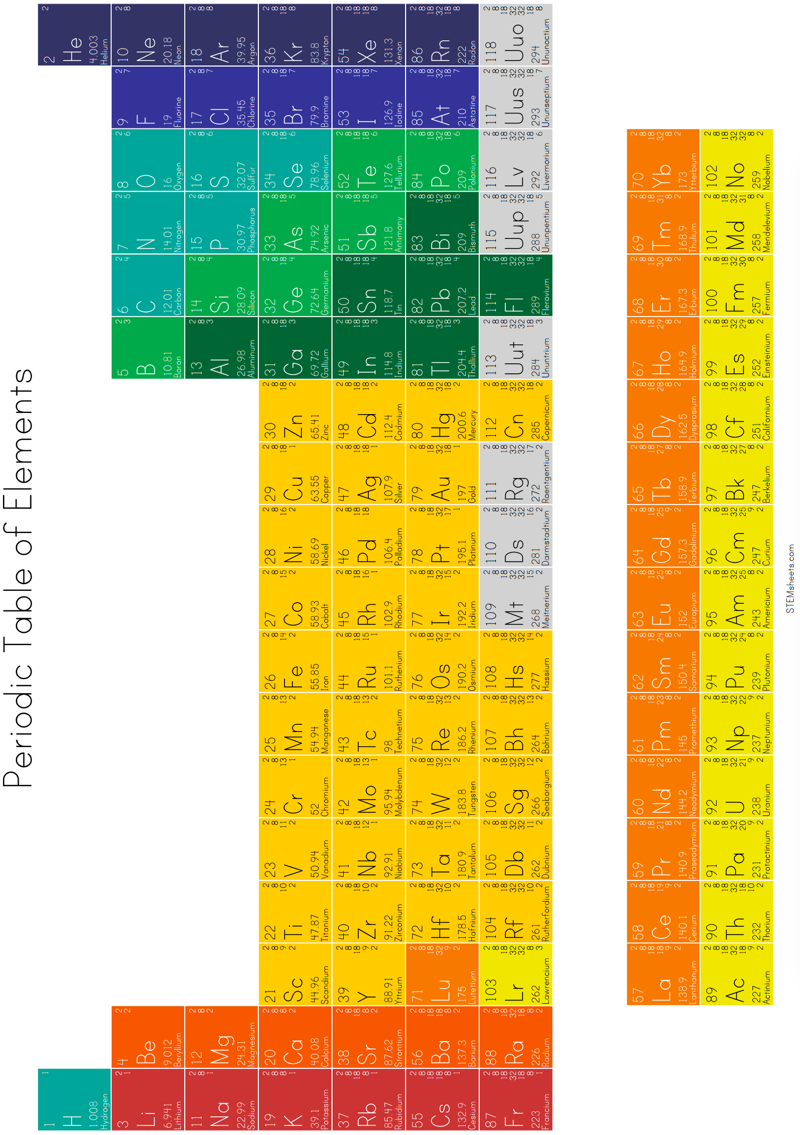
| Year: 2015 | PT id = 709, Type = review |
Mystery of Matter: Search for the Elements
The Mystery of Matter: Search for the Elements is a multimedia project about one of the great adventures in the history of science: the long (and continuing) quest to understand what the world is made of – to identify, understand and organize the basic building blocks of matter. In a nutshell, the project is about the human story behind the Periodic Table of the Elements.
The centerpiece of the project is a three-hour series that premieres Aug. 19, 2015 on PBS. The Mystery of Matter introduces viewers to some of history's most extraordinary scientists:
The Mystery of Matter will show not only what these scientific explorers discovered but also how, using actors to reveal the creative process through the scientists' own words, and conveying their landmark discoveries through re-enactments shot with replicas of their original lab equipment. Knitting these strands together into a coherent, compelling whole is host Michael Emerson, a two-time Emmy Award-winning actor best known for his roles on Lost and Person of Interest. Eric Scerri appears as the expert.
| Year: 2016 | PT id = 940, Type = non-chem |
90 Global Issues, Periodic Table of
Indian Schoolgirl Perfectly Reproduces Periodic Table by Inserting 90 Global Issues, a headline from The Epoc Times.
"Below is the periodic table, with the elemental symbols as they should be. But rather than a chemical, each symbol abbreviates a relevant social problem affecting the world today. Meet Kaanchi Chopra, the creator of this unique period table of elements. Chopra is a 17-year-old student and artist from Delhi, India. Because she uses her art as a platform to incite change, Chopra refers to herself as an ardent artivist.
From Kaanchi Chopra's ART AND ACTIVISM blog:
"As I flipped through the pages of [my chemistry textbook] trying to decipher the meaning of the title, a flashback to Grade 10 suddenly reminded me of the Periodic Table. How we used to make numerous mnemonics to memorize the Alkali metals, Alkaline Earth metals, Halogens, Noble gases and Transitional metals.
"In this entire rote learning process, I found something different and probably something as meaningful as those elements. I realized that each and every symbol of the elements in the Periodic Table was an acronym of a global issue. It could be expanded to form a word which represented one of humanity's worst vices. A few words in this table also represent the various movements and social issues which have gained a lot of attention in the recent times. That was when I decided to make a periodic table of 90 global issues and here it is!"

Thanks to Carel Kusters for the tip!
| Year: 2016 | PT id = 719, Type = data misc |
Collective Work of Chemists
From an article on LinkedIn:
Twelve elements were known from the Ancient Times, and were described by Romans and Greeks. The remaining 106 elements have been discovered by scientists of 15 different countries during the last 4 centuries. In addition, 19 elements of those 106 (18%) have been co-discovered by researchers of two countries.
Although some of them (like Bromine or Thallium) were isolated separately at the same time by chemists of different nationalities within the race to discover new elements in 18th-21st centuries, most of them have been obtained since then through collaborative research, like the recently discovered Ununpentium, Ununseptium and Ununoctium.
Another example is the isolation of Radium and Polonium by the Polish Maria Skłodowska-Curie and her French husband, Pierre Curie.
Thus, Periodic Table is the result of a collective and long-term work of hundreds of scientists.
It is noteworthy to see that Russia and United States have discovered mainly artificial elements.
| Year: 2016 | PT id = 720, Type = non-chem |
Genetic Codon Periodic Table
Heinrich Ferreira, splicejunction.blogspot.com, has created a 'periodic table' for the 20 amino acids, organized by generic code and hydrophobic value.
"I realized that when one orders the codons in the genetic code by Hamming distance of base changes between the 64 different codons for the 20 amino acids, that the amino acids with similar hydrophobic/polar nature automatically cluster together.
"This shows how the genetic code is optimized to minimize the production of incorrectly folded proteins.
"Thus, in the case of a single base change which results in a different amino acid being used, the chances are that incorrect amino acid will have the same or similar hydrophobic qualities are actually quite high due to the codons appearing next to each other on the Genetic Code periodic table.
"This representation, like the periodic table, is a torus where the adjacent codons wrap around from bottom to top and left to right."
| Year: 2016 | PT id = 722, Type = misc |
Philatelic Table of The Elements
Larry French writes:
"I created and first displayed [this Philatelic Table of the Elements] at the ACS National Meeting in San Diego.
"The table has been assembled with each element is represented by a single (or in a few cases a pair) of postage stamps. The table offers a platform for discussions of people, places, sources and applications associated with 114 elements. A total of 73 stamp issuing entities are represented. The table runs from hydrogen, with a North Vietnamese stamp celebrating the test of first Chinese H bomb, to livermorium, represented by a Soviet issue marking the 25th anniversary of the Nuclear Research Institute at Dubna. The table travels from Bolivia's Salar de Uyuni (lithium) to the Enewetak Atoll of the Marshall Islands (einsteinium) and spotlights environmental impacts of phosphate extraction in Nauru and lead mining in Peru. Discoverers and inventors from Moissan and Soddy to Auer and the Curies are met along the way. A range of applications including cesium formate brines in North Sea oil and gas drilling, indium in solar energy conversion, lanthanum in electric cars and technetium in positron emission tomographic medical imaging is included.
"Eventually, my aim is to produce a book which includes an essay for each element and stamp. I have made significant headway with the writing but there is much still to be done."
Larry French
Baker Professor of Chemistry
St. Lawrence University
Click here for full size version
| Year: 2016 | PT id = 730, Type = formulation misc |
KAS Periodic Table
The KAS periodic table reproduces and depicts the nuclear properties of chemical elements. This periodic table depicts not only the trends of nuclear properties, but also reproduces their numerical values that remain very close to the experimental values (difference less than 4%).
The Segre Chart is based on the number of protons, Z, and the number of neutrons, N. It is like a library of nuclei and shows the recorded data only. The Segre Chart can not work when the number of neutrons is not given. But KAS Periodic Table works when the number of neutrons is not given.It does not require the number of neutrons to produce the results.This is a simple chart based on the number of protons of chemical element. We identify the following properties of elements:-
Read more here, here and here.
| Year: 2016 | PT id = 731, Type = formulation non-chem |
Harrington Periodic Tables
So we start this effort tabula rasa (without preconceived ideas).
1) All atoms have a default "common denominator" structure at 270 mass units, irrespective of the element under discussion. Therefore, no elements seen as wisps and glints past this point are of consequence. Ergo, the bizarre stability of Dubnium 270.
2) This common structure is divided up by the exact same divisors as are the electron orbitals - i.e. the prime numbers of 2, 3, 5, and 7.
3) Pi as a divisor produces its own, unique and dominating organizational patterns.
4) Each of these sets of plotted nuclide "boxes" use identical formats, but are arranged in vertical columns based on the set of 270 AMUs being divided by these prime numbers. So the 5D Table is 270/5 or 54 AMUs per vertical column/"tower".
5) Each system reinforces unique elemental parameters. The system based on 3/Pi, and its second "harmonic" at 6/2Pi reflects physical properties. The 2Pi configuration almost exactly emulates the "conventional" / Mendeleevian element-based table, except the periods are based upon mass not element count, and these periods do not organize in rows of 18 elements, but rather rows of 44 mass units. The organization/configuration of this default structure is: Pi(Pi^2 + Pi + 1) = 44 This is the primary physical default structure of the periodic table and spectrum of elements, as projected in 3D space, and as perceptible to humans.
6) 5D determines everything with magnetic properties. This disproves every single theory that attributes electron shell behavior as determining magnetic parameters. Clearly here we see that the nucleus is "calling the shots", with electron orbitals conforming as driven. The various red and blue shaded boxes are found at extremes of top and bottom.
7) The system of 7D determines most of all physical parameters of surface and molecular behavior. Here we see surface tension, density, softness and hardness, malleability, boiling and melting points and a few other behaviors. This system of correlation is fully unknown to conventional theory. Notice how superlative parameters bunch at the top and bottom of this configuration.
8) When this system of 270 mass units is divided by 12, for 22 mass units per period, the periodic cycle rate precisely correlates with known Type 1 and 2 elemental superconductors. The physical correlations between periodic repetition at 22 mass units, the 270 count system, and superconductors is also completely novel and not compatible to conventional BCS theory. The correlation between this 22 count system and the three largest cross section nuclides known to man (113Cd, 157Gd and 135Xe) is also completely heretical, however mathematically symmetrical and perfect it may actually be organized.
9) The center portion of this common 270 count structure is named the "Cordillera", for the habit of multiple parallel mountain ridges sharing a common alignment. This area is profoundly affected by Pi-based organizations. The very center at 135Xe indicates that the overall table should terminate at element 108 Hassium at 270 mass units. This has a Proton/Neutron ratio of 3:2. This actual nuclide has very poor stability, unlike Dubnium 105 with 270 mass counts. This nuclide has a ratio of precisely 1:Pi/2, indicating the entire table describes a spectrum of mass organizational states spanning the integer ratio of 1:1 (Deuterium) to 3:2, then on through to 1:Pi/2. Current accepted atomic theories concerning "Islands of Stability" are ridiculous.
WAH
Click on the image to see the full size version
| Year: 2016 | PT id = 742, Type = formulation 3D spiral |
Instructables 3D Periodic Table
From Makendo on the Instructables website:
The first periodic table was developed in 1862 by a French geologist called Alexandre-Émile Béguyer de Chancourtois. He plotted the elements on a cylinder with a circumference of 16 units, and noted the resulting helix placed elements with similar properties in line with each other. But his idea - which he called the "Telluric Spiral" (see here), because the element tellurium was near the middle - never caught on, perhaps because it was published in a geology journal unread by chemists, and because de Chancourtois failed to include the diagram and described the helix as a square circle triangle.
Mendeleev got all the glory, and it is his 1869 version (dramatically updated, but still recognizable) that nearly everyone uses today.
This instructable [project] documents my efforts to reimagine a 3D periodic table of the elements, using modern making methods. It's based on the structure of a chiral nanotube, and is made from a 3D printed lattice, laser cut acrylic, a lazy susan bearing, 118 sample vials and a cylindrical lamp.
| Year: 2017 | PT id = 1120, Type = formulation data review |
Restrepo's Similarity Landscape
Building Classes of Similar Chemical Elements from Binary Compounds and Their Stoichiometries by Guillermo Restrepo, Chapter 5 from: Elements Old and New: Discoveries, Developments, Challenges, and Environmental Implications p 95-110.
From the abstract:
Similarity is one of the key concepts of the periodic table, which was historically addressed by assessing the resemblance of chemical elements through that of their compounds. A contemporary approach to the similarity among elements is through quantum chemistry, based on the resemblance of the electronic properties of the atoms involved. In spite of having two approaches, the historical one has been almost abandoned and the quantum chemical oversimplified to free atoms, which are of little interest for chemistry. Here we show that a mathematical and computational historical approach yields well-known chemical similarities of chemical elements when studied through binary compounds and their stoichiometries; these similarities are also in agreement with quantum chemistry results for bound atoms. The results come from the analysis of 4,700 binary compounds of 94 chemical elements through the definition of neighbourhoods for every element that were contrasted producing similarity classes. The method detected classes of elements with different patterns on the periodic table, e.g. vertical similarities as in the alkali metals, horizontal ones as in the 4th-row platinum metals and mixed similarities as in the actinoids with some transition metals. We anticipate the methodology here presented to be a starting point for more temporal and even more detailed studies of the periodic table.
Thanks to René for the tip!
| Year: 2017 | PT id = 739, Type = formulation |
Alternative Periodic Table
From Useful Charts:
You'll notice that this periodic table looks quite a bit different from the one you're used to. The traditional periodic table is designed to emphasize the concept of valence, which is important for knowing which elements can easily combine with others to form compounds. In contrast, the periodic table below is designed to simply emphasize the way in which atoms are "built" (specifically, how electrons group together into shells and subshells).
It's based on a design proposed by Edward Mazurs in the 1960s. Like the traditional table, this alternative version can be used to find an elements name, number, atomic weight, state of matter, period, group, and block. However, it also contains detailed information on electron configurations and the different types of electron subshells.
| Year: 2017 | PT id = 741, Type = formulation |
New Rendering of ADOMAH Periodic Table
From Valery Tsimmerman, of the PerfectPeriodicTable.com and the ADOMAH Periodic Table:
"I received email from Dr. Marcus Wolf who is a chemist, working on renewable energy and electrochemical storage in Germany, near Nuremberg. He also lectures at Georg Simon Ohm, Technische Hochschule Nürnberg. Attached to his email was new version of ADOMAH Periodic Table that he created. In this new rendering he is using Jensen's Valence Manifold (VM)."
This is what Dr. Marcus Wolf wrote:
"The first one to come up with the idea of using a valence manifold VM = [e + v] as a label for the groups, was Will B. Jensen. He derived it from the very early attempts of Richard Abegg, who, at around 1904, brought up the hypothesis of 'main- and counter-valences', derived from the observable behavior of elements and their compounds in electrochemical experiments. Eric Scerri is citing Jensen in his latest book, in the chapter about Richard Abegg. But Jensen's proper article from 1983 or so is far more detailed and in his later publications he then introduces the valence manifold concept. Last weekend I accidentally observed another consistency between the G-values and their ordering and the valence electron counts, e. If you fix the e value of the starting group in a given l-block as e(initial), you could generate every G-number of a given group by adding the valence vacancy count, v, to it:
G = e(initial) + v.
"That is another hint for the consistency of the VM labelling concept."

| Year: 2018 | PT id = 1309, Type = review |
Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements
Robinson, Ann E., "Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements" (2018). Doctoral Dissertations. 1385.
https://doi.org/10.7275/12706048 https://scholarworks.umass.edu/dissertations_2/1385. Download and view the PDF.
See Ann E. Robinson's ORCID page.
Mark Leach writes:
"An excellent, comprehensive study that is full of details and references."

| Year: 2018 | PT id = 919, Type = review |
Mendeleev to Oganesson: A Multidisciplinary Perspective on the Periodic Table
Since 1969, the international chemistry community has only held conferences on the topic of the Periodic Table three times, and the 2012 conference in Cusco, Peru was the first in almost a decade. The conference was highly interdisciplinary, featuring papers on geology, physics, mathematical and theoretical chemistry, the history and philosophy of chemistry, and chemical education, from the most reputable Periodic Table scholars across the world. Eric Scerri and Guillermo Restrepo have collected fifteen of the strongest papers presented at this conference, from the most notable Periodic Table scholars. The collected volume will contain pieces on chemistry, philosophy of science, applied mathematics, and science education.
Eric Scerri is a leading philosopher of science specializing in the history and philosophy of chemistry and especially the periodic table. He is the author of numerous OUP books including A Tale of Seven Scientists and a New Philosophy of Science (2016) and The Periodic Table: A Very Short Introduction (2012). Scerri has been a full-time lecturer at UCLA for the past eighteen years where he regularly teaches classes in history and philosophy of science.
Guillermo Restrepo is a chemist specializing in mathematical and philosophy of chemistry with more than sixty scientific papers and book chapters on these and related areas. Restrepo was a professor of chemistry at the Universidad de Pamplona (Colombia) between 2004 and 2017, and since 2014 has been in Germany as an Alexander von Humboldt Fellow at Leipzig University and more recently as researcher at the Max Planck Institute for Mathematics in the Sciences.
Preface
1. Heavy, Superheavy...Quo Vadis?
2. Nuclear Lattice Model and the Electronic Configuration of the Chemical Elements
3. Amateurs and Professionals in Chemistry: The Case of the Periodic System
4. The Periodic System: A Mathematical Approach
5. The "Chemical Mechanics" of the Periodic Table
6. The Grand Periodic Function
7. What Elements Belong in Group 3 of the Periodic Table?
8. The Periodic Table Retrieved from Density Functional Theory Based Concepts: The Electron Density, the Shape Function and the Linear Response Function
9. Resemioticization of Periodicity: A Social Semiotic Perspective
10. Organizing the Transition Metals
11. The Earth Scientist's Periodic Table of the Elements and Their Ions: A New Periodic Table Founded on Non-Traditional Concepts
12. The Origin of Mendeleev's Discovery of the Periodic System
13. Richard Abegg and the Periodic Table
14. The Chemist as Philosopher: D. I. Mendeleev's "The Unit" and "Worldview"
15. The Philosophical Importance of the Periodic Table
| Year: 2018 | PT id = 920, Type = formulation 3d |
Telluric Remix
Philip Stewart writes:
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
The printable version is available (click here for the full size version) to make your own:
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
| Year: 2018 | PT id = 938, Type = formulation |
Puddenphatt & Monagham Periodic Table
Jeries Rihani's version of R. J. Puddenphatt and P. K. Monaghan, published in1989, but is not an exact copy. The differences are as follows:
Puddephatt and Monaghan say "their table is after Philips and Williams":
Ref, Phillips CSG & Williams RJP 1965, Inorganic Chemistry, I: Principles and Non-metals, Clarendon Press, Oxford, p. 40.
| Year: 2018 | PT id = 1202, Type = formulation |
Beylkin's Periodic Table of The Elements
René Vernon writes: Beylkin's Periodic Table of The Elements has 4n2 periods, where n = 2,3..., and shows symmetry, regularity, and elegance, more so than Janet's left step table.
Beylkin (an applied mathematician) writes:
"Let us take a continuous strip of paper and, on one side of the strip, write all the elements in the order of their atomic numbers. We then form a spiral with the strip such that the two most chemically distinct groups, the group of halogens (in which we include hydrogen) and the group of noble gases, are properly aligned. By flattening the strip on a plane and folding it in the middle, we obtain the new periodic table..."
Other features:
There are four new(ish) groups: Ti-Zr-Ce-Th, V-Nb-Pr-Pa, Cr-Mo-Nd-U and Mn-Tc-Pm-Np. For the actinide elements of these groups, the resemblance of the earlier actinides to their lighter transition metal congeners is well known. For the lanthanide elements, Johansson et al. (2014) wrote a nice article about Ce and its cross-road position. For Pr, Nd, and Pm, all of these are known in multiple oxidations states (+2, +3, +4 excl. Pm, and +5 for Pr only), just as the transitions metals are so known.
| Year: 2018 | PT id = 951, Type = formulation |
Janet's Left-Step with Ground Level Microstates
By Valery Tsimmerman, who writes:
Janet's LST with ground level microstate information and total spin graph shown for each group of elements. The top line represents number of electrons in open sub-shells (with exception of six anomalous elements). Information shows physical (spectroscopic) basis of the groups.
The zigzag line on top is a graphic representation of Hund's rule showing the total inherent spins of atoms and the total spin of Cu is 1/2, same as for Ag and Au. When it comes to ground level atomic microstates and Hund's rule Cu is not anomalous (2S1/2), despite its anomalous electron configuration.
The diagram represents Hund's Rule that states that "the lowest energy atomic state is the one that maximizes the total spin quantum number for the electrons in the open subshell" (Wikipedia). Y-axis is the total spin and x-axis is number of electrons in open shells (with exception of six anomalous elements).
First, I would like to make couple of general comments. When discussing periodicity, they typically talk about chemical properties and electron configurations/differentiating electrons, etc, but those are not specific enough. For each electron configuration there are multiple microstates. For example, for single electron configuration of carbon there are over 30 microstates and only one of them corresponds to ground level. So, microstates express combined physical/spectroscopic properties of whole atoms and, the most important, combined properties of electrons located in open subshells.
Now, look at ground level term symbols in each group. I see amazing consistency, especially in the main groups. It tells me that groups are not only chemical, but physical!
Looking at periods one can see that all periods in s, p & d blocks begin with elements that have multiplicity M=2 and end with M=1. This is also true for f-block if it starts with La and Ac and ends with Yb and No. This puts Lu and Lr firmly in group 3. Placing La and Ac in group three ruins spectroscopic consistency.
Click here image to enlarge the PT below.
| Year: 2018 | PT id = 1261, Type = formulation |
Kurushkin's 32-Column Periodic Table & Left-step Periodic Table United
Dr Mikhail V. Kurushkin, 32-column Periodic Table & Left-step Periodic Table United: https://bernalinstitute.com/events/bernal-seminar-by-dr-mikhail-v-kurushkin-itmo-universityrussia/
ABSTRACT
The pursuit of optimal representation of the Periodic Table has been a central topic of interest for chemists, physicists, philosophers and historians of science for decades (Leigh, 2009; Scerri, 2009). Should the Periodic Table of Chemical Elements first and foremost serve the needs of chemists as implied by its name? Or should it start from considerations of before quantum mechanics and thus be more appealing to physicists (Scerri, 2010, 2012b)? Is there a representation which overcomes this problem? The Periodic Table is from a fundamental point of view a graphic representation of periodicity as a phenomenon of nature. While the 32-column Periodic Table, popularized by Glenn T. Seaborg, is considered by chemists the most scientifically correct representation (Scerri, 2012a), physicists apparently prefer the Left-step Periodic Table above all (Scerri, 2005; Stewart, 2010). Alternatively, it is suggested that a rigorously fundamental representation of periodicity could only take the form of a spiral as, evidently, the abrupt periods of 2-D Periodic Tables contradict the gradual increase of atomic number, and the spiral representation reconciles this debate (Imyanitov, 2016). An optimal representation is eagerly sought after both for the needs of philosophy of chemistry and chemical education as their never-ending dialogue secures a thorough methodology of chemistry. The aim of the present work is to show that the 32-column Periodic Table and the Left-step Periodic Table can co-exist in mutual tolerance in a form of what Philip Stewart has already called Kurushkin’s Periodic Table (Kurushkin, 2017), Figure 1 below.
René Vernon writes:
"Kurushkin reminds us that the Janet left step table (with Sc-Y-Lu-Lr, and He over Be), and the version of the table with the s-elements on the right (also with Sc-Y-Lu-Lr, and He over Be) are interchangeable.
"For an earlier paywall version which includes a short video see:
Kurushkin M 2018, Building the periodic table based on the atomic structure, Journal of Chemical Education, vol. 94, no. 7, pp. 976–979, https://pubs.acs.org/doi/10.1021/acs.jchemed.7b00242
"Kurushkin’s interchangeable approach extends to tables with group 3 as either Sc-Y-La-Ac or Sc-Y-Lu-Lr. See Vernon's Yin Yang of The Periodic Table https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=1252"
| Year: 2019 | PT id = 1024, Type = formulation review |
5 Periodic Tables We Don't Use (And One We Do)
SciShow says:
"From Mendeleev's original design to physicist-favorite "left-step" rendition, the periodic table of elements has gone through many iterations since it was first used to organize elements 150 years ago - each with its own useful insights into the patterns of the elements":
| Year: 2019 | PT id = 1027, Type = formulation data |
Chemical Bonds, Periodic Table of
The Max Planck Society (M-P-G, Max-Planck-Gesellschaft) has an article about the hidden structure of the periodic system.
Guillermo Restrepo, MPI for Mathematics in the Sciences:
"A periodic table of chemical bonds: Each of the 94 circles with chemical element symbols represents the bond that the respective element forms with an organic residue. The bonds are ordered according to how strongly they are polarized. Where there is a direct arrow connection, the order is clear: Bonds of hydrogen, for example, are more polarized than bonds of boron, phosphorus, and palladium. The same applies to rubidium in comparison to caesium, which has particularly low polarized bonds and is therefore at the bottom of the new periodic table. If there is no direct arrow between two elements, they may still be comparable – if there is a chain of arrows between them. For example, the bonds of oxygen are more polarized than the bonds of bromine. Bonds represented by the same colour have the same binding behaviour and belong to one of the 44 classes.":

Thanks to René for the tip!
| Year: 2019 | PT id = 1028, Type = formulation |
Slightly Different Periodic Table
The Max Planck Society (M-P-G, Max-Planck-Gesellschaft) has an article about the hidden structure of the periodic system.
Guillermo Restrepo, MPI for Mathematics in the Sciences:
"A slightly different periodic table: The table of chemical elements, which goes back to Dmitri Mendeleev and Lothar Meyer, is just one example of how objects – in this case the chemical elements – can be organized in such a system. The researchers from Leipzig illustrate the general structure of a periodic table with this example: The black dots represent the objects ordered by the green arrows. Using a suitable criterion, the objects can be classified into groups (dashed lines) in which the red arrows create a sub-order":
Thanks to René for the tip!
| Year: 2019 | PT id = 1046, Type = formulation data |
Group 3 of The Periodic Table
There are several ways in which the 'common/modern medium form' periodic table are shown with respect to the Group 3 elements and how the f-block is shown. Indeed, there is even some dispute about which elements constitute Group 3. There are three general approaches to showing Group 3:
(See Scerri's take and Thyssen's view on this matter.)
So, which one of the three options is 'better'?
The general feeling amongst the knowledgeable is that leaving a gap is not an option, so it comes down to:
Sc, Y, La, Ac vs. Sc, Y, Lu, Lr
René Vernon has looked as the properties of the potential Group 3 elements, including: densities, 1st ionisation energies, ionic radii, 3rd ionisation energies, melting points & electron affinity:
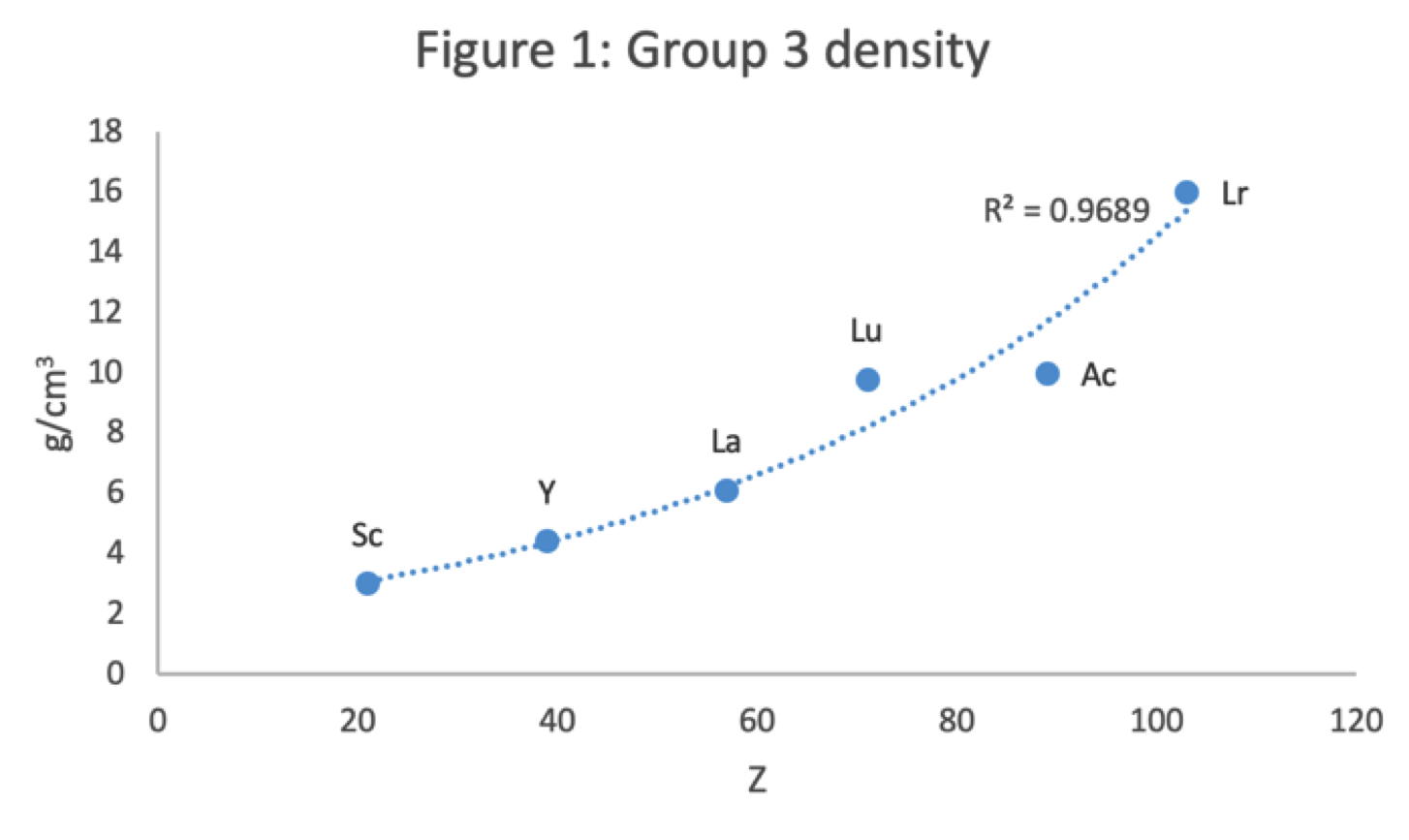
Figure 1 shows that a Z plot of the density values for Sc, Y, La, Lu Ac and Lr follows a smooth trendline.
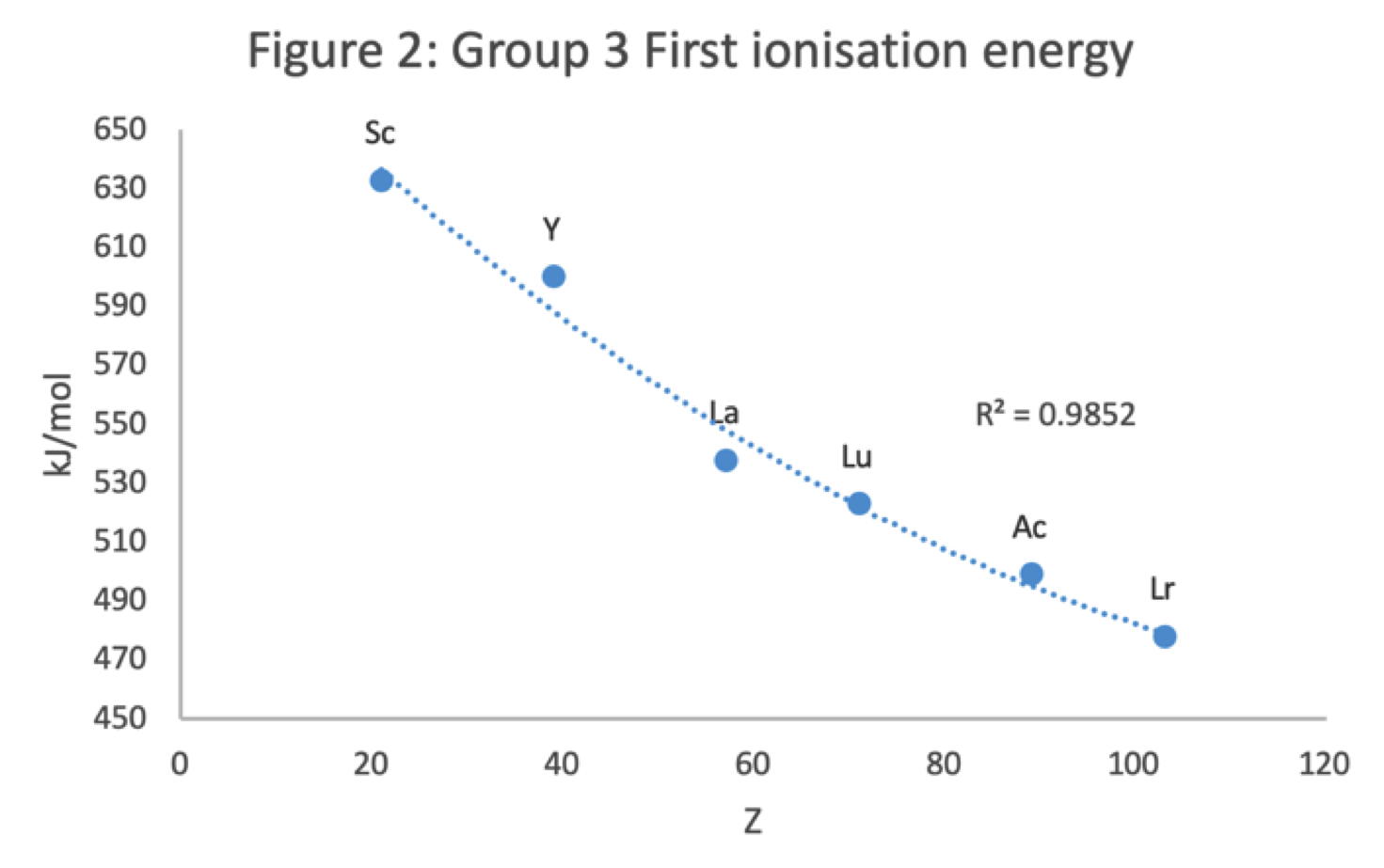
Figure 2 shows that a Z plot of the first ionization energy values follows a smooth trendline.
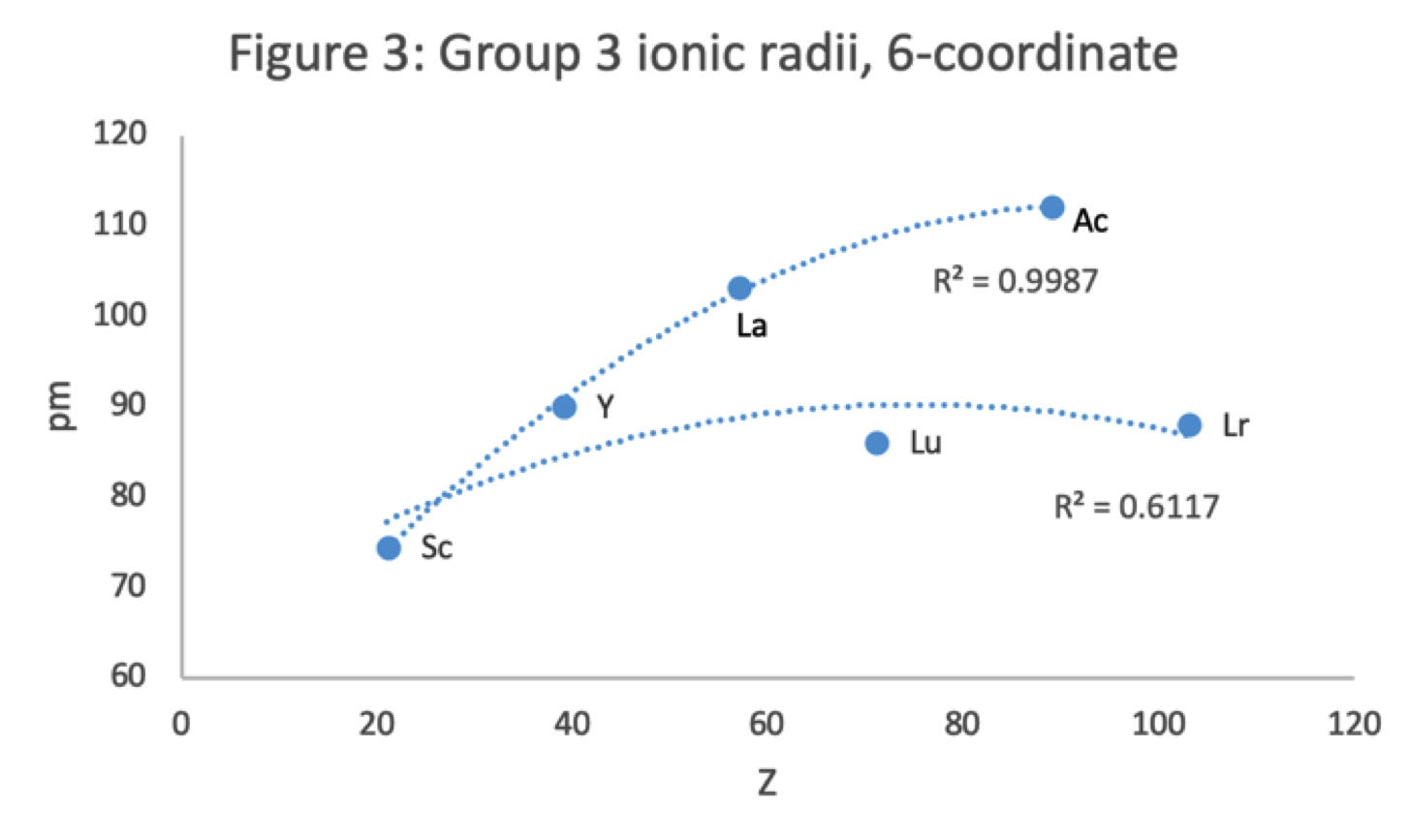
Figure 3 shows that a Z plot of the 6-coordinate ionic radii for the subject elements bifurcates after Y into an -La-Ac tranche (R2 = 0.99) and a -Lu-Lr branch (0.61). The trendline for -La-Ac is smoother.
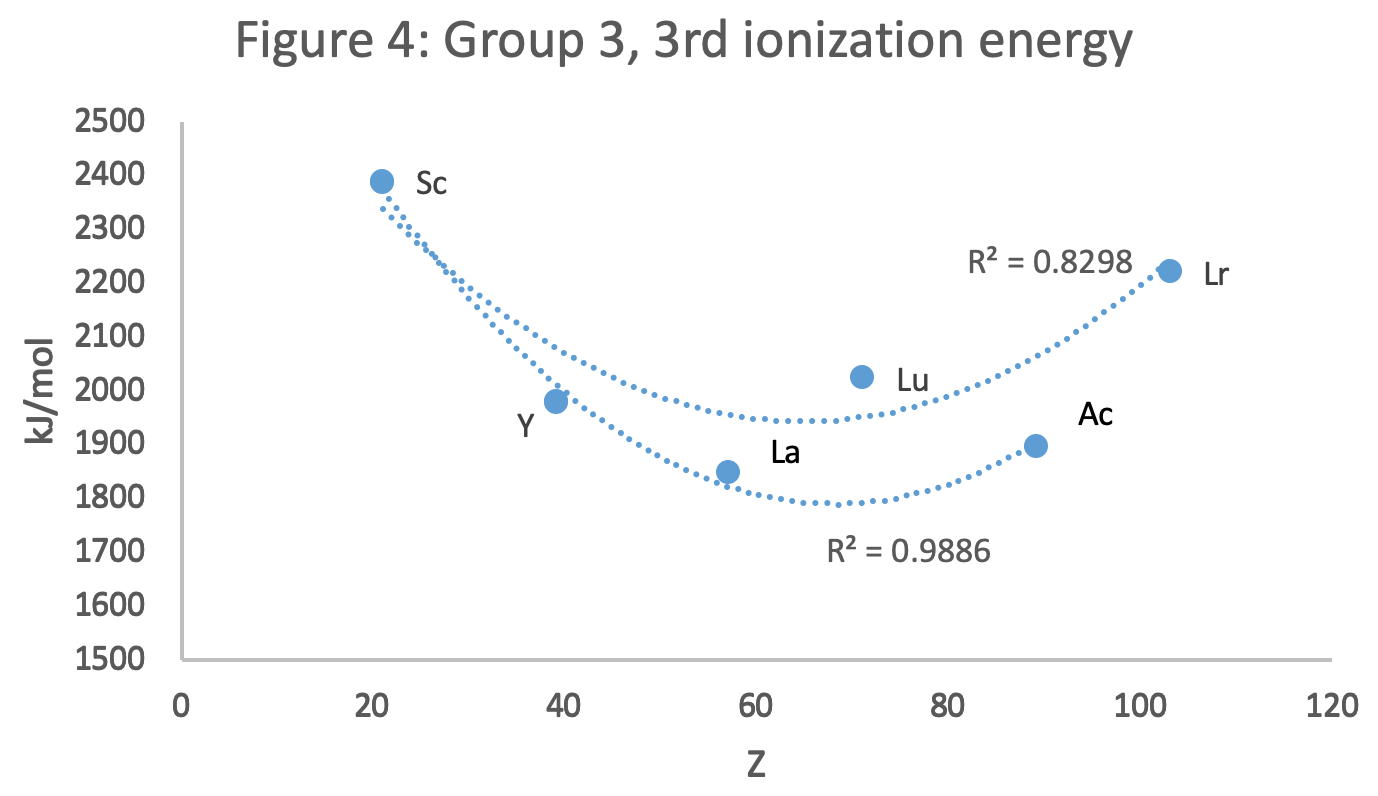
Figure 4 shows a Z plot of 3rd ionisation energy values bifurcating after Y into a -Lu-Lr tranche (R2 = 0.83) and a -La-Ac branch (0.98). The trendline for -La-Ac is smoother.
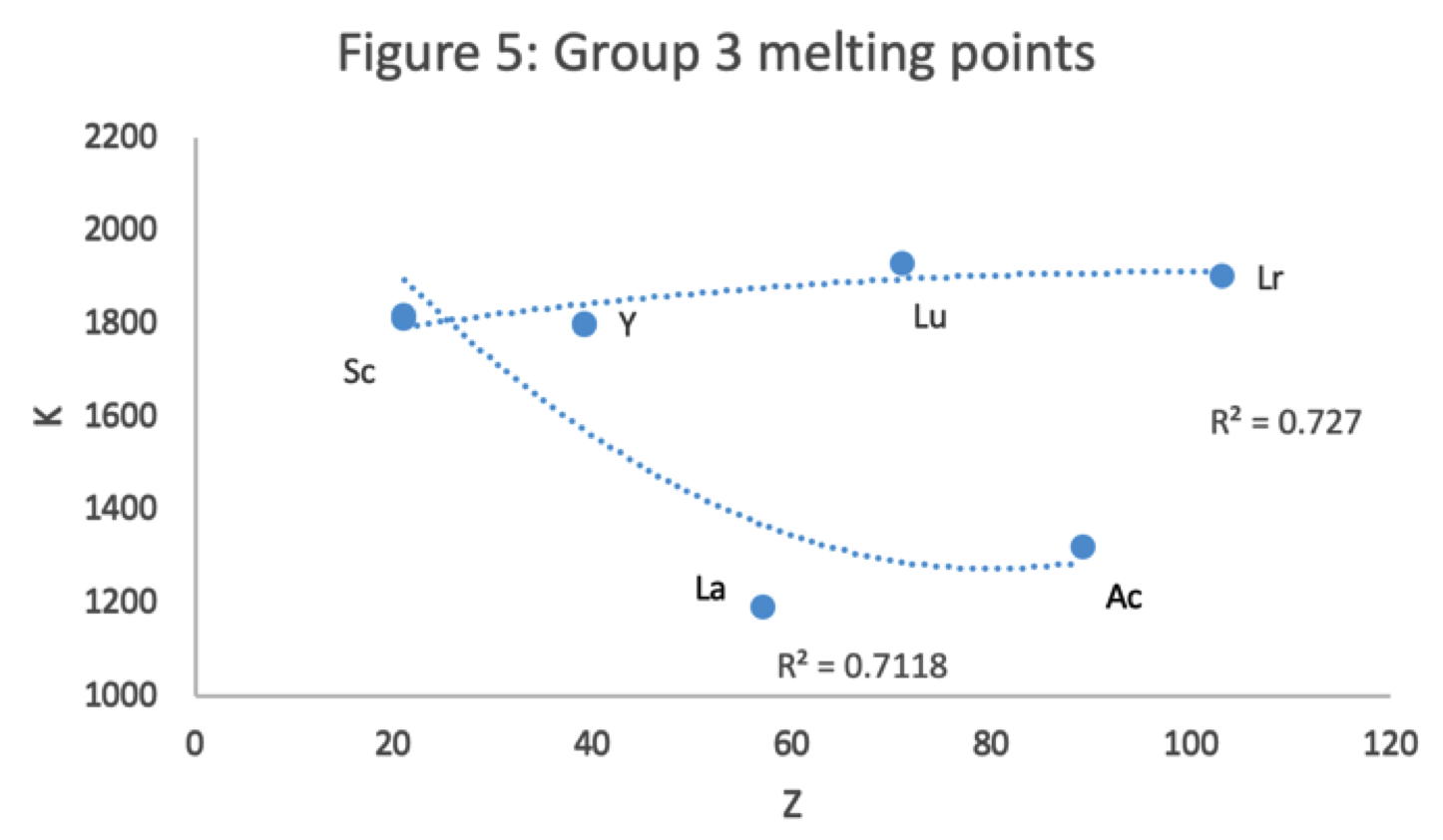
Figure 5 shows that a Z plot of the melting points bifurcates after Y into an -Lu-Lr (R2 = 0.72) tranche and a -La-Ac (0.71) branch. While the fit values for the two options are comparable, -Lu-Lr is preferred since Y and La show a greater departure from trend.
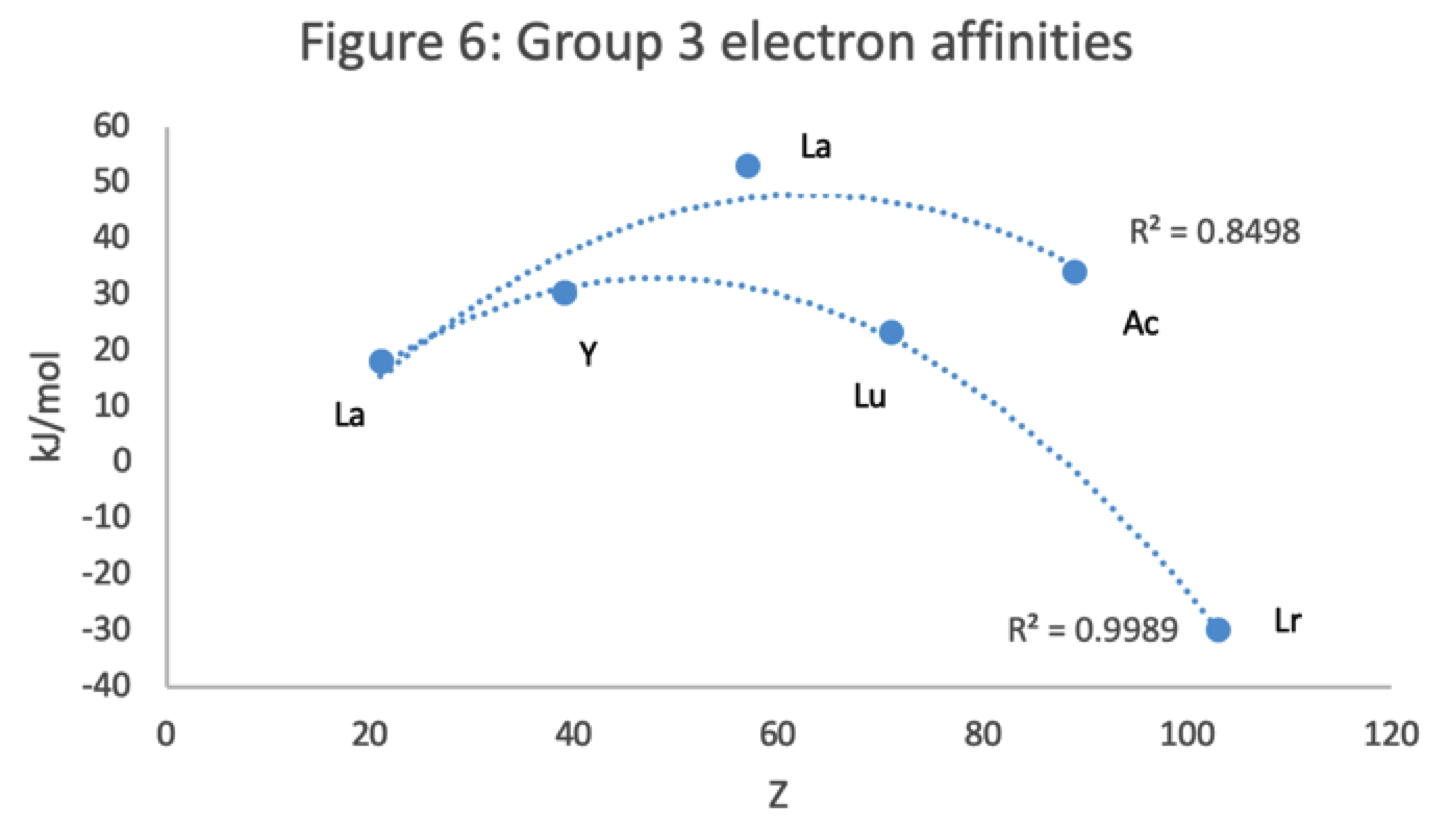
Figure 6 has a Z plot of electron affinity values bifurcating after Y into an -La-Ac tranche (R2 = 0.85) and a -Lu-Lr branch (0.99).[iii] The trendline supports Lu-Lr. The trend-lines by themselves are inconclusive: two show no difference; two support -La-Ac; two support -Lu-Lr.
Upon reviewing the data, René's comment is that: "The net result is that the two options seem inseparable" and he proposes that IUPAC adopt the following periodic table numbering system:
Professor Sir Martyn Poliakoff's [of the Periodic Videos YouTube channel & Nottiningham University] take on this matter:
| Year: 2019 | PT id = 1053, Type = formulation |
Chavhan's Third Generation Periodic Table of the Elements
Randhir Bhavial Chavhan's Third Generation Periodic Table of the Elements poster, as presented 4th International Conference on Periodic Table at St. Petersburg, Russia.
Click here, or on the image, to enlarge:
| Year: 2019 | PT id = 1064, Type = formulation |
Samanez's Binodic Periodic System, New Mathematical Paradigm Poster
Julio Antonio Gutierrez Samanez (Master's student in chemical engineering at the San Antonio Abad National University of Cusco, Peru) presented a poster at the 4th International Conference on the Periodic Table arranged by IUPAC, Saint Petersburg, Russian Federation, July 2019. See the high resolution .PDF file.
More here and at kutiry.com
| Year: 2019 | PT id = 1065, Type = formulation review misc |
Mendeleev 150
Mendeleev 150 is the 4th International Conference on the Periodic Table. The event welcomed nearly 300 guests from over 30 countries and has become one of the key events of IUPAC's International Year of the Periodic Table.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1071, Type = data |
Toma's Periodic Tables
Henrique E. Toma writes:
"I will be delighted if I could have a chance to contribute for the fantastic moment expressed by 2019 IYPT.
"I am senior Professor of Chemistry at the University of São Paulo, and great Periodic Table enthusiast since the beginning of my career about 50 years ago. This interest actually came from my supervisor and mentor, Professor Henry Taube (Nobel Prize, 1983), who taught me the beauty of the elements."
"As an inorganic chemist, I have been collecting the elements and minerals for a long time, and I built up the Periodic Table with the real elements shown below. It is one of the attractions of the campus, and has been reported in many publications1. It was visited by colleagues from IUPAC, including the President. I wouldn't be surprised if it inspired IUPAC the similar Table exposed in Paris, this year. The difference is that our table is that it also places the typical minerals together with the elements, and I believe that this is very important aspect for teaching and discussing the history behind them:

"Next, is my personal version of the IUPAC Periodic Table, shown in Figure 2, with the isotopes distributed in a column right to the element symbol. This Table is very practical, and particularly useful when you are dealing with mass spectrometry or isotopes. It is in my book of Elements2.
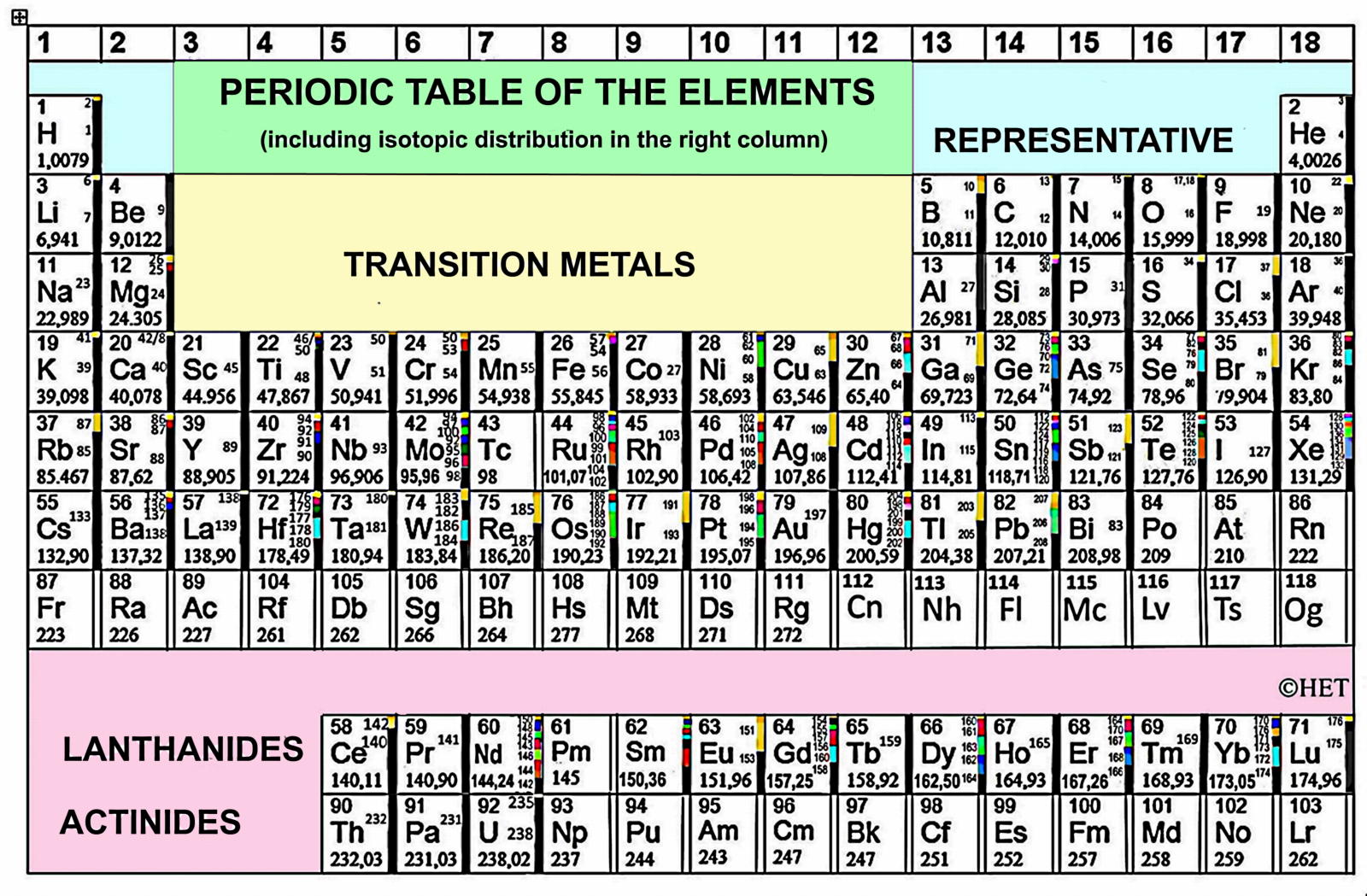
"Another is the Periodic Table of the Elements for Life, with the essential elements and abundance expressed by colors, including those used in medicine. This Table will be changing with the progress of Bioinorganic Chemistry, and is in my book of Bioinorganic Chemistry3.
"Finally, I have adapted the periodic table of elemental sustainability, using the colors to call attention for this issue. In this form, it is can be more easily understood by the public. Elemental Sustainability is a very important issue, as discussed in Green Chemistry Journal4.
References:
| Year: 2019 | PT id = 1073, Type = data |
Medicines, Periodic Table of
From C. Van Cleave 1 and D. C. Crans, The First-Row Transition Metals in the Periodic Table of Medicine, Inorganics 2019, 7, 111 (Inorganics 2019, 7, 111; doi:10.3390/inorganics7090111, www.mdpi.com/journal/inorganics).
From the paper, specifically the text associated with the figure:
The periodic table with known medicinal uses of each main group or transition metal element when available. In the following, we list the use of each element.
| Year: 2019 | PT id = 1094, Type = formulation data review |
Physical Origin of Chemical Periodicities in the System of Elements
From de Gruyter: Physical origin of chemical periodicities in the system of elements, Chang-Su Cao, Han-Shi Hu, Jun Li* and W. H. Eugen Schwarz*, Pure Appl. Chem. 2019; 91(12).
Published Online: 2019-11-30 | DOI: https://doi.org/10.1515/pac-2019-0901 (open access)
Abstract:
The Periodic Law, one of the great discoveries in human history, is magnificent in the art of chemistry. Different arrangements of chemical elements in differently shaped Periodic Tables serve for different purposes. "Can this Periodic Table be derived from quantum chemistry or physics?" can only be answered positively, if the internal structure of the Periodic Table is explicitly connected to facts and data from chemistry.
Quantum chemical rationalization of such a Periodic Tables is achieved by explaining the details of energies and radii of atomic core and valence orbitals in the leading electron configurations of chemically bonded atoms. The coarse horizontal pseudo-periodicity in seven rows of 2, 8, 8, 18, 18, 32, 32 members is triggered by the low energy of and large gap above the 1s and nsp valence shells (2 ≤ n ≤ 6 !). The pseudo-periodicity, in particular the wavy variation of the elemental properties in the four longer rows, is due to the different behaviors of the s and p vs. d and f pairs of atomic valence shells along the ordered array of elements. The so-called secondary or vertical periodicity is related to pseudo-periodic changes of the atomic core shells.
The Periodic Law of the naturally given System of Elements describes the trends of the many chemical properties displayed inside the Chemical Periodic Tables. While the general physical laws of quantum mechanics form a simple network, their application to the unlimited field of chemical materials under ambient 'human' conditions results in a complex and somewhat accidental structure inside the Table that fits to some more or less symmetric outer shape. Periodic Tables designed after some creative concept for the overall appearance are of interest in non-chemical fields of wisdom and art.
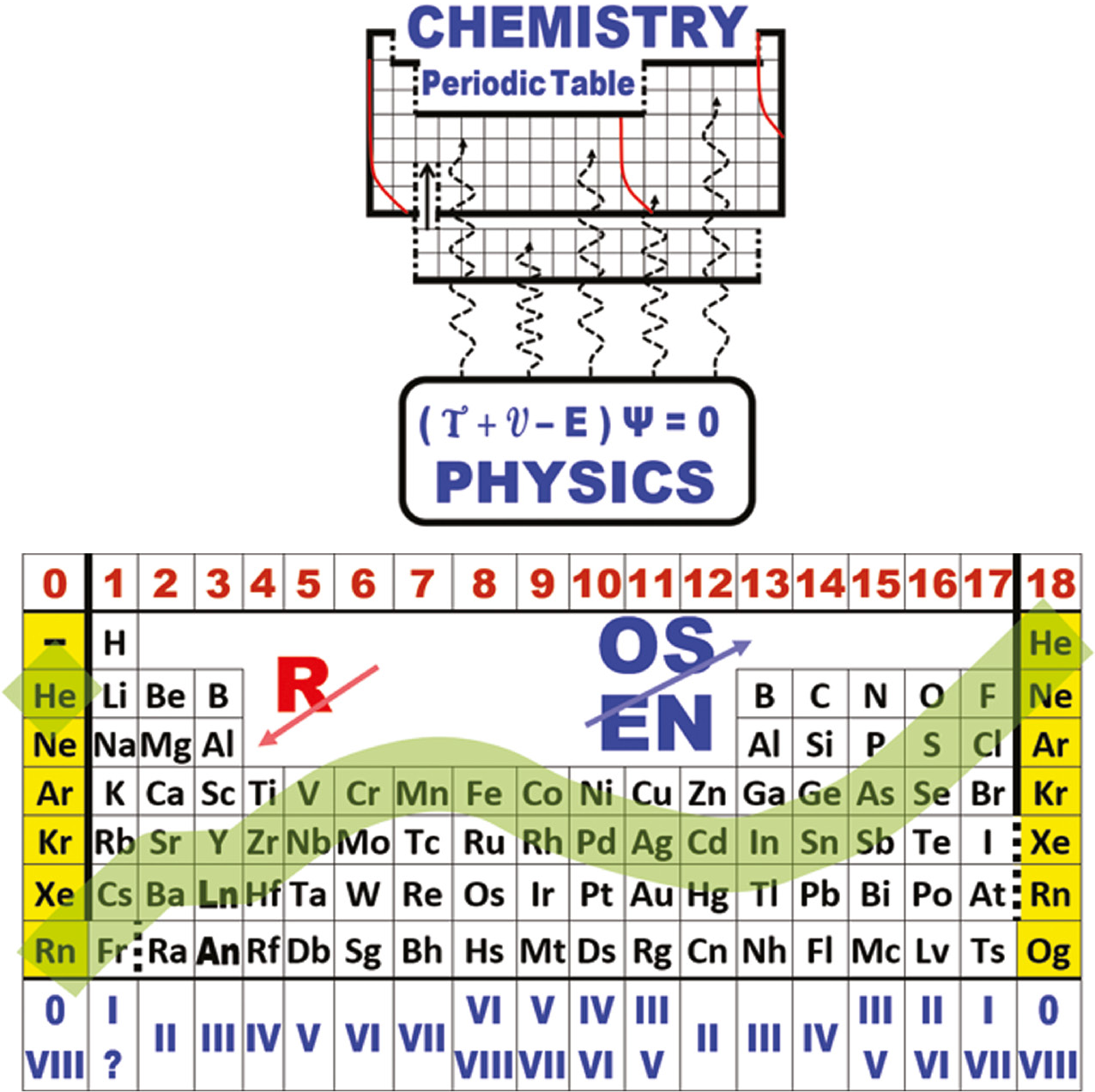
| Year: 2019 | PT id = 1099, Type = formulation |
Colburn's 2019 Periodic Table of The Elements
Justin Lee Colburn writes:
"What is unique to my Periodic Table is the fact that any Elements Electron Spin can be identified for Orbital Diagrams using a technique I have called 'Element Shifting'.
"Elements with an Up spin Valence Electron are shifted up and Elements with a down spin are shifted down. Also, the Hund's rule Exceptions are Highlighted in the Transition Metals so their orbital diagrams can also be identified easily.
"In addition, an accurate numbering system can be applied to all the elements with Helium placed in Group 2 instead of Group 18. I believe that quantitative data should take priority when giving elements their position, but this system is meant to be dynamic rather than static. In my Periodic Table System, (1-8) corresponds to Valence Electrons in the s and p orbitals and then the 9-18 and 19-32 corresponds to core electron in d and f orbitals .
I believe that it is important to begin by showing students the first 20 Elements FIRST because they all add Outer Valence Electrons which makes the Periodic Table logic easy to follow. Also explaining that Hydrogen and Helium are anomalies with more than one logical position, can really help clear up confusion for new students.
"After element 20, the Transition Elements such as scandium 21 begin adding core electrons in the d orbital the current standard (1-18) numbering system does not reflect this. One of the reasons why I prefer keeping the s and p block elements on the outside of the table and the f and d block elements on the inside is because of how they add electrons to their orbitals.
"I have been developing a curriculum based on this system that I believe will help students learn and understand the logic and trends of The Periodic Table more efficiently than the standard. Rather than memorizing Element information, Students will truly be able to follow the logic based on the location of the Elements, simple counting and using the numbering system."
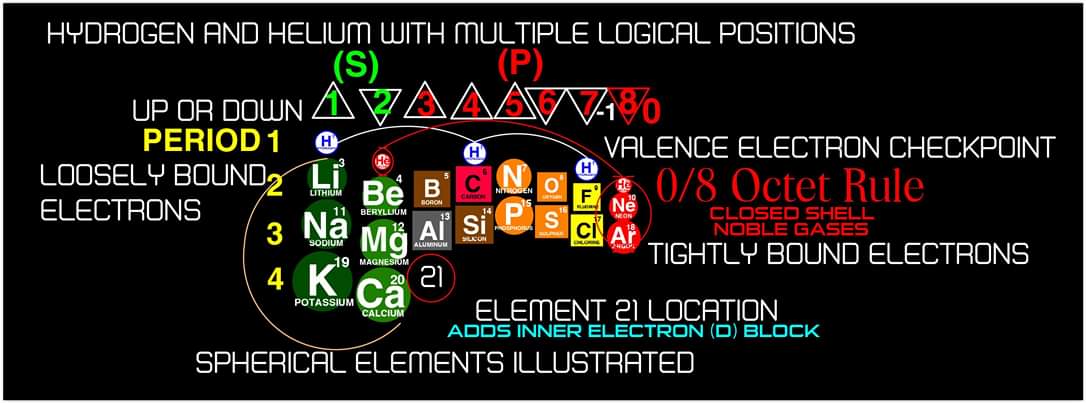
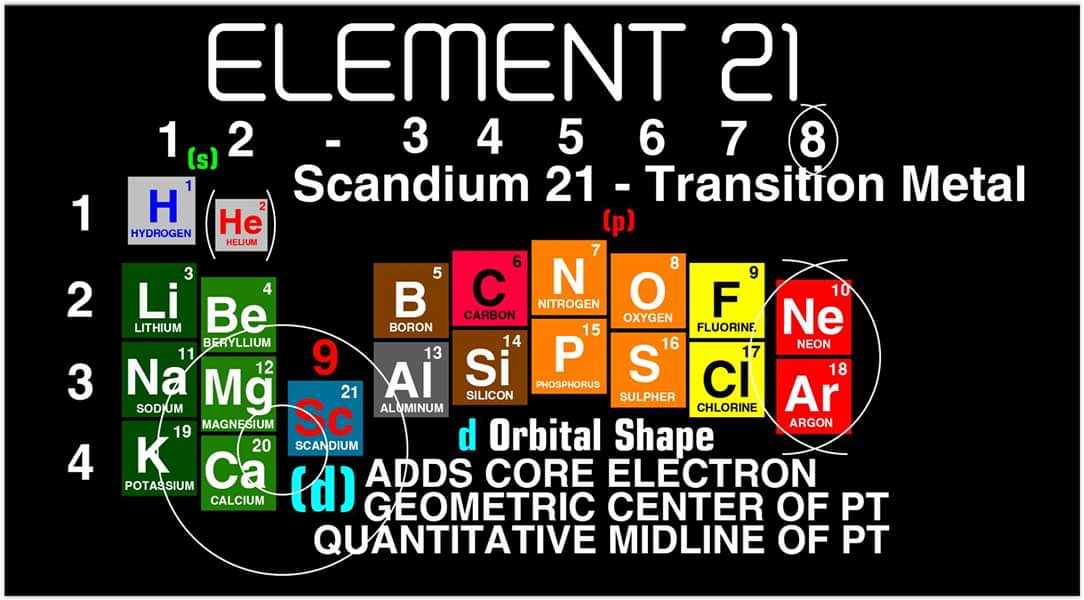
| Year: 2019 | PT id = 1127, Type = data misc |
Geological Periodic Table
Alvarez & Cordoba's Periodic Table of the Elements Associated with Geology [from Spanish using Google Translate]
"It is a simple and innovative table where each element has the shape of its respective crystalline system. It also has several novelties linked to earth sciences such as: illustrative images that show where the element can be found naturally on our planet, geochemical classification and different types of relevant characterizations (radioactivity, synthetics, alloys, majority elements in bark and mantle). Likewise, various useful tools were included in the area such as the well-known Bowen series, categorizations of compatible and incompatible elements, typical cases of the Piper diagram and Stiff diagrams.
"To increase the interaction and understanding between the user and the table, it has elements external to it (letters) that incorporate augmented reality, which allows learning in a simpler, didactic and entertaining way about the atomic structure of chemical elements in 3D. Just scan the back of the letter with your cell phone to see its structure."
Click the image to see the PDF file
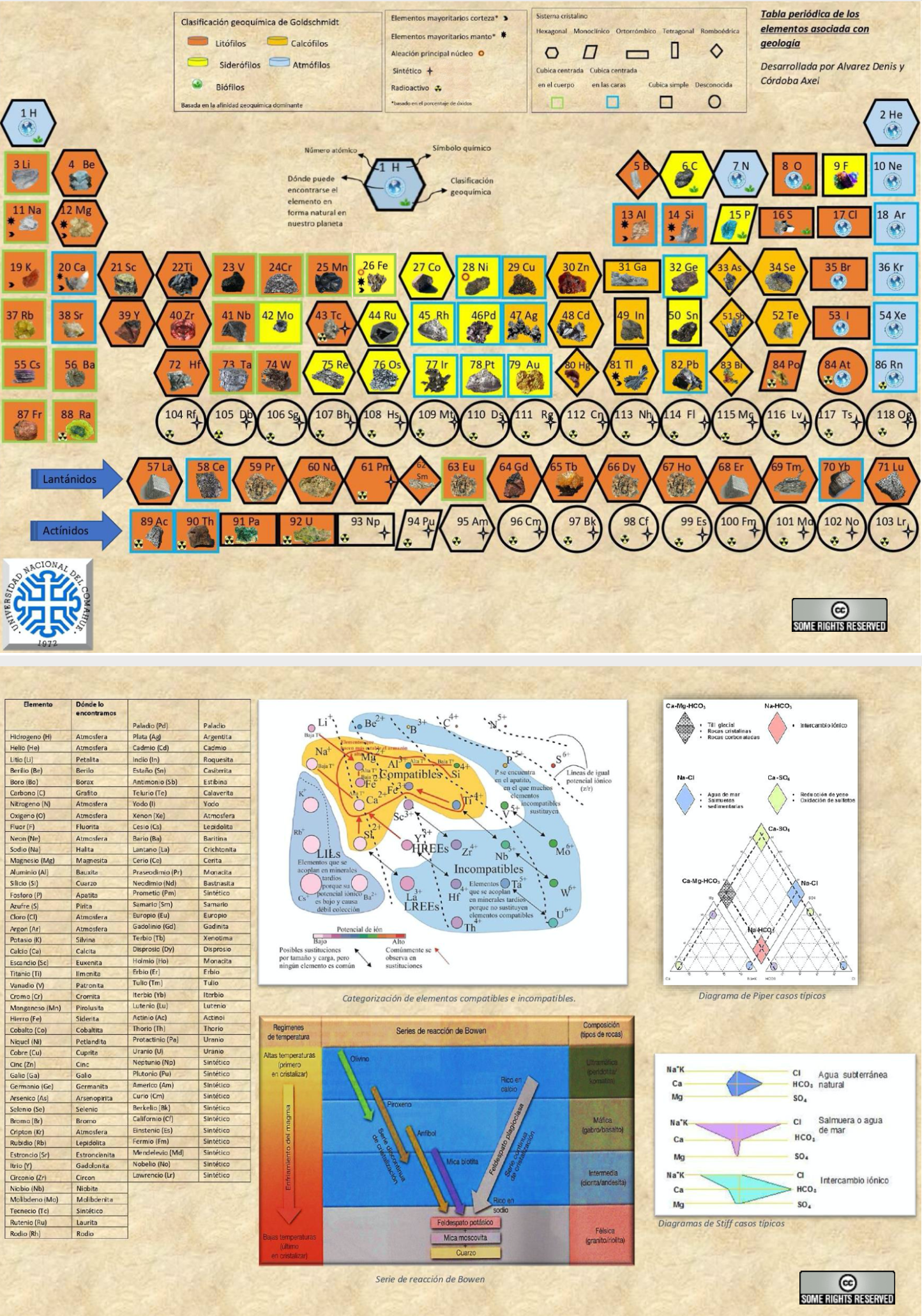
| Year: 2019 | PT id = 1133, Type = formulation data |
Vernon's Oxidation Number Periodic Table
René Vernon's periodic table showing oxidation number trends.
René writes:

| Year: 2019 | PT id = 1178, Type = data |
Abundance by Atomic Number, Z
An article in De Gruyter Conversations: The Periodic Table & The Lanthanides by Simon Cotton has this interesting chart of elemental abundance with respect to 106 atoms of Si.
The image source is http://upload.wikimedia.org/wikipedia/commons/0/09/Elemental_abundances.svg
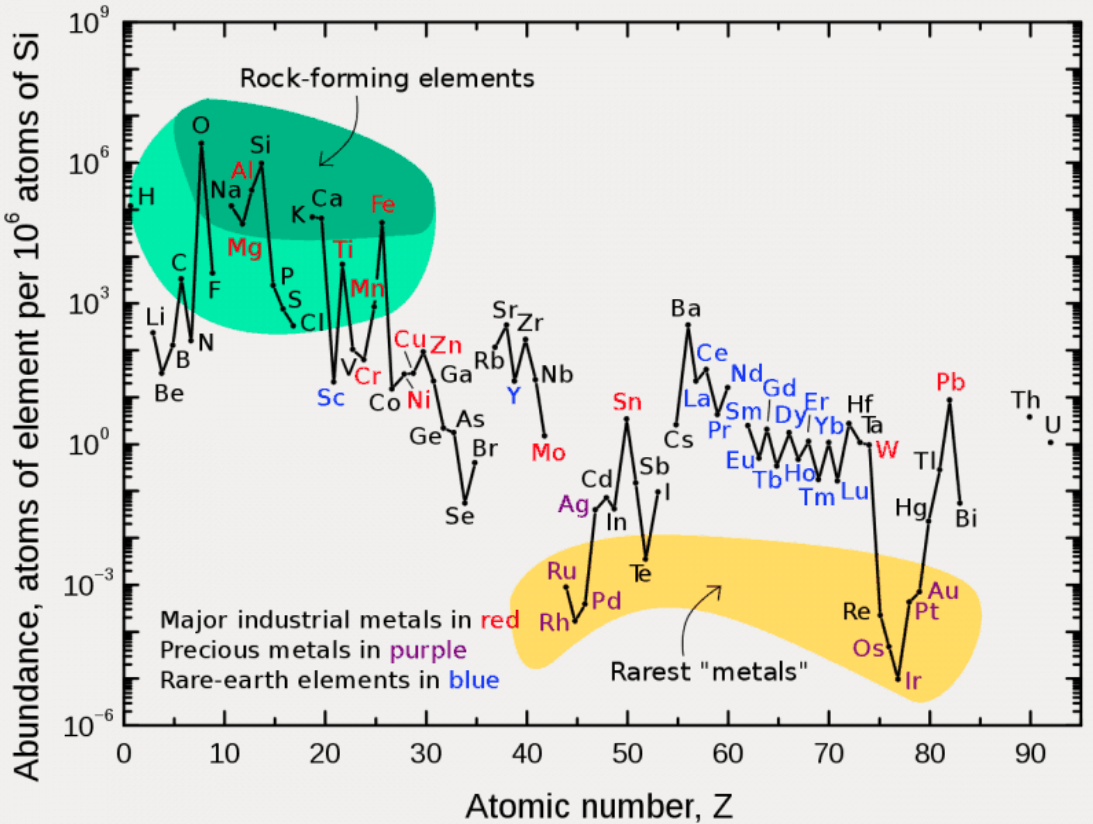
Thanks to René Vernon for the tip.
| Year: 2019 | PT id = 959, Type = formulation |
UCLA Periodic Table (Proposed)
Eric Scerri writes:
During an office hour here at UCLA with a couple of students – Annelise Gazale & Chidinma Onyeonwu – we came up with a 'new periodic table'.
The basis of it is related to a point you frequently make against the Left Step formulation, namely that it messes up trends in atomic radius etc.
So how about this: Traditionally on the right side of the table elements become less reactive as we move down, but on the right side of the table elements become more reactive as we move down. Consequently, the noble gases are anomalous in the way they usually sit since they become more reactive as we move down the table.
Ergo: Move the noble gases to the left edge of the table. (Yes, this has been done before of course but not for this reason.)
Thanks to Eric Scerri for the tip! See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 962, Type = formulation review misc |
Möbius-Escher Periodic Table
A comment article in Nature by Prof. Eric Scerri about quantum mechanics and the periodic table:
"Can quantum ideas explain chemistry's greatest icon? Simplistic assumptions about the periodic table lead us astray.
"Such has been the scientific and cultural impact of Dmitri Mendeleev's periodic table of the elements that many people assume it is essentially complete. [But] in its 150th year, can researchers simply raise a toast to the table's many dividends, and occasionally incorporate another heavy synthetic element?
"No – this invaluable compilation is still not settled. The placements of certain elements, even hydrogen and helium, are debated."
The article is accompanied by a fantastic illustration by Señor Salme with ideas from the Möbius strip and M.C. Escher:
| Year: 2019 | PT id = 984, Type = formulation 3D spiral |
Telluric Remix in Colour
Philip Stewart writes (this is the same text that accompanies the 2018 B/W version):
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
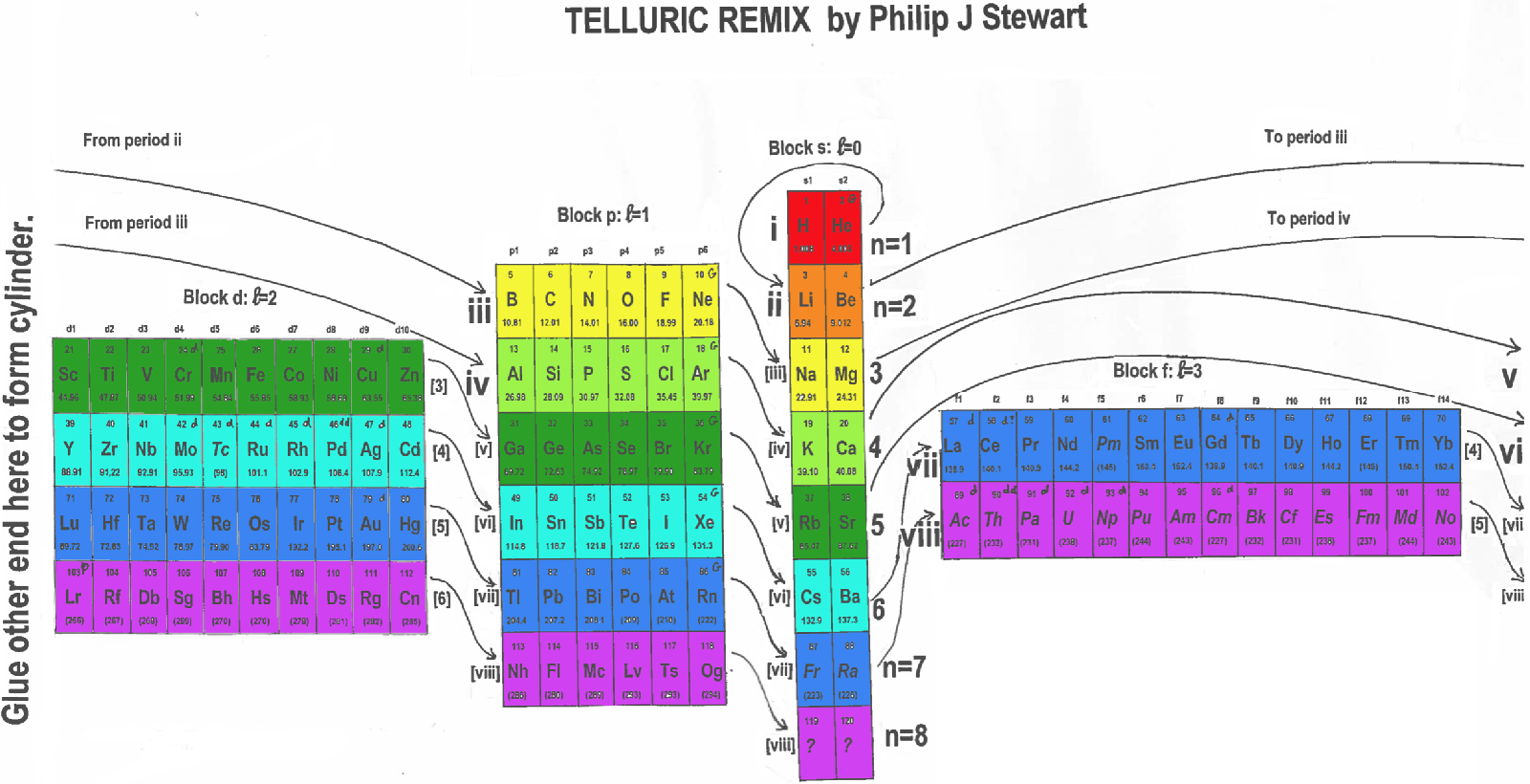
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
| Year: 2019 | PT id = 1246, Type = misc element |
Periodic Table of the Elements Coloring Book
Periodic Table of the Elements Coloring Book
Project managing and chemistry overseen by Yann Brouillette (Faculty, Chemistry, Dawson College). Element representations and cover by Dawson College Illustration & Design students (2nd year)* overseen by Meinert Hansen (Faculty, Illustration & Design, Dawson College).
Thanks to René for the tip!
| Year: 2019 | PT id = 996, Type = formulation spiral 3D |
Grainger's Elemental Periodicity with "Concentric Spheres Intersecting Orthogonal Planes" Formulation
From Tony Grainger, an Elemental Periodicity formulation with concentric spheres intersecting orthogonal planes.
Tony writes:
"I hand sketched this periodic table about a decade ago and placed it on my cubicle window at UTAS, with minimal comments from work mates. It bears some similarity to other formulations in the database, especially when cut along the left axis and laid flat. The concept of all elements of a period being aligned along orthogonal planes cutting a sphere was inherent in the original sketch. When I began using SVG about five years ago I realised I could draw this as a real projection of the 3D model. It was on the back burner, until I found the original sketch during a tidy up."
There are two images of this 3D formulation: an "inside_corner_below/outside_corner_above" (top image) and an "outside_corner_below/inside_corner_above" lower image.
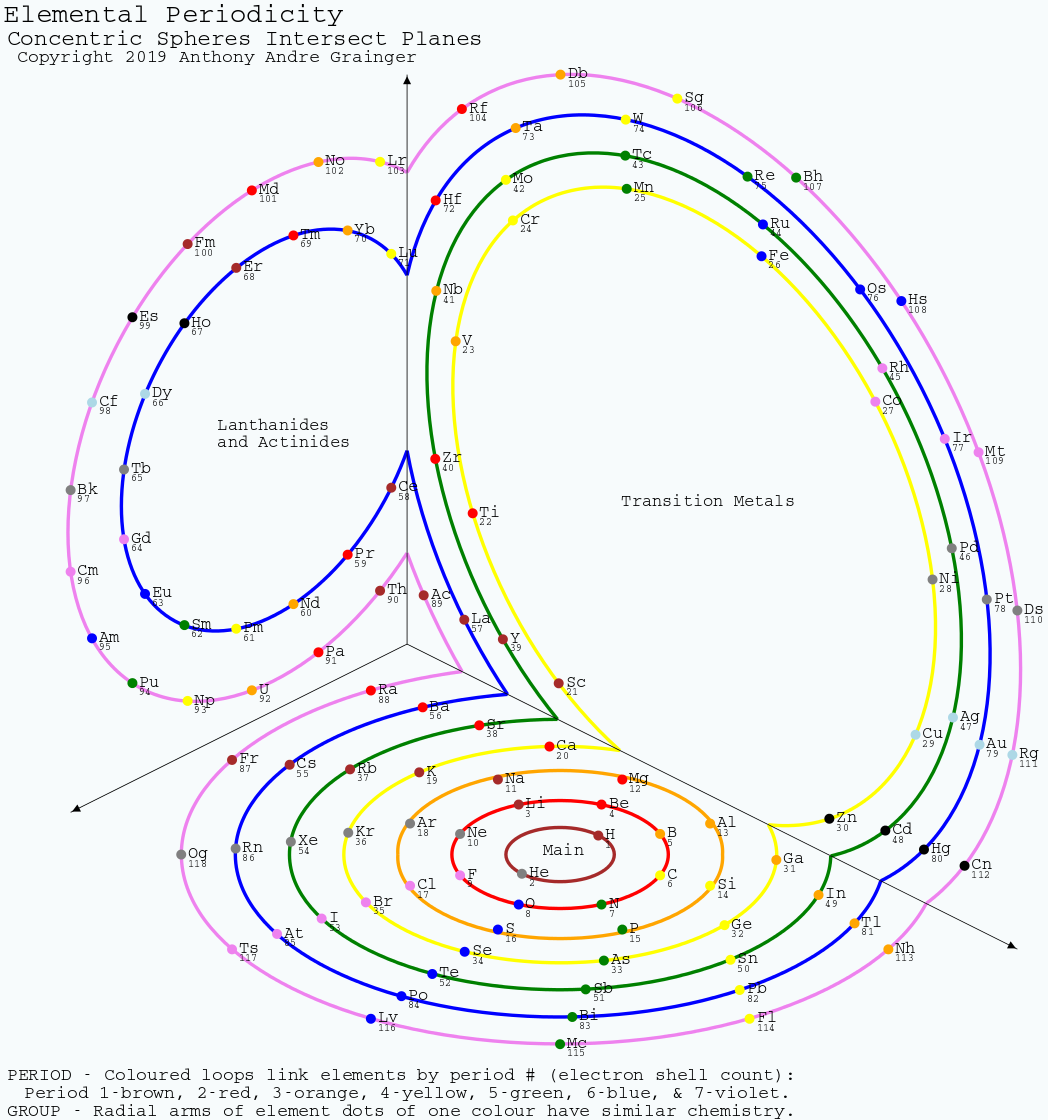
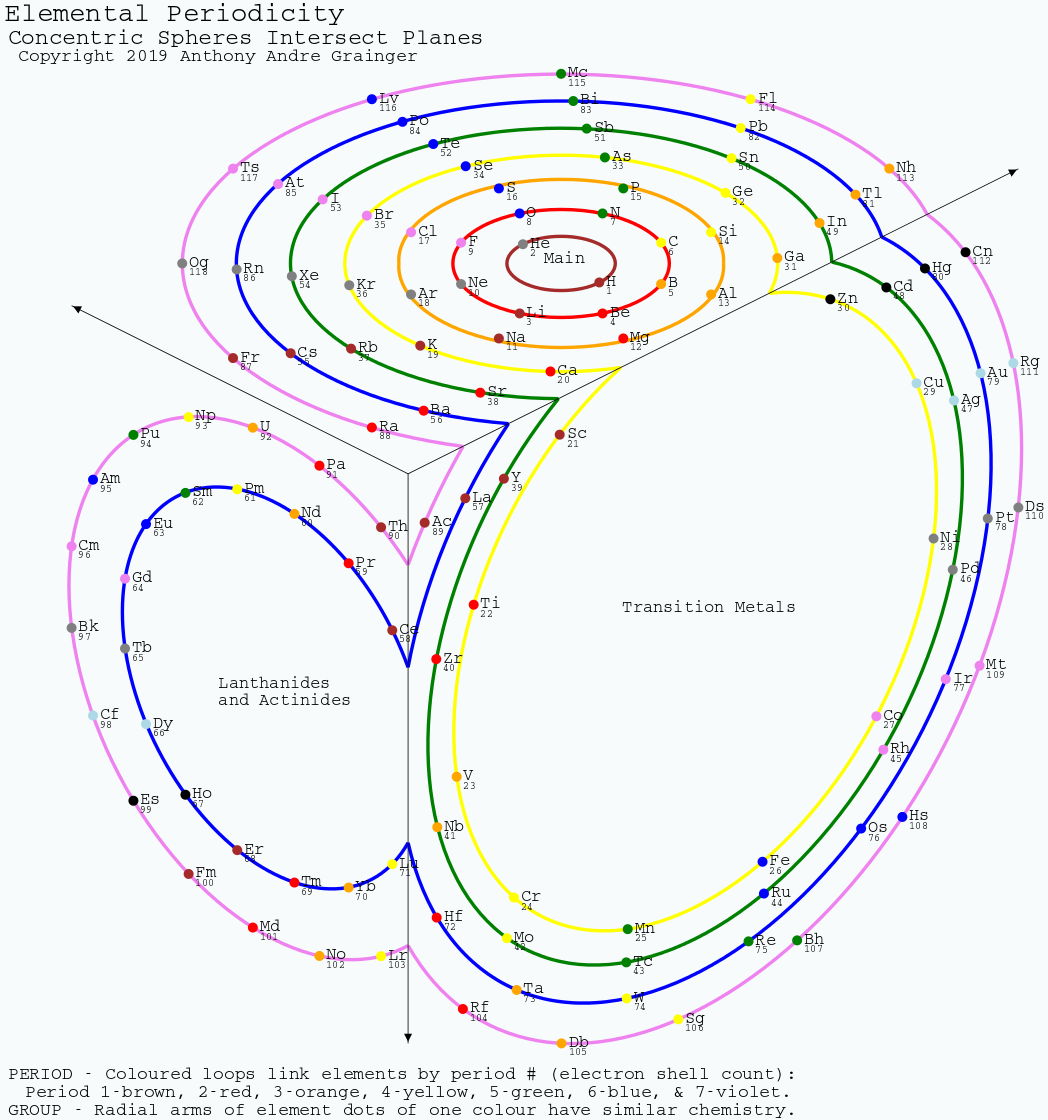
| Year: 2019 | PT id = 1004, Type = review |
St Catharine's College: Celebrating the Periodic Table
The United Nations have proclaimed 2019 to be the International Year of the Periodic Table of Chemical Elements since it is the 150th anniversary of the publication of Dmitri Mendeleev's first Periodic Table. But was it really the first?
St Catharine's College, Cambridge, in the UK, is proud to exhibit its fine collection of material relating to the early development of the Periodic Table. Starting from the first list of elements which emerged around the time of the French Revolution in the late 1780s, and the first list of atomic masses drawn up by Manchester chemist John Dalton, we explore why six different chemists from around the world each came up with their own versions of the iconic table in the 1860s.

From the RSC Website:
"Curated by periodic table superfan Peter Wothers, the main body of the exhibition is a staggering collection of historic books that trace the creation of chemistry's roadmap.
"This is an unprecedented record of the periodic table's origins, from early alchemical texts through to original copies of Antoine Lavoisier's 1789 Elementary Treatise of Chemistry – the first true list of elements – and notes on the discoveries of (among others) John Newlands, Julius Lothar Meyer through to Dmitri Mendeleev".
Celebrating the periodic table – the first edition of Mendeleev's textbook from Chemistry World on Vimeo.
| Year: 2019 | PT id = 1005, Type = formulation misc 3D spiral |
Schaltenbrand's Helical Gathering of the Elements
From the RSC Website:
"A glistering, shining spiral made of silver, gold, platinum, palladium and a diamond forms the show-stopping apex of the tribute from the University of Cambridge's St Catharine's college to the International Year of the Periodic Table.
"Commissioned to match George Schaltenbrand's 1920 design for a helical gathering of the elements – albeit extended to all 118 current elements – and signed by Yuri Oganessian, it is almost certainly the most expensive periodic table in the world."


Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1019, Type = formulation 3D |
Stewart's Quantahedron Formulation
From Philip Stewart, here & here, comes a three dimensional Quantahedron Formulation.
Philip writes:
"The Quantahedron is based on Tsimmerman's Adomah cube, realised in transparent plastic, in the usual order in which Z values are read, printed on separable blocks so that it can be assembled."
| Year: 2020 | PT id = 1316, Type = formulation |
Ziggurat Formulation
Thanks to René Vernon for finding this "Ziggurat" formulation (with a dash of Segrè Chart, upper left) on the RSC page for Oganesson:

| Year: 2020 | PT id = 1097, Type = data |
Annotated Periodic Table
From René Vernon's paper, Vernon, R.E. Organising the metals and nonmetals. Found Chem (2020). https://doi.org/10.1007/s10698-020-09356-6 (in the supplementary material).
Click image to enlarge.
| Year: 2020 | PT id = 1098, Type = review |
What Is A Chemical Element?
A Collection of Essays by Chemists, Philosophers, Historians, and Educators Edited by Eric Scerri and Elena Ghibaudi published by Oxford University Press
The concept of a chemical element is foundational within the field of chemistry, but there is wide disagreement over its definition. Even the International Union for Pure and Applied Chemistry (IUPAC) claims two distinct definitions: a species of atoms versus one which identifies chemical elements with the simple substances bearing their names. The double definition of elements proposed by the International Union for Pure and Applied Chemistry contrasts an abstract meaning and an operational one. Nevertheless, the philosophical aspects of this notion are not fully captured by the IUPAC definitions, despite the fact that they were crucial for the construction of the Periodic Table. Although rich scientific literature on the element and the periodic table exists as well as a recent growth in the philosophy of chemistry, scholars are still searching for a definitive answer to this important question: What is an element?
Eric Scerri and Elena Ghibaudi have teamed up to assemble a group of scholars to provide readers an overview of the current state of the debate on chemical elements from epistemological, historical, and educational perspectives. What Is A Chemical Element? fills a gap for the benefit of the whole chemistry community-experimental researchers, philosophers, chemistry educators, and anyone looking to learn more about the elements of the periodic table.
Foreword
Introduction
CHAPTER 1: The many questions raised by the dual concept of 'element' Eric R. Scerri
CHAPTER 2: From simple substance to chemical element Bernadette Bensaude-Vincent
CHAPTER 3: Dmitrii Mendeleev's concept of the chemical element prior to the Periodic Law Nathan M. Brooks
CHAPTER 4: Referring to chemical elements and compounds: Colourless airs in late eighteenth century chemical practice Geoffrey Blumenthal, James Ladyman, and Vanessa Seifert
CHAPTER 5: The Changing Relation Between Atomicity and Elementarity: From Lavoisier to Dalton Marina P. Banchetti-Robino
CHAPTER 6: Origins of the Ambiguity of the Current Definition of Chemical Element Joseph E. Earley
CHAPTER 7: The Existence of Elements, and the Elements of Existence Robin F. Hendry
CHAPTER 8: Kant, Cassirer, and the Idea of Chemical Element Farzad Mahootian
CHAPTER 9: The Operational Definition of the Elements: A Philosophical Reappraisal Joachim Schummer
CHAPTER 10: Substance and Function: The case of Chemical Elements Jean-Pierre Llored
CHAPTER 11: Making elements Klaus Ruthenberg
CHAPTER 12: A formal approach to the conceptual development of chemical element Guillermo Restrepo
CHAPTER 13: Chemical Elements and Chemical Substances: Rethinking Paneth's Distinction Sara N. Hjimans
CHAPTER 14: The dual conception of the chemical element: epistemic aspects and implications for chemical education Elena Ghibaudi, Alberto Regis, and Ezio Roletto
Appendix: Reference list on the philosophy of chemistry Index.

| Year: 2020 | PT id = 1104, Type = misc non-chem |
FReNeTic
FReNeTiC is the multi-Award winning 'Frenzied word game of the Elements' where players race against the clock to form as many words as possible using the Element Symbols of The Periodic Table.
In this fast and furious word game players score points equivalent to the atomic numbers of each tile used to create the word, for example Ba Na Na = Banana = 78 points.
The first player to score 1000 points wins!
Everyone plays all the time, quick set up and easy-to-follow rules with FRaNTiC FUN AcTiON! (And no, you don't need to know the Periodic Table or be a GeNiUS to play).

Thanks to Marcus for the tip!
| Year: 2020 | PT id = 1114, Type = formulation data |
Vernon's Periodic Table showing the Idealized Solid-State Electron Configurations of the Elements
René Vernon writes:
"I've attached a periodic table showing the solid-state electron configurations of the elements. Among other things, it provides a first order explanation as to why elements such as Ln (etc.) like the +3 oxidation state.
"The table includes two versions of the f-block, the first starting with La-Ac; the second with Ce-Th. The table with the first f-block version has 24 anomalies [with respect to Madelung's rule]; the table with the second f-block version has 10 anomalies.
"In the case of the Sc-Y-La-Ac form, I wonder if such a solid-state table is more relevant these days than a table based on gas phase configurations, which has about 20 anomalous configurations.
"Partly we use gas phase configurations since, as Eric Scerri mentioned to me elsewhere, configurations were first obtained (~100 years ago?) from spectroscopy, and this field primarily deals with gas phase atoms. That said, are gas phase configurations still so relevant these days – for this purpose – given the importance of solid-state physics?
"I've never been able to find a periodic table of solid-state electron configurations. Perhaps that has something to do with it? Then again, surely I'm not the first person to have drawn one of these?"
Click image below to enlarge:
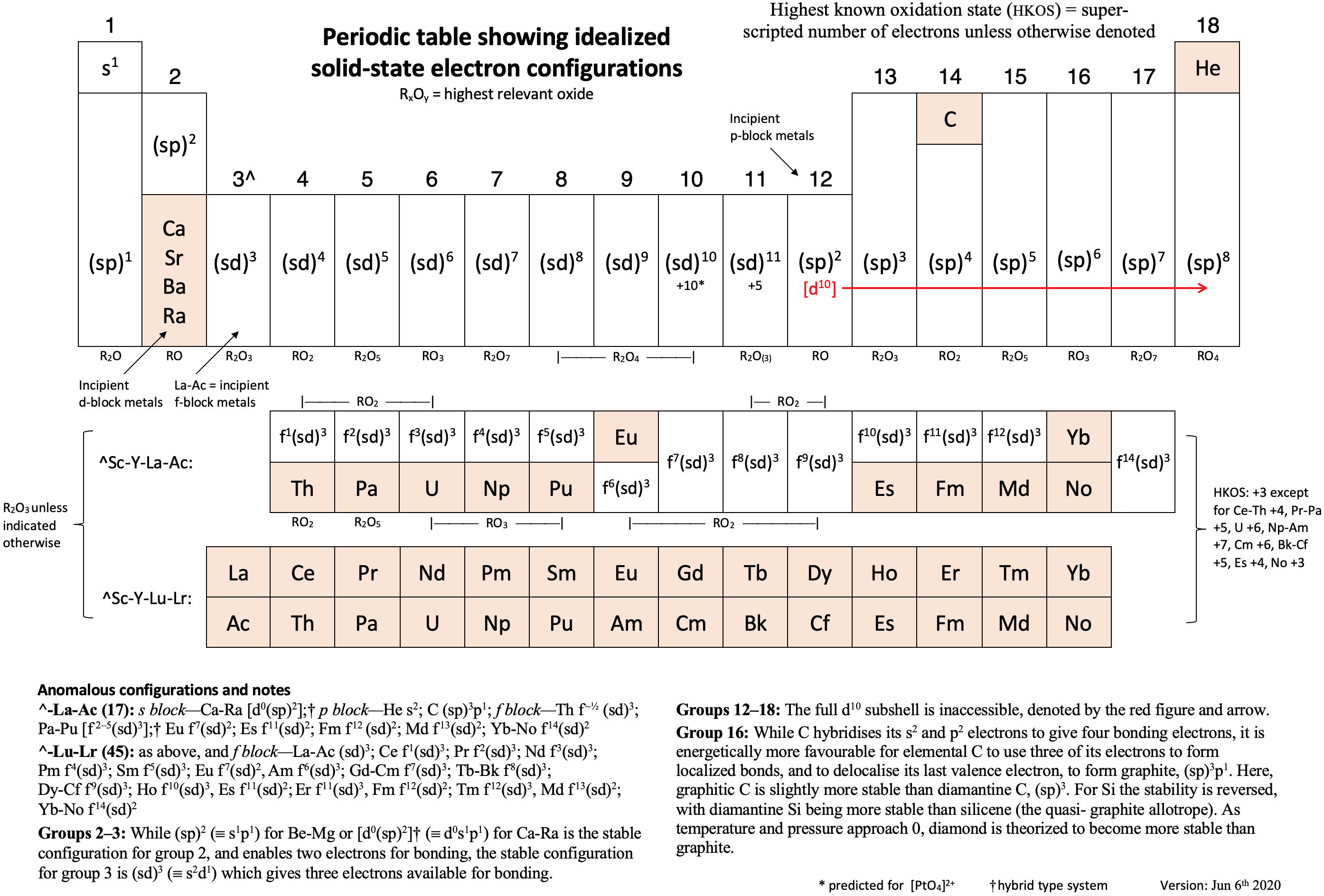
| Year: 2020 | PT id = 1117, Type = formulation data |
Correlation of Electron Affinity (F) with Elemental Orbital Radii (rorb)
From Jour. Fac. Sci., Hokkaido Univ., Ser. IV. vol. 22, no. 2, Aug., 1987, pp. 357-385, The Connection Between the Properties of Elements and Compounds; Mineralogical-Crystallochemical Classification of Elements by Alexander A. Godovikov & Yu Hariya and expanded by René Vernon who writes.
René Vernon writes:
I was delighted to read about two properties that account for nearly everything seen in the periodic table.
Two properties
While researching double periodicity, I happened upon an obscure article, which simply correlates electron affinity with orbital radius, and in so doing reproduces the broad contours of the periodic table. Having never thought much about the value or significance of EA, and its absence of easily discernible trends, I was suitably astonished. The authors left out the Ln and An and stopped at Bi. They were sitting on a gold mine but provided no further analysis.Development
I added the data up to Lr, updated the EA values, and have redrawn their graph. It is a thing of beauty and wonderment in its simplest sufficient complexity and its return on investment. I've appended 39 observations, covering all 103 elements.
Observations
Conclusion
So there it is, just two properties account for nearly everything.
Click images below to enlarge:
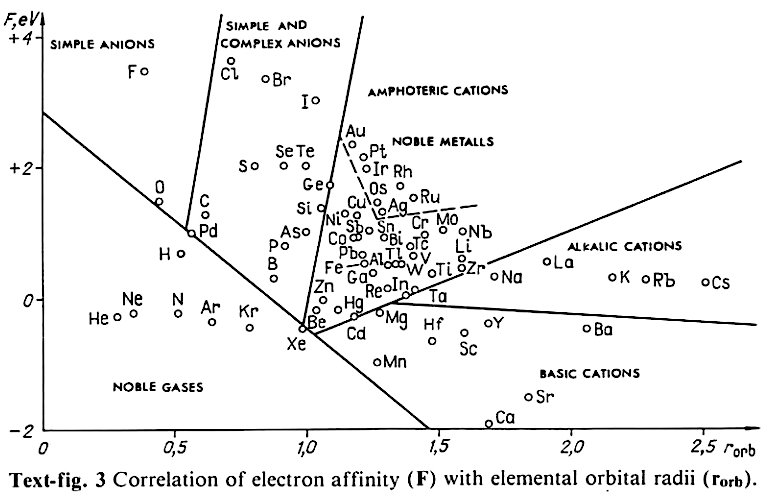
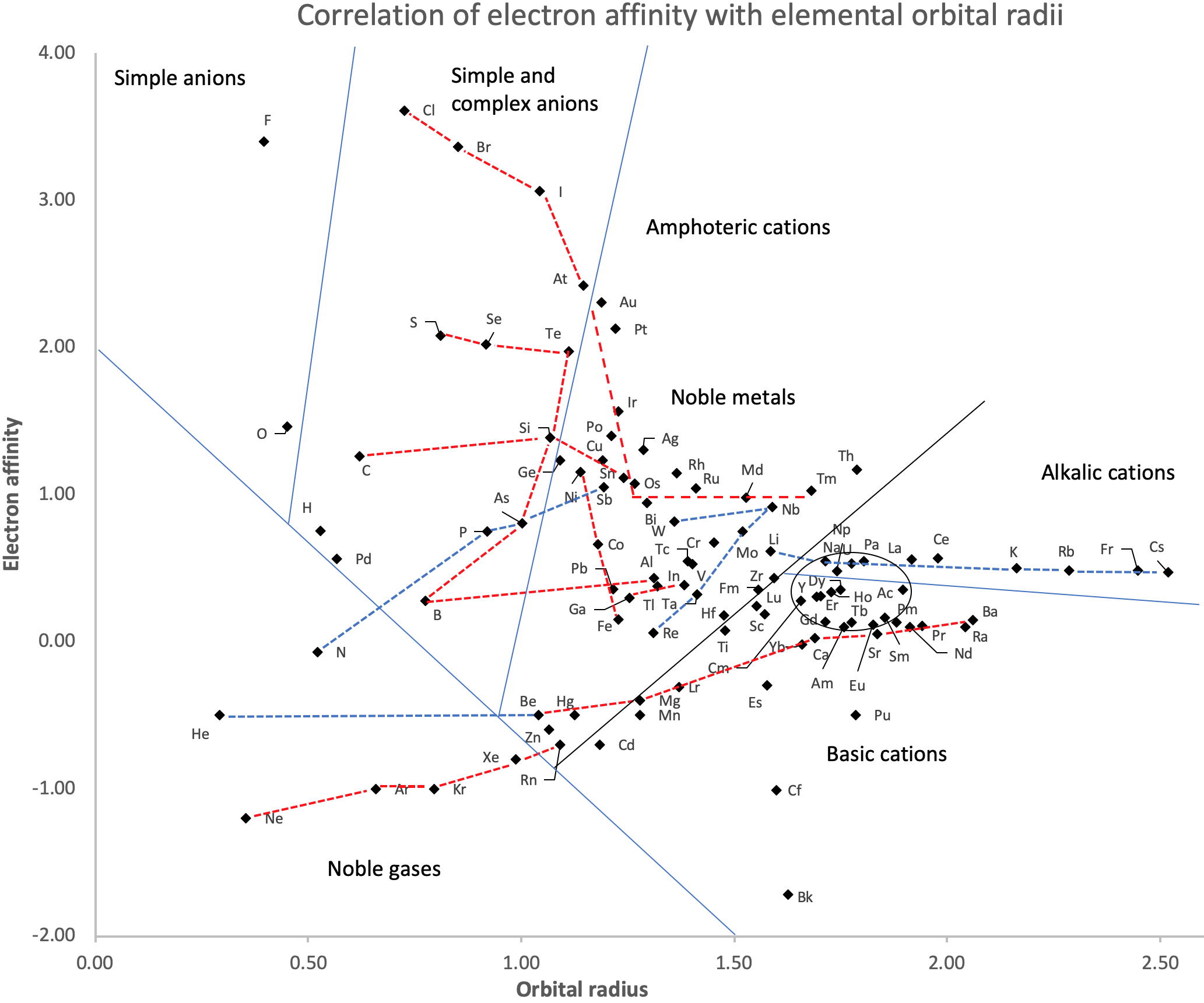
| Year: 2020 | PT id = 1122, Type = formulation data |
Vernon's Constellation of Electronegativity
René Vernon has created a "Constellation of Electronegativity" by plotting Electronegativity against Elemental Orbital Radii (rorb)
Observations on the EN plot:
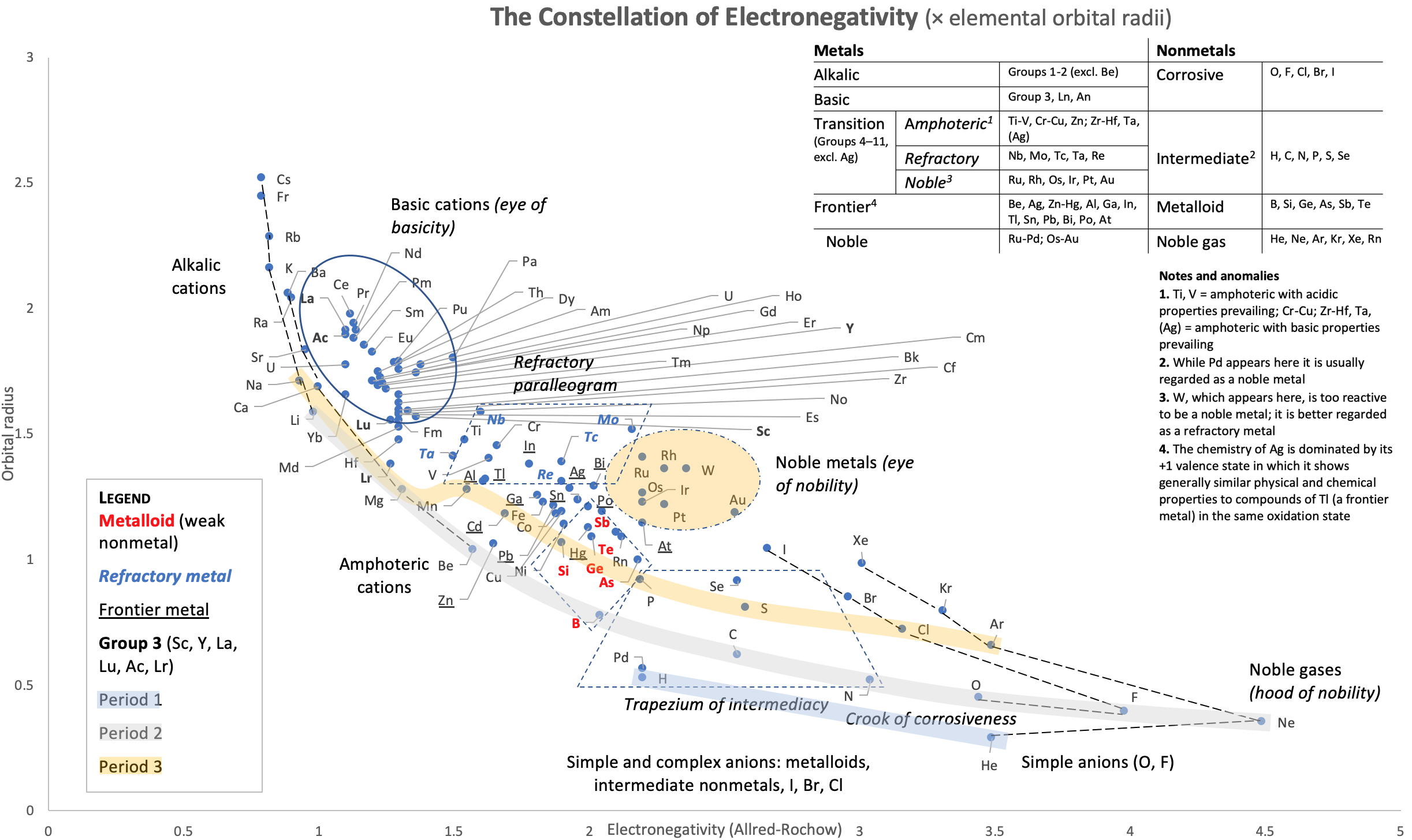
| Year: 2020 | PT id = 1131, Type = formulation |
Jodogne's Periodic Table of The Elements
Dr.Ir.Jodogne Jean Claude writes:
"I have the pleasure to send to you my paper on the PT which appears in Chimie Nouvelle 133 of the Soc.Royale de Chimie. However for the moment it is in French. The paper contains and explains the ultimate evolution of my preceding PT but it is the most scientifically based. Pedagogically, I believe it is interesting and easy. As you will see it keeps most of the chemical usual properties of the traditional one."
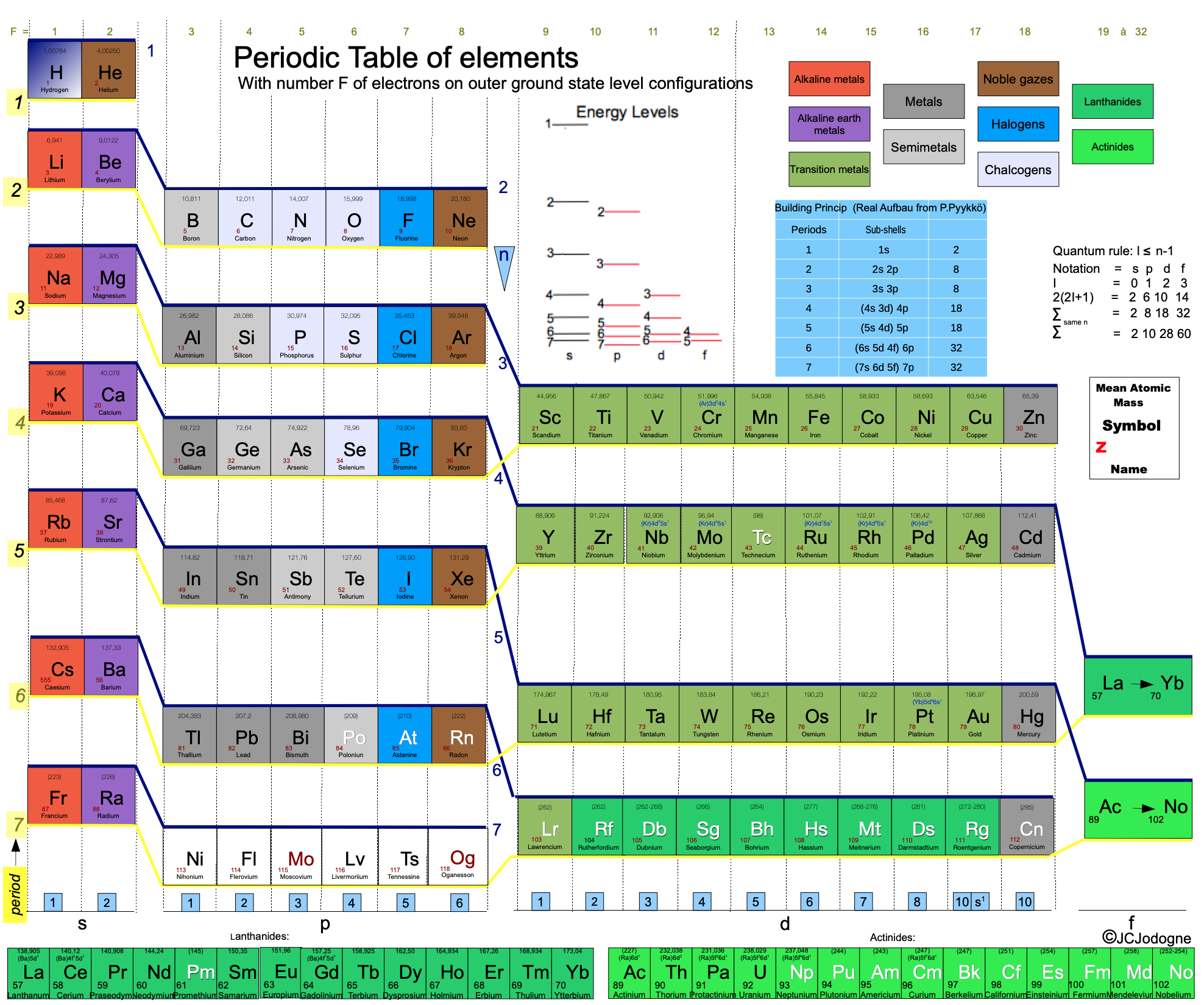
| Year: 2020 | PT id = 1147, Type = formulation |
Periodic Ziggurat of The Elements
By René Vernon, the Periodic Ziggurat of the Elements. Click to enlarge:
| Year: 2020 | PT id = 1149, Type = misc review formulation |
Scerri's Periodic Table of Books About The Periodic Table & The Chemical Elements
From Eric Scerri, a periodic table of books about the periodic table & the chemical elements... many by Eric Scerri himself.
Eric Scerri, UCLA, Department of Chemistry & Biochemistry. See the website EricScerri.com and Eric's Twitter Feed.
There is no particular connection between each of the elements and the book associated with it in the table, with the exception of: H, He, N, Ti, V, Nb, Ag, La, Au, Ac, U, Pu & Og.
The following is a list of references for each of the 118 books featured on Periodic Table of Books About The Periodic Table & The Chemical Elements. Books published in languages other than English are. They include the Catalan, Croatian, French, German, Italian, Norwegian & Spanish languages:
| 1 | H | J. Ridgen, Hydrogen, the Essential Element, Harvard University Press, Cambridge, MA, 2002. |
| 2 | He | W.M. Sears Jr., Helium, The Disappearing Element, Springer, Berlin, 2015. |
| 3 | Li | K. Lew, The Alkali Metals, Rosen Central, New York, 2009. |
| 4 | Be | S. Esteban Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009. (Spanish) |
| 5 | B | E.R. Scerri. The Periodic Table, Its Story and Its Significance, 2nd edition, Oxford University Press, New York, 2020. |
| 6 | C | U. Lagerkvist, The Periodic Table and a Missed Nobel Prize, World Scientific, Singapore, 2012. |
| 7 | N | W.B. Jensen, Mendeleev on the Periodic Law: Selected Writings, 1869–1905, Dover, Mineola, NY, 2005. |
| 8 | O | M. Kaji, H. Kragh, G. Pallo, (eds.), Early Responses to the Periodic System, Oxford University, Press, New York, 2015. |
| 9 | F | E. Mazurs, Graphic Representation of the Periodic System During One Hundred Years, Alabama University Press, Tuscaloosa, AL, 1974. |
| 10 | Ne | T. Gray, The Elements: A Visual Exploration of Every Known Atom in the Universe, Black Dog & Leventhal, 2009. |
| 11 | Na | N.C. Norman, Periodicity and the s- and p-Block Elements, Oxford University Press, Oxford, 2007. |
| 12 | Mg | M. Gordin, A Well-Ordered Thing, Dimitrii Mendeleev and the Shadow of the Periodic Table, 2nd edition, Basic Books, New York, 2019. |
| 13 | Al | S. Kean, The Disappearing Spoon, Little, Brown & Co., New York, 2010. |
| 14 | Si | P.A. Cox, The Elements, Oxford University Press, Oxford, 1989. |
| 15 | P | J. Emsley, The 13th Element: The Sordid Tale of Murder, Fire, and Phosphorus, Wiley, New York, 2002. |
| 16 | S | P. Parsons, G. Dixon, The Periodic Table: A Field Guide to the Elements, Qurcus, London, 2014. |
| 17 | Cl | P. Levi, The Periodic Table, Schocken, New York, 1995. |
| 18 | Ar | B.D. Wiker, The Mystery of the Periodic Table, Bethlehem Books, New York, 2003. |
| 19 | K | H. Alderesey-Williams, Periodic Tales, Viking Press, 2011. |
| 20 | Ca | P. Strathern, Mendeleyev's Dream, Hamish-Hamilton, London, 1999. |
| 21 | Sc | D. Scott, Around the World in 18 Elements, Royal Society of Chemistry, London, 2015. |
| 22 | Ti | E. W. Collings, Gerhard Welsch, Materials Properties Handbook: Titanium Alloys, ASM International, Geauga County, Ohio, 1994. |
| 23 | V | D. Rehder, Bioinorganic Vanadium Chemistry, Wiley-Blackwell, Weinheim, 2008. |
| 24 | Cr | K. Chapman, Superheavy, Bloomsbury Sigma, New York, 2019. |
| 25 | Mn | E.R. Scerri, E. Ghibaudi (eds.), What is an Element? Oxford University Press, New York, 2020. |
| 26 | Fe | M. Soon Lee, Elemental Haiku, Ten Speed Press, New York, 2019. |
| 27 | Co | J. Emsley, Nature's Building Blocks, An A-Z Guide to the Elements, Oxford University Press, Oxford, 2001. |
| 28 | Ni | T. James, Elemental, Robinson, London, 2018. |
| 29 | Cu | E.R. Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, New York, 2007. |
| 30 | Zn | H. Rossotti, Diverse Atoms, Oxford University Press, Oxford, 1998. |
| 31 | Ga | P. Ball, A Very Short Introduction to the Elements, Oxford University Press, 2004. |
| 32 | Ge | I. Asimov, The Building Blocks of the Universe, Lancer Books, New York, 1966. |
| 33 | As | J. Browne, Seven Elements that Changed the World, Weidenfeld and Nicholson, London, 2013. |
| 34 | Se | N. Raos, Bezbroj Lica Periodnog Sustava Elemenata, Technical Museum of Zagreb, Croatia, 2010. (Croatian) |
| 35 | Br | P. Strathern, The Knowledge, The Periodic Table, Quadrille Publishing, London, 2015. |
| 36 | Kr | A. Ede, The Chemical Element, Greenwood Press, Westport, CT, 2006. |
| 37 | Rb | A. Stwertka, The Elements, Oxford University Press, Oxford, 1998. |
| 38 | Sr | E.R. Scerri, A Tale of Seven Elements, Oxford University Press, New York, 2013. |
| 39 | Y | H.-J. Quadbeck-Seeger, World of the Elements, Wiley-VCH, Weinheim, 2007. |
| 40 | Zr | M. Fontani, M. Costa, M.V. Orna (eds.), The Lost Elements, Oxford University Press, New York, 2015. |
| 41 | Nb | M. Seegers, T. Peeters (eds.), Niobium: Chemical Properties, Applications and Environmental Effects, Nova Science Publishers, New York, 2013. |
| 42 | Mo | E.R. Scerri, Selected Papers on the Periodic Table, Imperial College Press, Imperial College Press, London and Singapore, 2009. |
| 43 | Tc | A. Dingle, The Periodic Table, Elements with Style, Kingfisher, Richmond, B.C. Canada, 2007. |
| 44 | Ru | G. Rudorf, Das periodische System, seine Geschichte und Bedeutung für die chemische Sysytematik, Hamburg-Leipzig, 1904. (German) |
| 45 | Rh | I. Nechaev, G.W. Jenkins, The Chemical Elements, Tarquin Publications, Publications, Norfolk, UK, 1997. |
| 46 | Pd | P. Davern, The Periodic Table of Poems, No Starch Press, San Francisco, 2020. |
| 47 | Ag | C. Fenau, Non-ferrous metals from Ag to Zn, Unicore, Brussells, 2002. |
| 48 | Cd | J. Van Spronsen, The Periodic System of the Chemical Elements, A History of the First Hundred Years, Elsevier, Amsterdam, 1969. |
| 49 | In | M. Tweed, Essential Elements, Walker and Company, New York, 2003. |
| 50 | Sn | M.E. Weeks, Discovery of the Elements, Journal of Chemical Education, Easton PA, 1960. |
| 51 | Sb | P. Wothers, Antimony Gold Jupiter's Wolf, Oxford University Press, Oxford, 2019. |
| 52 | Te | W. Zhu, Chemical Elements in Life, World Scientific Press, Singapore, 2020. |
| 53 | I | O. Sacks, Uncle Tungsten, Vintage Books, New York, 2001. |
| 54 | Xe | E.R. Scerri, (ed.), 30-Second Elements, Icon Books, London, 2013. |
| 55 | Cs | M. Jacob (ed.), It's Elemental: The Periodic Table, Celebrating 80th Anniversary, Chemical & Engineering News, American Chemical Society, Washington D.C., 2003. |
| 56 | Ba | J. Marshall, Discovery of the Elements, Pearson Custom Publishing, New York,1998. |
| 57 | La | K. Veronense, Rare, Prometheus Books, Amherst, New York, 2015. |
| 58 | Ce | N. Holt, The Periodic Table of Football, Ebury Publishing, London, 2016. |
| 59 | Pr | S. Alvarez, C. Mans, 150 Ans de Taules Périodiques a la Universitat de Barcelona, Edicions de la Universitat de Barcelona, Barcelona, 2019. (Catalan) |
| 60 | Nd | L. Garzon Ruiperez, De Mendeleiev a Los Superelementos, Universidad de Oviedo, Oviedo, 1988. (Spanish) |
| 61 | Pm | P. Ball, A Guided Tour of the Ingredients, Oxford University Press, Oxford, 2002. |
| 62 | Sm | S. Esteban Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009. (Spanish). |
| 63 | Eu | A. E. Garrett, The Periodic Law, D. Appleton & Co., New York, 1909. |
| 64 | Gd | M.S. Sethi, M. Satake, Periodic Tables and Periodic Properties, Discovery Publishing House, Delhi, India, 1992. |
| 65 | Tb | M. Eesa, The cosmic history of the elements: A brief journey through the creation of the chemical elements and the history of the periodic table, Createspace Independent Publishing Platform, 2012. |
| 66 | Dy | P. Depovere, La Classification périodique des éléments, De Boeck, Bruxelles, 2002. (French). |
| 67 | Ho | F. Habashi, The Periodic Table & Mendeleev, Laval University Press, Quebec, 2017. |
| 68 | Er | W.J. Nuttall, R. Clarke, B. Glowacki, The Future of Helium as a Natural Resource, Routledge, London, 2014. |
| 69 | Tm | R.D. Osorio Giraldo, M.V. Alzate Cano, La Tabla Periodica, Bogota, Colombia, 2010. (Spanish). |
| 70 | Yb | P.R. Polo, El Profeta del Orden Quimico, Mendeleiev, Nivola, Spain, 2008. (Spanish). |
| 71 | Lu | E.R. Scerri, A Very Short Introduction to the Periodic Table, 2nd edition, Oxford University Press, Oxford, 2019. |
| 72 | Hf | D.H. Rouvray, R.B. King, The Mathematics of the Periodic Table, Nova Scientific Publishers, New York, 2006. |
| 73 | Ta | P. Thyssen, A. Ceulemans, Shattered Symmetry, Oxford University Press, New York, 2017. |
| 74 | W | P.W. Atkins, The Periodic Kingdom, Basic Books, New York, NY, 1995. |
| 75 | Re | D.G. Cooper, The Periodic Table, 3rd edition. Butterworths, London, 1964. |
| 76 | Os | E. Lassner, W.-D. Schubert, Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds, Springer, Berlin, 1999. |
| 77 | Ir | J.C.A. Boeyens, D.C. Levendis, Number Theory and the Periodicity of Matter, Springer, Berlin, 2008. |
| 78 | Pt | R. Hefferlin, Periodic Systems and their Relation to the Systematic Analysis of Molecular Data, Edwin Mellen Press, Lewiston, NY, 1989. |
| 79 | Au | R.J. Puddephatt, The Chemistry of Gold, Elsevier, Amsterdam, 1978. |
| 80 | Hg | D.H. Rouvray, R.B. King, The Periodic Table Into the 21st Century, Research Studies Press, Baldock, UK, 2004. |
| 81 | Tl | R.E. Krebs, The History and Use of Our Earth's Chemical Elements, Greenwood Publishing Group, Santa Barbara, CA, 2006. |
| 82 | Pb | E. Torgsen, Genier, sjarlataner og 50 bøtter med urin - Historien om det periodiske system, Spartacus, 2018. (Norwegian). |
| 83 | Bi | K. Buchanan, D. Roller, Memorize the Periodic Table, Memory Worldwide Pty Limited, 2013. |
| 84 | Po | D. Morris, The Last Sorcerers, The Path from Alchemy to the Periodic Table, Joseph Henry Press, New York, 2003. |
| 85 | At | T. Jackson, The Elements, Shelter Harbor Press, New York, 2012. |
| 86 | Rn | R.J.P. Williams, J.J.R. Frausto da Silva, The Natural Selection of the Chemical Elements: The Environment and Life's Chemistry, Clarendon Press, Oxford, 1997. |
| 87 | Fr | G. Rudorf, The Periodic Classification and the Problem of Chemical Evolution, Whittaker & Co., London, New York, 1900. |
| 88 | Ra | L. Van Gorp, Elements, Compass Point Books, Manakato, MN, 2008. |
| 89 | Ac | G.T. Seaborg, J.J. Katz, L.R. Morss, Chemistry of the Actinide Elements, Springer, Berlin, 1986. |
| 90 | Th | G. Münzenberg, Superheavy Elements - Searching for the End of the Periodic Table, Manipal Universal Press, India, 2018. |
| 91 | Pa | A. Castillejos Salazar, La Tabla Periòdica: Abecedario de la Quimica, Universidad Autonoma de Mexico, D.F. Mexico, 2005. (Spanish). |
| 92 | U | T. Zoellner, Uranium, Penguin Books, London, 2009. |
| 93 | Np | J. Barrett, Atomic Structure and Periodicity, Royal Society of Chemistry, London, 2002. |
| 94 | Pu | J. Bernstein, Plutonium, Joseph Henry, Washington DC, 2007. |
| 95 | Am | S. Hofmann, Beyond Uranium, Taylor & Francis, London, 2002. |
| 96 | Cm | H.M. Davis, The Chemical Elements, Ballantine Books, New York, 1961. |
| 97 | Bk | P.González Duarte, Les Mils Cares de la Taula Periòdica, Universitat Autonoma de Barcelona, Bellaterra Barcelona, 2005 (Catalan). |
| 98 | Cf | R. Rich, Periodic Correlations, Benjamin, New York, 1965. |
| 99 | Es | E. Rabinowitsch, E. Thilo, Periodisches System, Geschichte und Theorie, Stuttgart, 1930. (German). |
| 100 | Fm | P.K. Kuroda, The Origin of the Chemical Elements, and the Oklo Phenomenon, Springer-Verlag, Berlin, 1982. |
| 101 | Md | G. Villani, Mendeleev, La Tavola Periodica Degli Elementi, Grandangolo, Milan, 2016. (Italian). |
| 102 | No | J. Russell, Elementary: The Periodic Table Explained, Michael O'Mara, London, 2020. |
| 103 | Lr | P. Enghag, Encyclopedia of the Elements, Wiley-VCH, Weinheim, 2004. |
| 104 | Rf | R.J. Puddephatt, The Periodic Table of the Elements, Oxford University Press, Oxford, 1972. |
| 105 | Db | L. Ohrström, The Last Alchemist in Paris, Oxford University Press, New York, 2013. |
| 106 | Sg | N.N. Greenwood, E. Earnshaw, Chemistry of the Elements, 2nd edition, Elsevier, Amsterdam, 1997. |
| 107 | Bh | R. Luft, Dictionnaire des Corps Simples de la Chimie, Association Cultures et Techniques, Nantes, 1997. (French) |
| 108 | Hs | Science Foundation Course Team, The Periodic Table and Chemical Bonding, The Open University, Milton Keynes, 1971. |
| 109 | Mt | W.W. Schulz, J. Navratil, Transplutonium Elements, American Chemical Society, Washington D.C., 1981. |
| 110 | Ds | I. Nechaev, Chemical Elements, Lindsay Drummond, 1946. |
| 111 | Rg | F. Hund, Linienspektren und Periodisches System Der Elemente, Springer, Berlin, 1927. |
| 112 | Cn | F.P. Venable, The Development of the Periodic Law, Chemical Publishing Co., Easton, PA, 1896. |
| 113 | Nh | O. Baca Mendoza, Leyes Geneticas de los Elementos Quimicos. Nuevo Sistema Periodico, Universidad Nacional de Cuzco, Cuzco, Peru, 1953 (Spanish). |
| 114 | Fl | B. Yorifuji, Wonderful Life with the Elements, No Starch Press, San Francisco, 2012. |
| 115 | Mc | D.I. Mendeléeff, The Principles of Chemistry, American Home Library, New York, 1902. |
| 116 | Lv | A. Lima-de-Faria, Periodic Tables Unifying Living Organisms at the Molecular Level: The Predictive Power of the Law of Periodicity, World Scientific Press, Singapore, 2018. |
| 117 | Ts | H.B. Gray, J.D. Simon, W.C. Trogler, Braving the Elements, University Science Books, Sausalito, CA, 1995. |
| 118 | Og | E.R. Scerri, G. Restrepo, Mendeleev to Oganesson, Oxford University Press, New York, 2018. |
| Year: 2020 | PT id = 1152, Type = review |
Rayner-Canham's The Periodic Table: Past, Present, and Future
A book by Geoff Rayner-Canham, The Periodic Table: Past, Present, and Future.
https://doi.org/10.1142/11775 | August 2020
Contents:
| Year: 2020 | PT id = 1157, Type = data |
Molar Magnetic Susceptibilities, Periodic Table of
Periodic Table of Molar Magnetic Susceptibilities by René Vernon, who writes:
I had read that the lanthanides were characterised by their magnetic properties, but never fully appreciated what this means. To this end, here is a table of Molar Magnetic Susceptibility (MMS) values (χ) for the elements, where MMS is a measure of how much a material will become magnetised in an applied magnetic field.
Formally, MMS is the ratio of magnetisation M (magnetic moment per unit volume) to the applied magnetising field of intensity H, allowing a simple classification into two categories of most materials responses to an applied magnetic field:
Alignment with the magnetic field, χ > 0, gives rise to paramagnetism
Alignment against the magnetic field, χ < 0, gives rise to diamagnetismSix observations:
1. The average value for each block is:
2. Lanthanides having unpaired 4f metals (Ce to Tm) have magnetic susceptibilities two to four orders of magnitude larger than those of "normal" metals.
3. Mn (511), Pd (540), O (3415) [this is actually the triplet diradical molecule O2] & Bi (-280) stand out. [A magnetic cross would be good for repelling a bismuth vampire.]
4. MMS reduces going down all groups of the d-block. The average reduction going from 4d to 5d is 50%.
5. In group 3 there is a reduction of 48% on going from Y to La. If Lu is instead placed under Y the reduction is 2%.
6. There are at least six, rather than three, ferromagnetic metals.
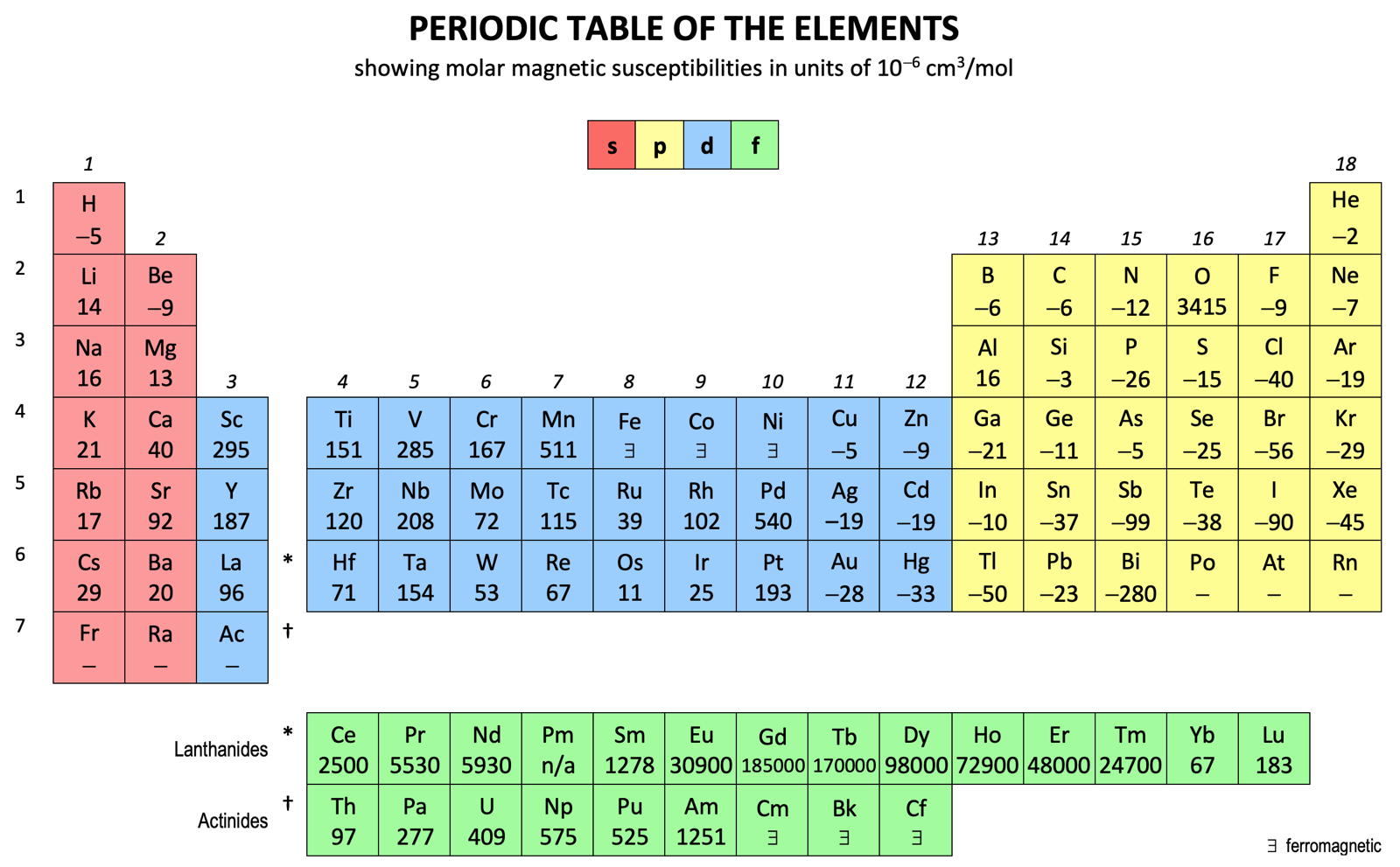
| Year: 2020 | PT id = 1161, Type = formulation |
Vernon's Periodic Treehouse
René Vernon's Periodic Treehouse of the Elements, fearuring the World's longest dividing line between metals and nonmetals.
René writes:
I can't remember what started me off on this one. It may have been Mendeleev's line, as shown on the cover of Bent's 2006 book, New ideas in chemistry from fresh energy for the periodic law.
There are a few things that look somewhat arbitrary, so I may revisit these:
| Year: 2020 | PT id = 1165, Type = formulation |
Vernon's (Partially Disordered) 15 Column Periodic Table
A formulation by René Vernon, who writes:
"Here is a 15-column table which is a hybrid of a Mendeleev 8-column table and an 18-column standard table. The key relocations are the p-block nonmetals to the far left; and the coinage and post-transition metals under their s and early d-block counterparts.
"Taking a leaf out of Mendeleev's playbook, I ignored atomic number order when this seemed appropriate. It's refreshing to see the traditional horizontal gaps between blocks disappear. (DIM did not like these.)
"Since Dias (2004, see references below) reckoned a periodic table is a partially ordered set forming a two-dimensional array, I believe I now have a partially ordered table that is partially disordered twice over.
"The table has some curious relationships. Equally, some relationships seen in the standard form are absent. The Group 2, 3, and aluminium dilemmas disappear. This confirms my impression that such dilemmas have no intrinsic meaning. Rather, their appearance or non-appearance is context dependent."
Notes & references below.
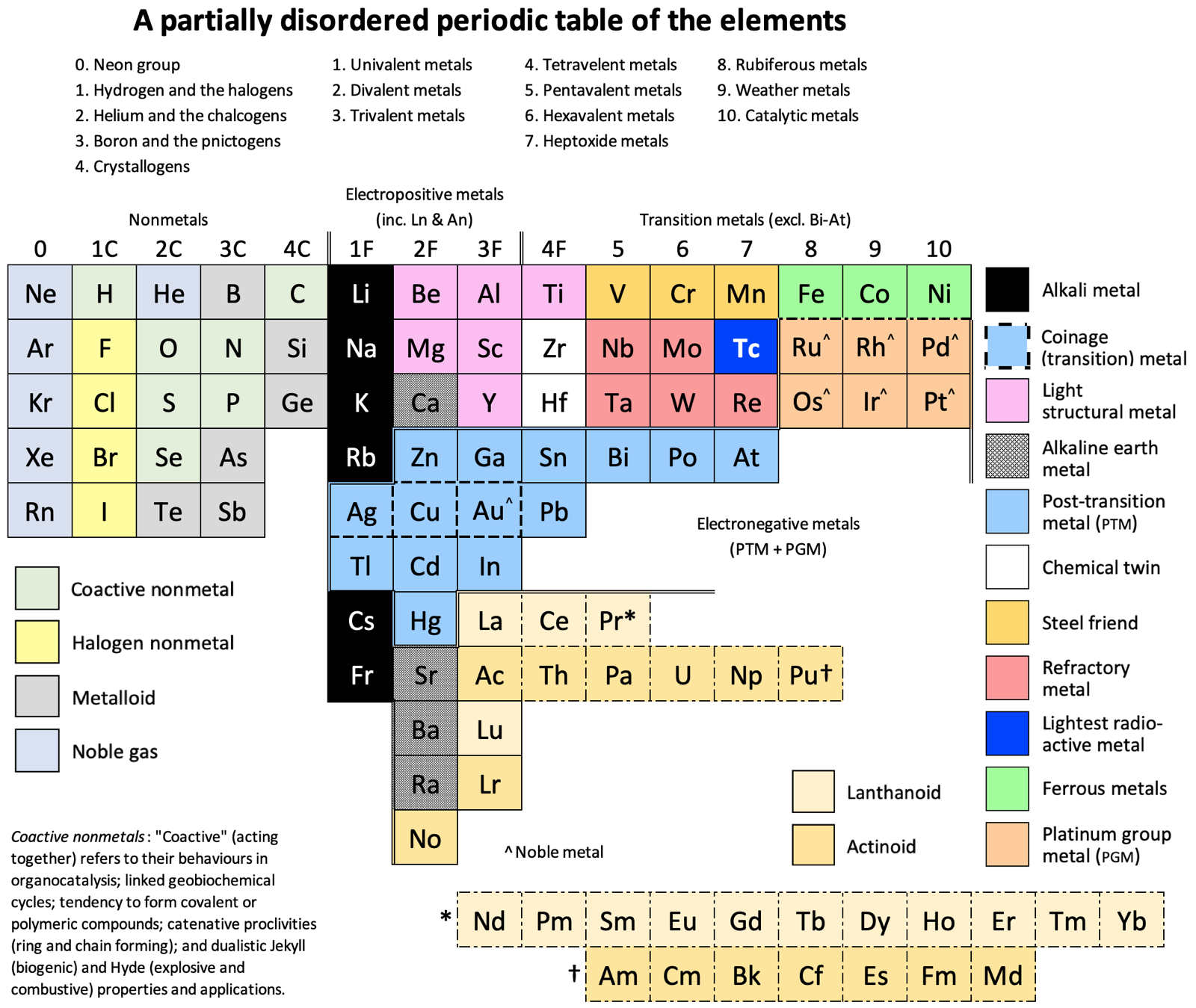
Groups 1 to 4 have either a C or F suffix where C (nonmetal) is after the importance of carbon to our existence; and F (metal) is for the importance of iron to civilisation.
Groups 1C and 1F present the greatest contrast in nonmetallic and metallic behaviour.
Coactive Nonmetals: They are capable of forming septenary heterogeneous compounds such as C20H26N4O10PSSe.
Group 2C: Helium is shaded as a noble gas. "Heliox" is a breathing gas mixture of helium and oxygen used in saturation diving, and as a medical treatment for patients with difficulty breathing.
Group 3C: Boron over nitrogen looks odd. Yet one boron atom and one nitrogen atom have the same number of electrons between them as two adjacent carbon atoms. The combination of nitrogen and boron has some unusual features that are hard to match in any other pair of elements (Niedenzu & Dawson 1965).
Boron and phosphorus form a range of ring and cage compounds having novel structural and electronic properties (Paine et al. 2005).
Metalloids. I treat them here as nonmetals given their chemistry is predominately that of chemically weak nonmetals.
Metals: The labels electropositive; transition; and electronegative are adapted from Kornilov (2008).
Group 1F: Monovalent thallium salts resemble those of silver and the alkali metals.
An alloy of cesium (73.71%), potassium (22.14%) and sodium (4.14%) has a melting point of –78.2°C (–108.76°F) (Oshe 1985).
Silver, copper, and gold, as well as being the coinage metals, are borderline post-transition metals.
Group 2F: Beryllium and magnesium are not in fact alkaline earths. Beryllium is amphoteric rather than alkaline; magnesium was isolated in impure form from its oxides, unlike the true alkaline earths. The old ambiguity over whether beryllium and magnesium should go over calcium or zinc has gone.
Nobelium is here since +2 is its preferred oxidation state, unlike other actinoids.
Group 3F: Aluminium is here in light of its similarity to scandium (Habishi 2010).
InGaAsP is a semiconducting alloy of gallium arsenide and indium phosphide, used in lasers and photonics.
There is no Group 3 "issue" since lanthanum, actinium, lutetium and lawrencium are in the same family.
Gold and aluminium form an interesting set of intermetallic compounds known as Au5Al2 (white plague) and AuAl2 (purple plague). Blue gold is an alloy of gold and either gallium or indium.
Lanthanoids: The oxidation state analogies with the transition metals stop after praseodymium. That is why the rest of lanthanoids are footnoted in dash-dot boxes.
Actinoids: The resemblance to their transition metal analogues falters after uranium, and peters out after plutonium.
Group 4F: It's funny to see titanium—the lightweight super-metal—in the same group as lead, the traditional "heavy" metal.
This is the first group impacted by the lanthanoid contraction (cerium through lutetium) which results in the atomic radius of hafnium being almost the same as that of zirconium. Hence "the twins".
The chemistry of titanium is significantly different from that of zirconium and hafnium (Fernelius 1982).
Lead zirconate titanate Pb[ZrxTi1–x]O3 (0 ≤ x ≤ 1) is one of the most commonly used piezo ceramics.
Group 5: Bismuth vanadate BiVO4 is a bright yellow solid widely used as a visible light photo-catalyst and dye.
Steel Friends: The name is reference to their use in steel alloys. They have isoelectronic soluble oxidizing tetroxoanions, plus a stable +3 oxidation state. (Rayner-Canham 2020).
Ferromagnetic Metals: The horizontal similarities among this triad of elements (as is the case among the PGM hexad) are greater than anywhere in the periodic table except among the lanthanides (Lee 1996). The +2 aqueous ion is a major component of their simple chemistry (Rayner-Canham 2020).
Group 8: "Rubiferous metals" (classical Latin rubēre to be red; -fer producing) is from the reddish-brown colour of rust; the most prevalent ruthenium precursor being ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically; and the red osmates [OsO4(OH)4]?2 formed upon reaction by osmium tetroxide with a base.
Group 9: "Weather metals" comes from the use of cobalt chloride as a humidity indicator in weather instruments; rhodium plating used to "protect other more vulnerable metals against weather exposure as well as against concentrated acids or acids fumes" (Küpfer 1954); and the "rainbow" etymology of iridium.
Group 10: "Catalytic metals" is after a passage in Greenwood and Earnshaw, "They are... readily obtained in finely divided forms which are catalytically very active." (2002). Of course, many transition metals have catalytic properties. That said, if you asked me about transition metal catalysts, palladium and platinum would be the first to come to mind. Group 10 appear to be particularly catalytic.
References:
| Year: 2020 | PT id = 1168, Type = formulation |
Shukarev's Periodic System (redrawn by Vernon)
Shukarev SA 1975, "On the image of the periodic system with the use of fifth move of late a-elements", Collection of Scientific and Methodological Articles on Chemistry. M.: Higher School, no 4, pp 3-12 (in Russian). Redrawn and commented upon by René Vernon:
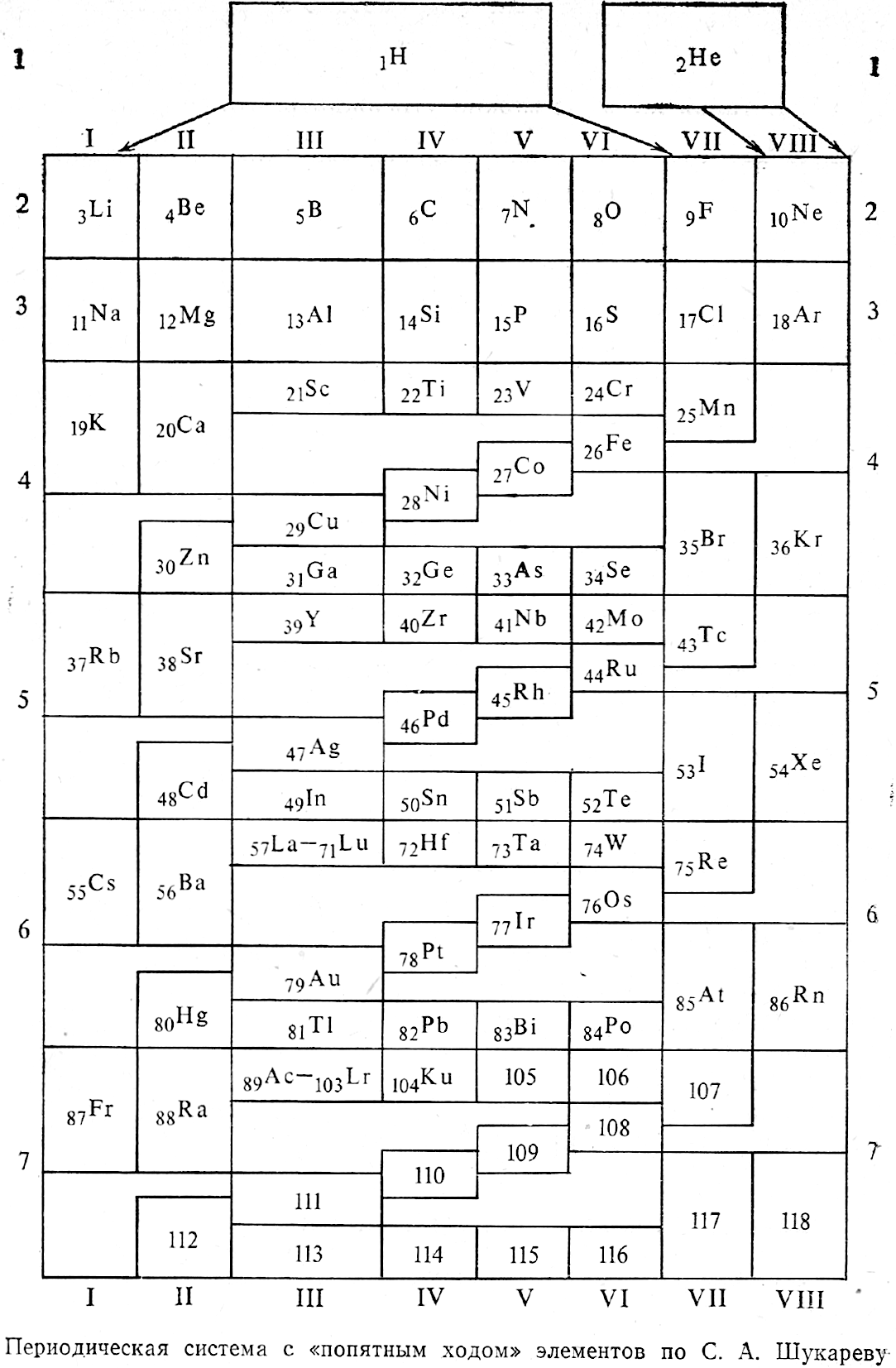
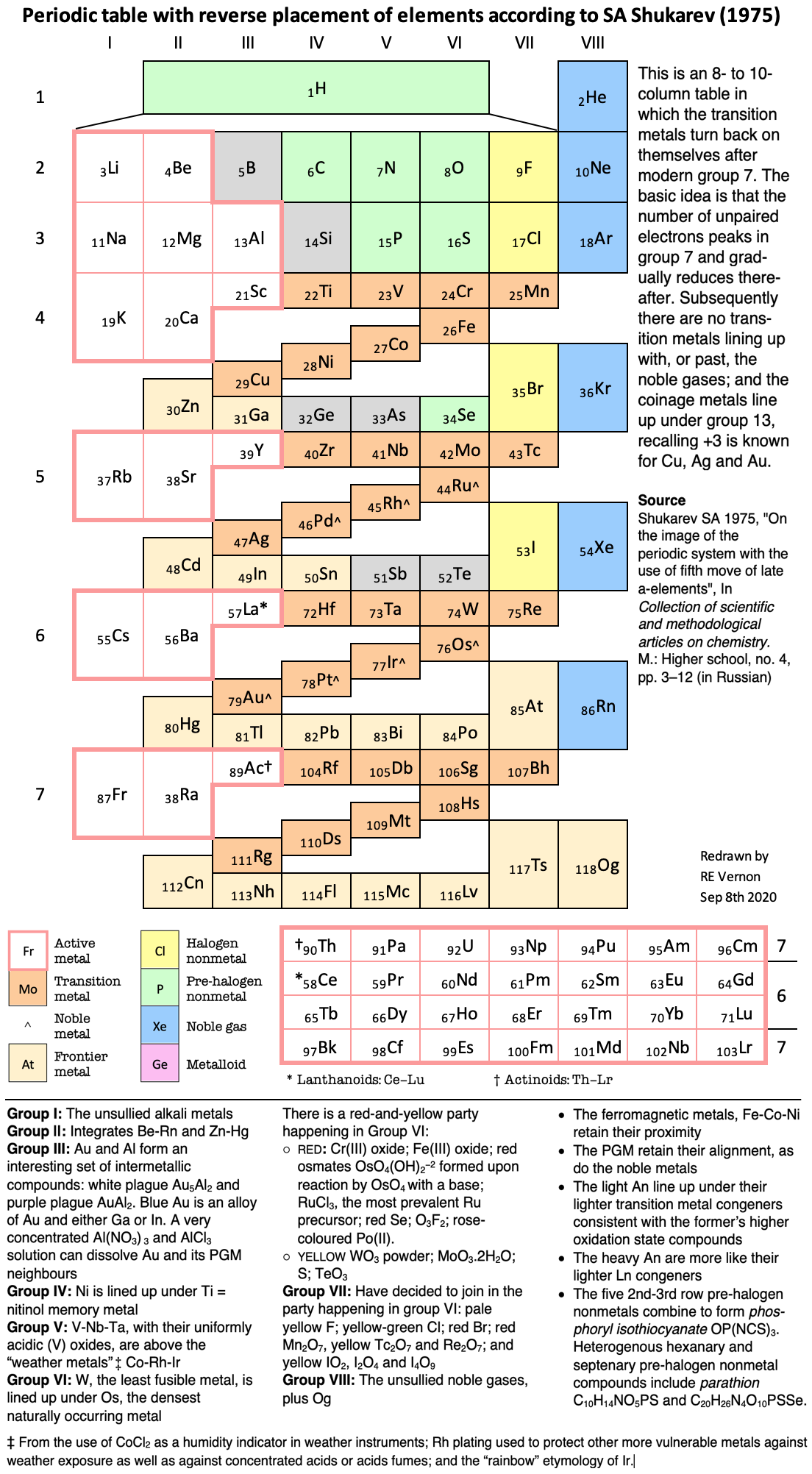
| Year: 2020 | PT id = 1169, Type = formulation |
Allahyari & Oganov: Mendeleev Numbers & Organising Chemical Space
This formulation may not look like a periodic table, but look again.
Zahed Allahyari & Artem R. Oganov, Nonempirical Definition of the Mendeleev Numbers: Organizing the Chemical Space, J. Phys. Chem. C 2020, 124, 43, 23867–23878, https://doi.org/10.1021/acs.jpcc.0c07857. A preprint version of the paper is available on the arxiv preprint server.
Abstract:
Organizing a chemical space so that elements with similar properties would take neighboring places in a sequence can help to predict new materials. In this paper, we propose a universal method of generating such a one-dimensional sequence of elements, i.e. at arbitrary pressure, which could be used to create a well-structured chemical space of materials and facilitate the prediction of new materials.
This work clarifies the physical meaning of Mendeleev numbers, which was earlier tabulated by Pettifor. We compare our proposed sequence of elements with alternative sequences formed by different Mendeleev numbers using the data for hardness, magnetization, enthalpy of formation, and atomization energy. For an unbiased evaluation of the MNs, we compare clustering rates obtained with each system of MNs.
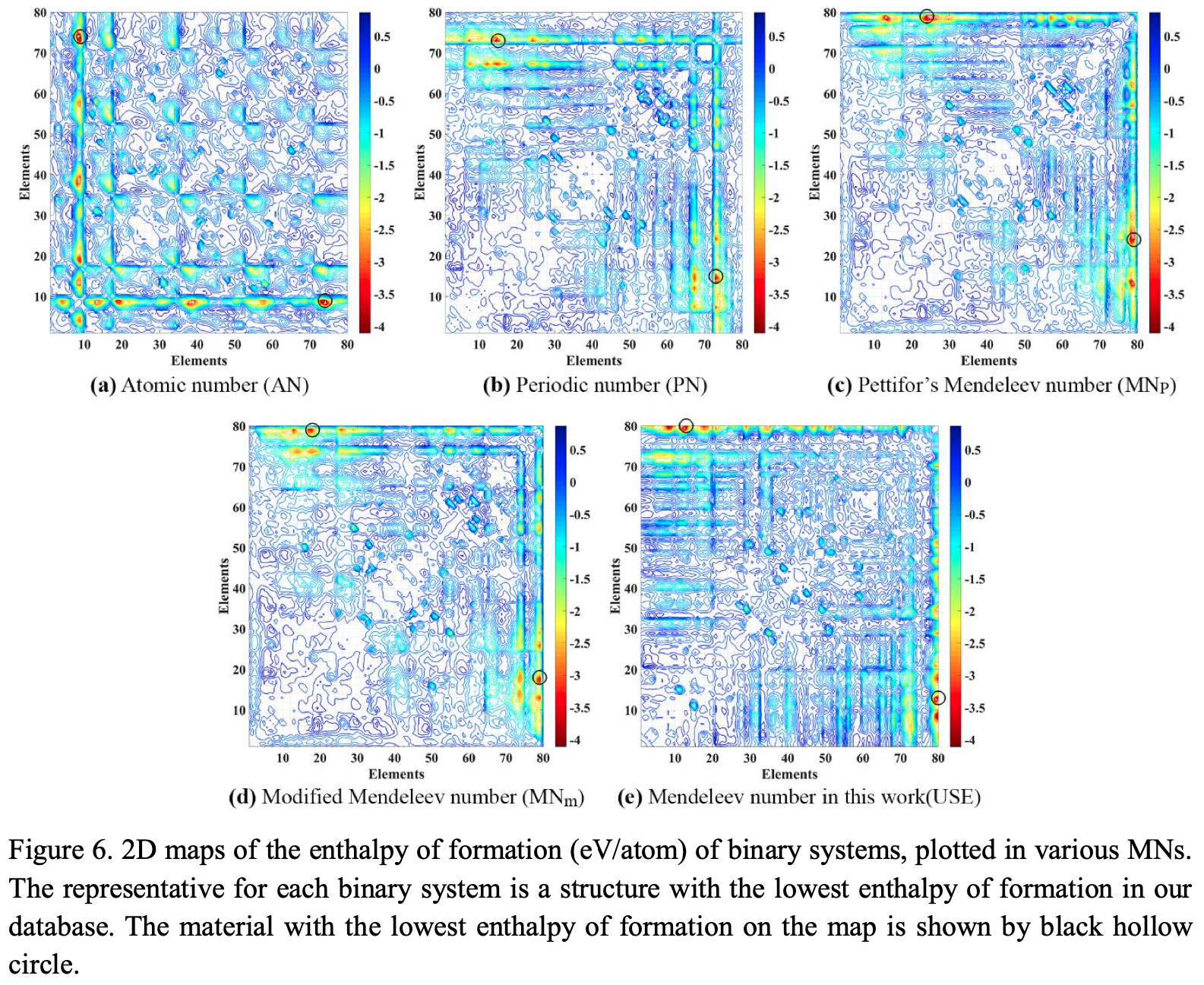
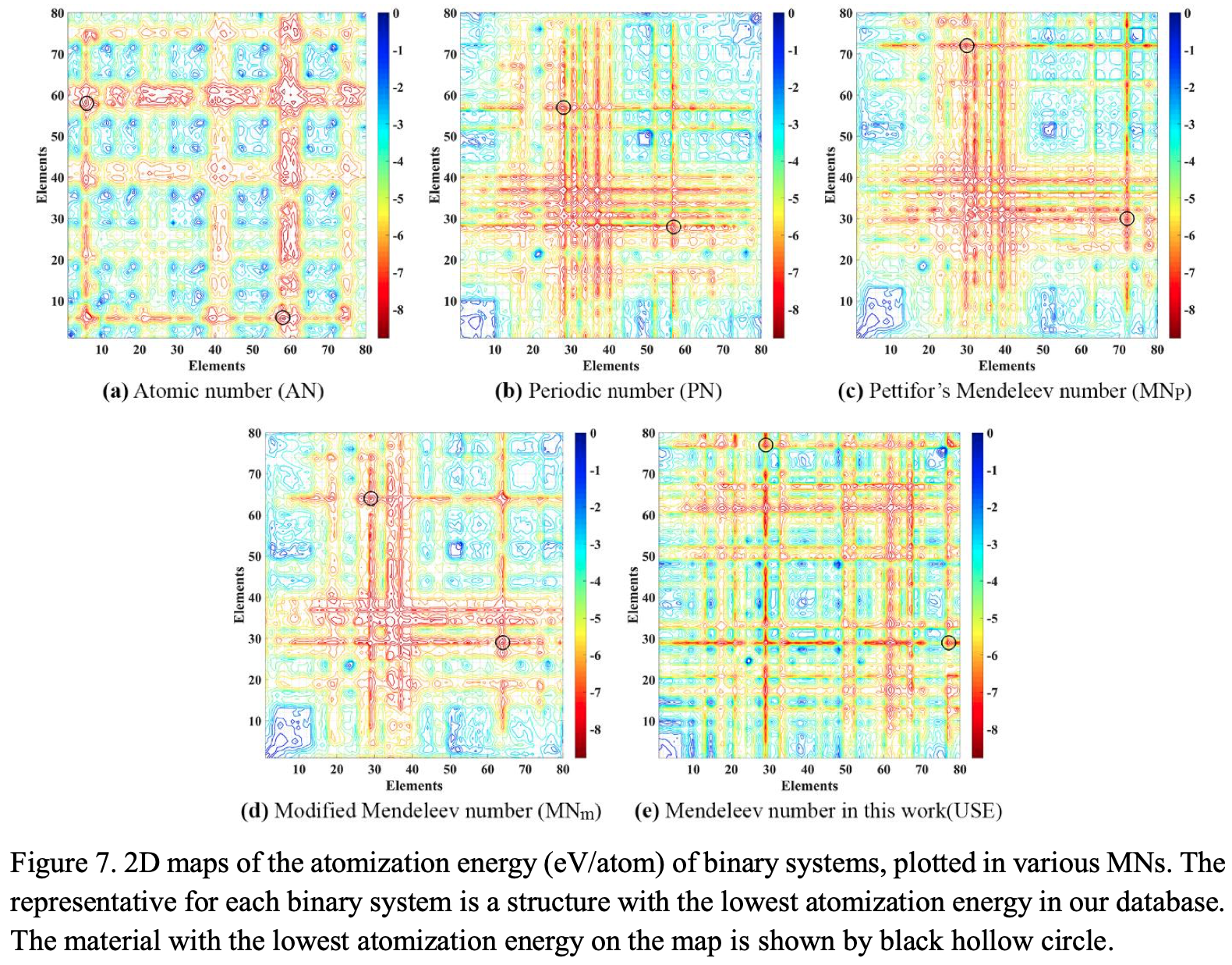
| Year: 2020 | PT id = 1170, Type = formulation |
Zig-Zag Line, Periodic Table
Periodic Table showing the (regular) zig-zag line by René Vernon who writes:
"It is curious that the full extent of the line has never been properly mapped (to my knowledge).
"Elements on the downside of the line generally display increasing metallic behaviour; elements on the topside generally display increasing nonmetallic behaviour.
"When you see the line you will usually see only about a quarter of it. The line actually runs all the way across the periodic table, as shown, for a total of 44 element box sides.
"Interpretations vary as to where the line runs. None of these is better than any other of them, provided the interpretation is explained to you. The thick black line (at least in the p-block) is the most common version. The metalloids tend to lie to either side of it.
"Polonium and astatine are shown here as post-transition metals although either or both of them are sometimes shown as metalloids (or, in the case of astatine, as a halogen). Polonium conducts electricity like a metal and forms a cation in aqueous solution. In 2013, astatine was predicted to be a centred cubic-metal Condensed Astatine: Monatomic and Metallic This prediction has been cited 35 times, with no dissenters. Astatine also forms a cation in aqueous solution. Oganesson is shown as having (as yet) unknown properties.
"The dashed lines show some alternative paths for the zigzag line.
"The lower one treats the metalloids as nonmetals since metalloid chemistry is predominately nonmetallic. The lower line and the upper line are sometimes shown together used when the metalloids are treated as neither metals nor nonmetals."
And in Janet Left-Step form:
| Year: 2020 | PT id = 1171, Type = data formulation |
16 Dividing Lines Within The Periodic Table
René Vernon points out that there are 16 dividing lines within the periodic table.
A-Z Dividing Lines:
48-crash line: Named after the dramatic reduction in physical metallic character after group 11, Cd being Z = 48. Group 12 show few transition metal attributes and behave predominantly like post-transition metals.
Big bang line: H makes up about 73% of the visible universe.
Corrosive line: O, F, Cl = most corrosive nonmetals.
d-Block fault line: Group 3 show little d-block behaviour; group 4 is the first in which characteristic d-block behaviour occurs.
Deming line: Demarcates the metalloids from the pre-halogen nonmetals. The "reactive" nonmetals to the right of the metalloids each have a sub-metallic appearance (C, O, Se, I).
Edge of the world line: No guesses for this one.
Klemm line: Klemm, in 1929, was the first to note the double periodicity of the lanthanides (Ce to Lu). Lockyer line: After the discoverer of He, the first element not found on Earth.
Ørsted line: After the magnetic effects believed to be responsible for Mn having a crystalline structure analogous to white P; Tc: First radioactive metal; Re: Last of the refractory metals; "most radioactive" of the naturally occurring elements with stable isotopes. Fe: First of the ferromagnetic metals; Ru: First noble metal; Os: Densest of naturally occurring metals. The number of unpaired d electrons peaks in group 7 and reduces thereafter.
Platypus line: Tl shows similarities to Rb, Ag, Hg, Pb.
Poor metal line: Most metals (80%) have a packing factor (PF)3 68%. Ga: Has a crystalline structure analogous to that of iodine. BCN 1+6.* PF 39.1%. Melts in your hand. In: Partly distorted structure due to incompletely ionised atoms. BCN 4+8. PE 68.6%. Oxides in preferred +3 state are weakly amphoteric; forms anionic indates in strongly basic solutions. Tendency to form covalent compounds is one of the more important properties influencing its electro-chemical behaviour. Sn: Irregularly coordinated structure associated with incompletely ionised atoms. BCN 4+2. PF 53.5%. Oxides in preferred +2 state are amphoteric; forms stannites in strongly basic solutions. Grey Sn is electronically a zero band gap semimetal, although it behaves like a semiconductor. Diamond structure. BCN 4. PF 34.0%. Pb: Close-packed, but abnormally large inter-atomic distance due to partial ionisation of Pb atoms. BCN 12. PF 74%. Oxide in preferred +2 state is amphoteric; forms anionic plumbates in strongly basic solutions. Bi: Electronic structure of a semimetal. Open-packed structure (3+3) with bonding intermediate between metallic and covalent. PF 44.6%. Trioxide is predominantly basic but will act as a weak acid in warm, very concentrated KOH. Can be fused with KOH in air, resulting in a brown mass of potassium bismuthate.
Seaborg line: No f electrons in gas phase La, Ac and Th atoms.
Triple line: N = gas; S = solid; Br = liquid.
Zigzag lobby: H needs no intro. Li: Many salts have a high degree of covalency. Small size frequently confers special properties on its compounds and for this reason is sometimes termed 'anomalous'. E.g. miscible with Na only above 380° immiscible with molten K, Rb, Cs, whereas all other pairs of AM are miscible with each other in all proportions. Be: Has a covalent component to its otherwise predominately metallic structure = low ductility. Lowest known Poisson's ratio of elemental metals. Amphoteric; predominately covalent chemistry atypical of group 2. Some aspects of its chemical properties are more like those of a metalloid.
Zigzag line: Eponymous metal-nonmetal dividing line.
Zintl line: Hypothetical boundary highlighting tendency for group 13 metals to form phases with a various stoichiometries, in contrast to group 14+ that tend to form salts with polymeric anions.
* BCN = bulk coordination number
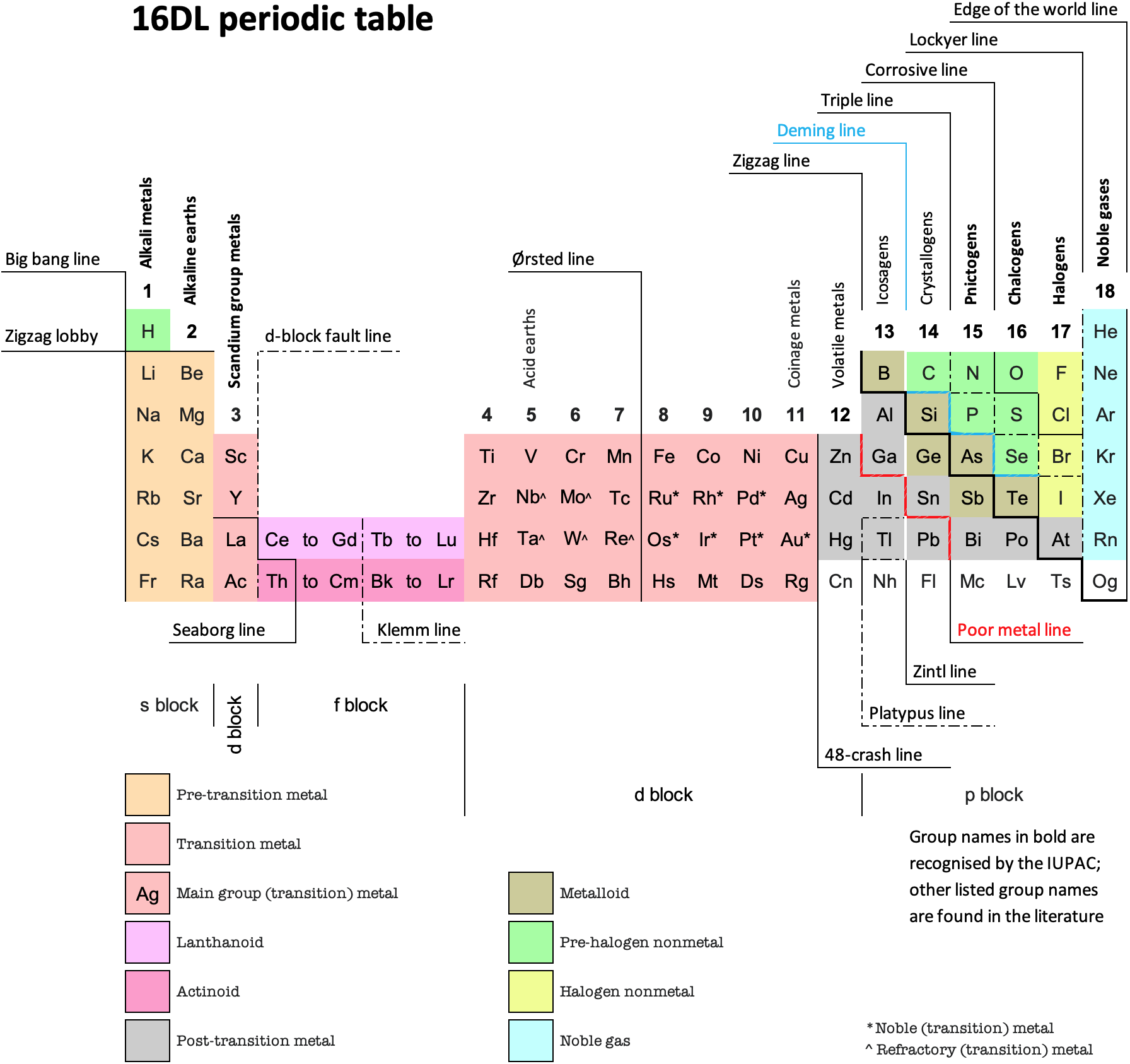
| Year: 2020 | PT id = 1174, Type = formulation |
Split s-, p- & d-Block Periodic Table
René Vernon presents a periodic table formulation with split s-, p- & d-blocks.
The details: Group 3 as B-Al-Ga-In-Tl
Al over Sc has some history, which seems to have been forgotten.
Here are some other tables with B-Al-Sc-Y-La:
What was it that these luminaries knew about B-Al-Sc-Y-La-Ac that is deemed to be no longer relevant, and why is that the case?
Deming (1947, Fundamental Chemistry, 2nd ed. p. 617) located Al with the pre-transition metals in groups 1?2. Cox (2004, Inorganic Chemistry, 2nd ed. p. 185) refers to the pre-transition metals as those in groups 1 and 2, and Al. Here's that 2019 periodic table (by me), recording oxidation number trends, further suggesting B and Al are better placed over Sc.
In this vein, Rayner-Canham (2020, The periodic table: Past, present, and future, pp. 178–181) writes:
"It was Rang in 1893 who seems to have been the first, on the basis of chemical similarity, to place boron and aluminum in Group 3.
"Such an assignment seems to have been forgotten until more recent times. Greenwood and Earnshaw have discussed the way in which aluminum can be considered as belonging to Group 3 as much as to Group 13 particularly in its physical properties. Habashi has suggested that there are so many similarities between aluminum and scandium that aluminum's place in the Periodic Table should actually be shifted to Group 3.
"In terms of the electron configuration of the tripositive ions, one would indeed expect that Al3+ (electron configuration, [Ne]) would resemble Sc3+ (electron configuration, [Ar]) more than Ga3+ (electron configuration, [Ar]3d10). Also of note, the standard reduction potential for aluminum fits better with those of the Group 3 elements than the Group 13 elements (Table 9.2) – as does its melting point.
"In terms of their comparative solution behavior, aluminum resembles both scandium(III) and gallium(III). For each ion, the free hydrated cation exists only in acidic solution. On addition of hydroxide ion to the respective cation, the hydroxides are produced as gelatinous precipitates. Each of the hydroxides redissolve in excess base to give an anionic hydroxo-complex, [M(OH)4]–... There does seem to be a triangular relationship between these three elements. However, aluminum does more closely resemble scandium rather than gallium in its chemistry. If hydrogen sulfide is bubbled through a solution of the respective cation, scandium ion gives a precipitate of scandium hydroxide, and aluminum ion gives a corresponding precipitate of aluminum hydroxide. By contrast, gallium ion gives a precipitate of gallium(III) sulfide. Also, scandium and aluminum both form carbides, while gallium does not."
To answer my own question as to why group 3 as B-Al-Sc-Y-La-Ac has been forgotten.
I suspect what happened is that it was historically known that group 3 was better represented as B-Al-Sc-Y-La-[Ac]. Then, with the advent and rise of modern electronic structure theory, B-Al- got moved to the p-block because, after all, they were p-block elements, never mind the damned chemistry. And La stayed in the d-block since it was the first element to show 5d electron, and 4f did not show until Ce. And Lu stayed where it was since even thought it was learnt that the f shell become full at Yb, rather than Lu, nothing changed about the chemistry of Lu. Nowadays, this has all been forgotten.
The modern periodic table is a chemistry-physics hybrid.
Lu in group 3 demands He over Be. La in group 3 demands B-Al over Sc. Neither option gets up. The more important consideration is to teach the history and have students and chemists appreciate both perspectives.
| Year: 2021 | PT id = 1181, Type = formulation review |
Understanding Periodic and Non-periodic Chemistry in Periodic Tables
Cao C, Vernon RE, Schwarz WHE and Li J (2021). Front. Chem. 8:813. https://doi.org/10.3389/fchem.2020.00813
Abstract:
The chemical elements are the "conserved principles" or "kernels" of chemistry that are retained when substances are altered. Comprehensive overviews of the chemistry of the elements and their compounds are needed in chemical science. To this end, a graphical display of the chemical properties of the elements, in the form of a Periodic Table, is the helpful tool. Such tables have been designed with the aim of either classifying real chemical substances or emphasizing formal and aesthetic concepts. Simplified, artistic, or economic tables are relevant to educational and cultural fields, while practicing chemists profit more from "chemical tables of chemical elements."
Such tables should incorporate four aspects:
(i) typical valence electron configurations of bonded atoms in chemical compounds (instead of the common but chemically atypical ground states of free atoms in physical vacuum);
(ii) at least three basic chemical properties (valence number, size, and energy of the valence shells), their joint variation across the elements showing principal and secondary periodicity;
(iii) elements in which the (sp)8, (d)10, and (f)14 valence shells become closed and inert under ambient chemical conditions, thereby determining the "fix-points" of chemical periodicity;
(iv) peculiar elements at the top and at the bottom of the Periodic Table.
While it is essential that Periodic Tables display important trends in element chemistry we need to keep our eyes open for unexpected chemical behavior in ambient, near ambient, or unusual conditions. The combination of experimental data and theoretical insight supports a more nuanced understanding of complex periodic trends and non-periodic phenomena.
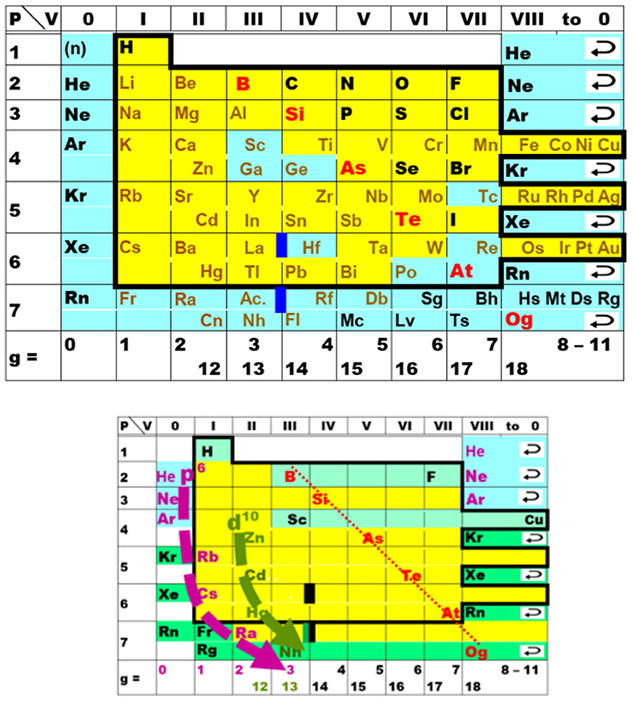
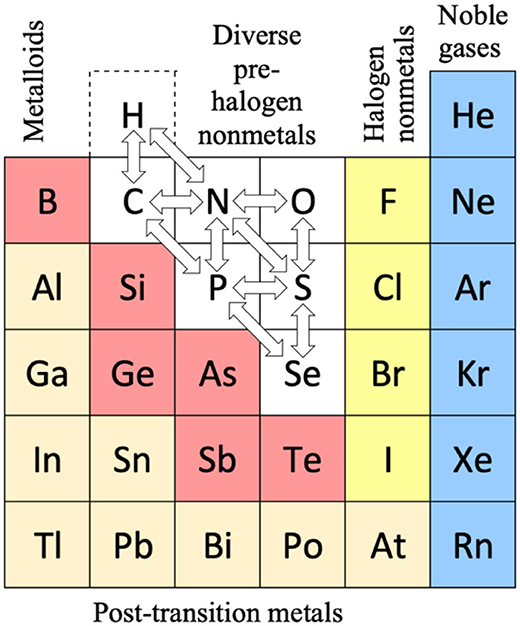
Thanks to René Vernon for the tip.
| Year: 2021 | PT id = 1183, Type = formulation data |
Crustal Abundance vs. Electronegativity
A chart by René Vernon of Elemental Abundance (g/kg log10) vs. Electronegativity, H to Bi.
René writes:
Below is a remarkable XY chart where x = electronegativity and y = crustal abundance (log10). It stops at the end of the s-process, at Bi. The abundance figures are from the CRC Hanbook of Physics and Chemistry (2016-2017).
I say remarkable as I had little idea what the chart would end up looking like when I started plotting the values.
As well as its coloured regions, I've marked out track lines for six of the main groups and one for group 3.
Observations
The rose-coloured arc on the left encompasses the pre-transition metals i.e. the alkali and alkaline earth metals and aluminium, followed by, in the orange rectangle, the rare earth metals. Opposite these regions, along the southern boundary of the green paddock, are the halogens.
In the pale yellow field sheltered by the pre-transition metals and the REM, are the 3d transition metals and, in the white corral, are 4d and 5d base transition metals. Opposite these regions, in the green paddock, are the core nonmetals H, C, N, O, P and S, with Se as an outlier.
Following in the grey blob are the post-transtion or poor metals, immediately adjacent to the bulk of the metalloids or poor nonmetals.
Finally, in the light blue patch, the noble metals are complemented by the noble gases frolicking in the open.
Abundance tends to decrease with increasing Z. Notable exceptions are Li, B, N and Si.
Curiosities
Comment
I was intrigued by the article referring to Ni and Ar, and the suggestion of Ar becoming somewhat anionic, albeit in extreme conditions (140 GPa, 1500 K)
References
Correlations
I wasn't looking for these but they at least exist as follows:
My references are:
Thus the abundance of the metals in the crust tends to fall with increasing EN.
An answer from L. Bruce Railsback, creator of the Earth Scientist's Periodic Table https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=142:
"I think I can answer one of the questions. 'Why is Si good at forming a planetary crust?' – because it's so bad at staying in the core. Silicon isn't sufficiently metallic to stay in the core. Even in the mantle and crust, it doesn't go into non-metal solids well: in cooling magmas, it's only a lesser member of the early-forming minerals (e.g., Mg2SiO4, forsterite, where it's outnumbered two to one). The mineral only of Si as a cation, SiO2 (quartz), is the LAST mineral to form as a magma cools, in essence the residuum of mineral-forming processes. At least some this thinking is at Bowen's Reaction Series and Igneous Rocks at http://railsback.org/FundamentalsIndex.html#Bowen"
Which Electronegativity Scale?
The wide variety of methods for deriving electronegativities tend to give results similar to one another.
| Year: 2021 | PT id = 1184, Type = formulation |
van Spronsen's Periodic Table: Update
René Vernon writes:
I'd never before realised how clever van Spronsen's 1969 Periodic Table is. It seems to be the ultimate logical electronic version, informed by the actual filling sequence in the gas phase atoms, rather than the idealised sequence.
So, H-He are over Li-Be.
Group 3 is Sc-Y-La-Ac since that is where the d-shell starts filling. In the rest of the d-block, there are (4+1) x d5 and (4+2) x d10.
The f-block starts with Ce, as that is where the f-shell starts filling. Notice the high degree of regularity with the 4 x f7 and the 4 x f14, and how Th is treated i.e. as 5f0.
After DIM's 8-column form, I believe the periodic family tree now looks like this:
Three split-blocks
1a. He over Ne; B-Al over Sc-Y-La-Ac = old school form
1b. H-He over F-Ne; ditto = e.g. Soddy 1914?, Kipp 1942?Two split blocks
2a. He over Ne; group 3 as Sc-Y-La-Ac = popular formOne split-block
3a. He over Ne; group 3 as Sc-Y-Lu-Lr = Lu form 3b. He over Be; group 3 as Sc-Y-La-La = forgotten van Spronsen formNo split blocks
4. He over Be = Janet equivalent
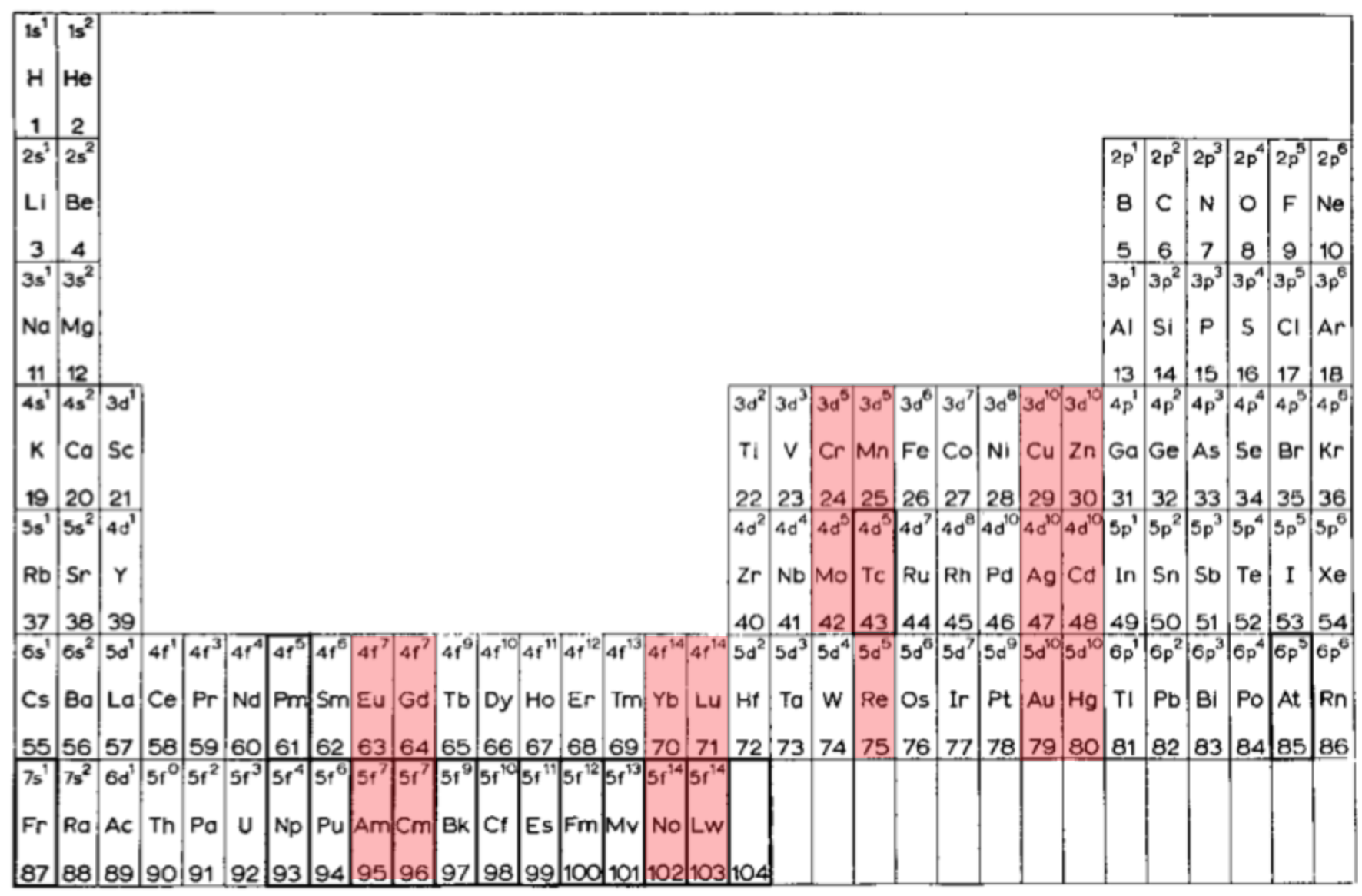
| Year: 2021 | PT id = 1186, Type = formulation |
Helix vs. Screw
Julio Antonio Gutierrez Samanez writes:
Until today, when they write about the work of Chancourtois and his telluric helix wound in a cylinder, still no one alludes to this other telluric helix wound in a cone or screw, the idea is the same: a rope that winds a geometric solid.
The first was devised in 1862, the other in 2008 (146 years later). But, there is a big epistemological difference. In the first, the elementary series presented was: 8, 8, 8, 8, 8 ..., etc., in the second it is: 2, 2, 8, 8, 18, 18, 32, 32. Furthermore, the division of conical radii is regulated by the function 2 (n ^ 2) = 2, 8, 18, 32...
Each binode has two spirals or two periods with the same number of elements, which correspond to the function 4 (n ^ 2). I don't think it is a discovery, it is just the conclusion of the contributions of Rydberg, Janet, and, of course, Chancourtois.
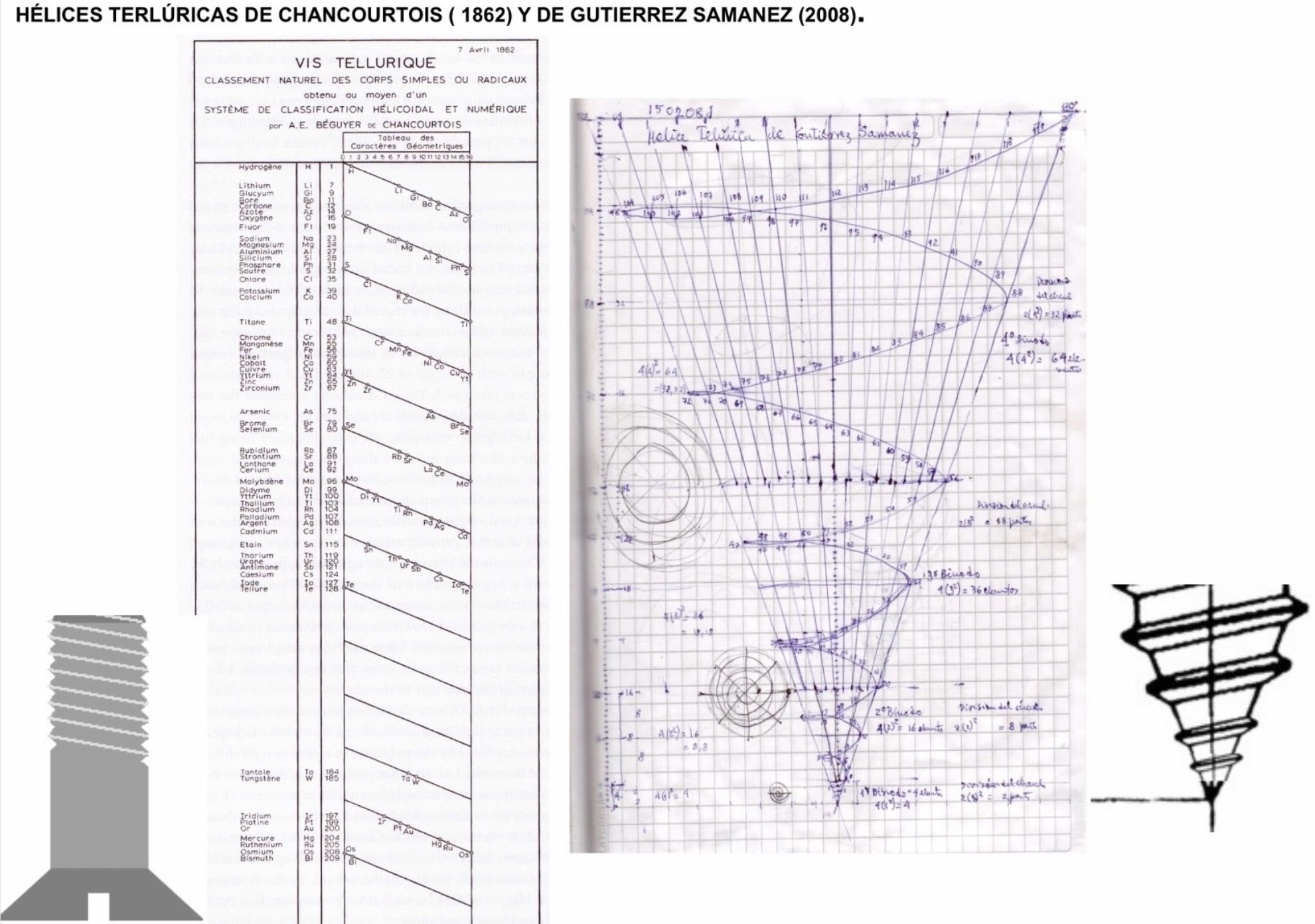
| Year: 2021 | PT id = 1190, Type = data |
Electronegativity: A Three-Part Wave
René Vernon points out that although there is a general trend in increasing electnegativity from Cs to F, there is actually an s-curve in the data.
Electronegativity across groups 1 to 18 appears to a show a three-part wave-like pattern.
There is a rise from group 1 to group 6, followed by a fall at group 7. I guess for group 7 that the EN for Mn is based on +2 and in this state Mn has five 3d electrons. The EN for Tc and Re are presumably based on +7, in which they notionally have underlying [Kr] and [Xe] cores.
There is rise from 7 to 8 (why?); a mesa from 8 to 11 (why?) that includes the PGM; and a fall at group 12. The fall may be influenced by group 12 having a full d shell; ditto group 13.
There is a rise from 13 to 18. Whereas in group 13 there is ionic chemistry in the form of the cations of Al to Tl this is not the case for C, Si, and Ge in group 14. Sn is reluctant to form a cation expect at pH < 1, and there is no Pb4+ cation.
The R2 value of 0.9739 is a best fit value for a second order polynomial. R2 for a straight line is 0.786
| Year: 2021 | PT id = 1203, Type = formulation |
Vernon's CSF Left-Step Periodic Table.
René Vernon's CSF Left-Step Periodic Table.
"I was prompted to switch to He-Be and [to develop a Janet type] left-step periodic table. I suggest it remediates concerns about H and He, and Lu in group 3.
Pros
Cons
| Year: 2021 | PT id = 1204, Type = formulation 3D |
Cubical-Stair Periodic Table
Sarthak Gupta's Cubical-Stair Periodic Table (Into a Whole New Dimension):
"Looking at the Modern periodic Table, somethings always bug you. The huge gap between the s and p-block when they should be side by side. The whole f-block floating around in air when it should be there in period 6 and 7. So why not experiment with shapes and structures and come up with something more space efficient?
"The cubical Periodic table paves the way taking the periodic table into a whole new dimension. Yes! from the 118 squares, we are going to transition into 67 cubes stacked onto each other like stairs."
The Cubical-Stair Periodic Table Explained:
Advantage over the Modern PT

| Year: 2021 | PT id = 1210, Type = formulation data |
Vernon's Eight-Fold Way Periodic Table
René Vernon suggests that the chemical elements can be grouped into eight classes: four metallic (Active, Transition, Post-Transition and Noble) and four non-metallic (Halogen, Biogen, Metalloid and Noble gas):
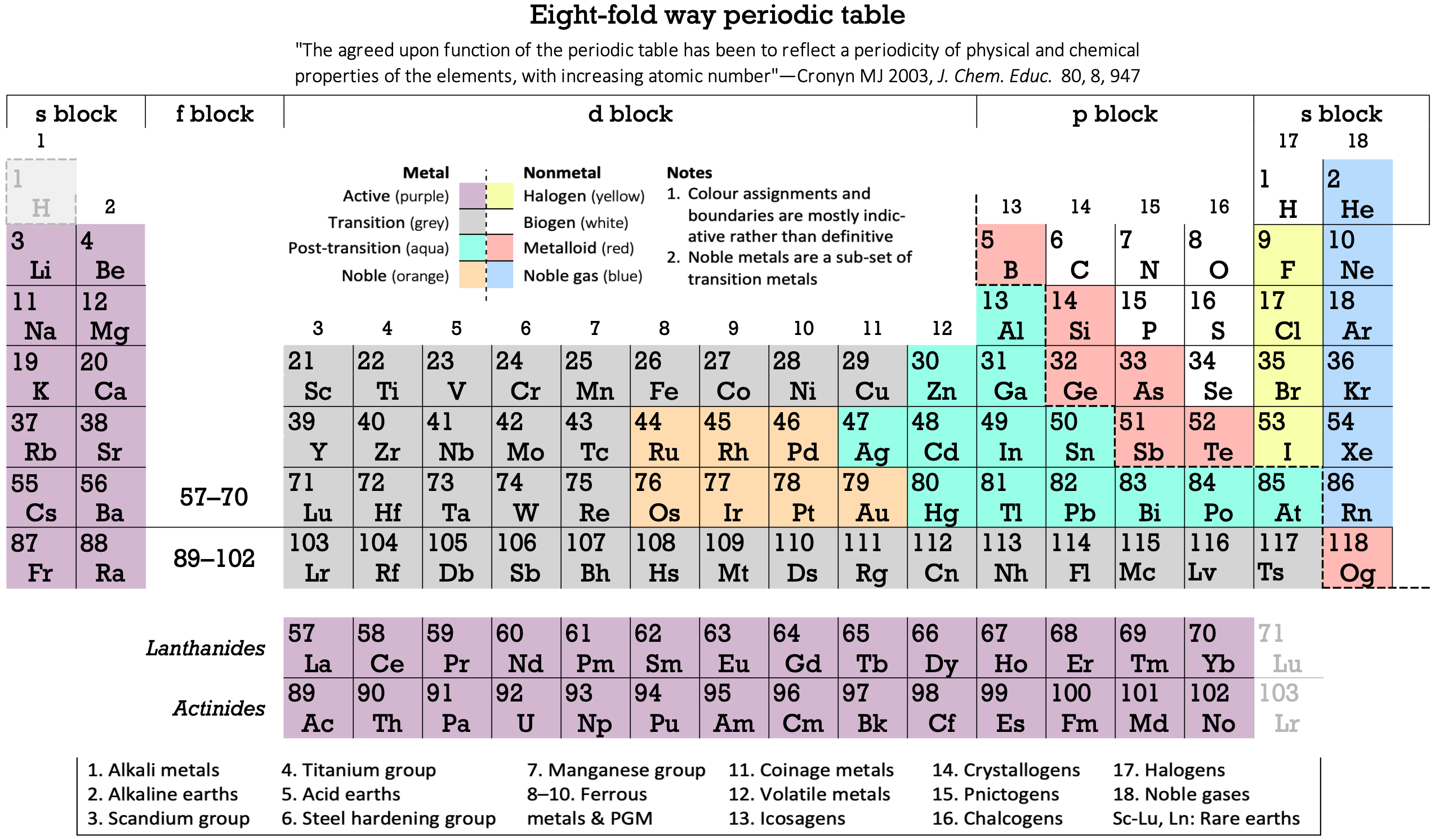
| Year: 2021 | PT id = 1244, Type = data |
Vernon's ABC Periodic Table
The Annotated Blocks & Categories (ABC) Periodic Table by René Vernon.
| Year: 2022 | PT id = 1330, Type = non-chem |
Phonics, Periodic Table of
A Periodic Table of Phonics from dyslexiclogic.com.
"The Periodic Table of Phonics allows us to see the relationship between the different sounds in the English language, but also how they relate to the most common spelling patterns. Children with dyslexia often have poor phonological awareness, meaning that they may struggle to perceive the difference between sounds that sound similar (eg. /th/ and /v/) or are articulated in a similar way (eg. /d/ and /t/)."

Thanks to René Vernon for the tip!
| Year: 2022 | PT id = 1231, Type = formulation 3D |
Kaleidocycle of the Periodic Table
Pablo Cassinello provides a "Three-dimensional figure to improve the didactics of the Periodic Table", a Kaleidocycle of the Periodic Table.
There is a full article about this dynamic, three dimensional formulation in Pablo's blog in Chem Ed Xchange, including instructions on how to make the object.
Pablo writes:
"A kaleidocycle has four different faces, each one made of a juxtaposition of rhombuses. By turning it you can easily choose one of the four faces. On these, four different pictures can be displayed. In this three-dimensional figure of the periodic table are the elements organized in four blocks according to their final electronic structure. It is intended that students with this playful figure actively participate in classes by rotating their kaleidocycle looking for the groups or elements that are being studied. The entire periodic table fits in one palm of their hands. It is also a didactic device because students only focus their attention on one block or group of elements from the entire Periodic Table. It can be a more entertaining, motivating and exciting way of learning about the subject of the Periodic Table."
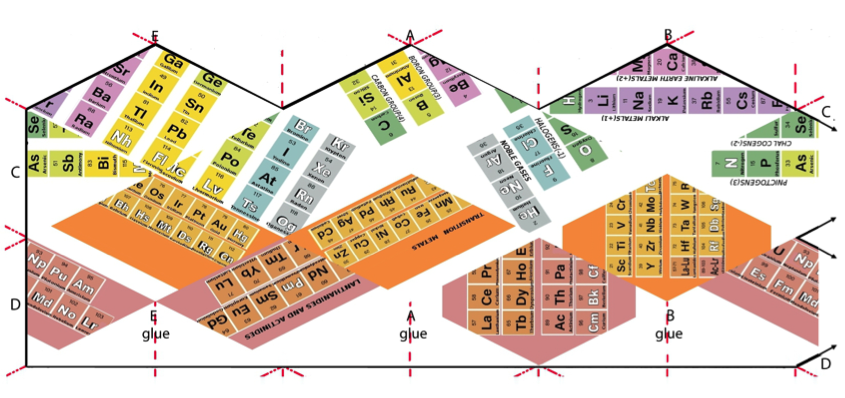
Thanks to René for the tip!
| Year: 2022 | PT id = 1241, Type = data |
Electronegativity Seamlessly Mapped Onto Various Formulations of The Periodic Table
A discussion on the Google Groups Periodic Table Discussion List, involving a René Vernon, Nawa Nagayasu & Julio Samanez (all contributors this database) lead to the development of the representations below, showing electronegativity seamlessly mapped onto a modified Left-Step Periodic Table:
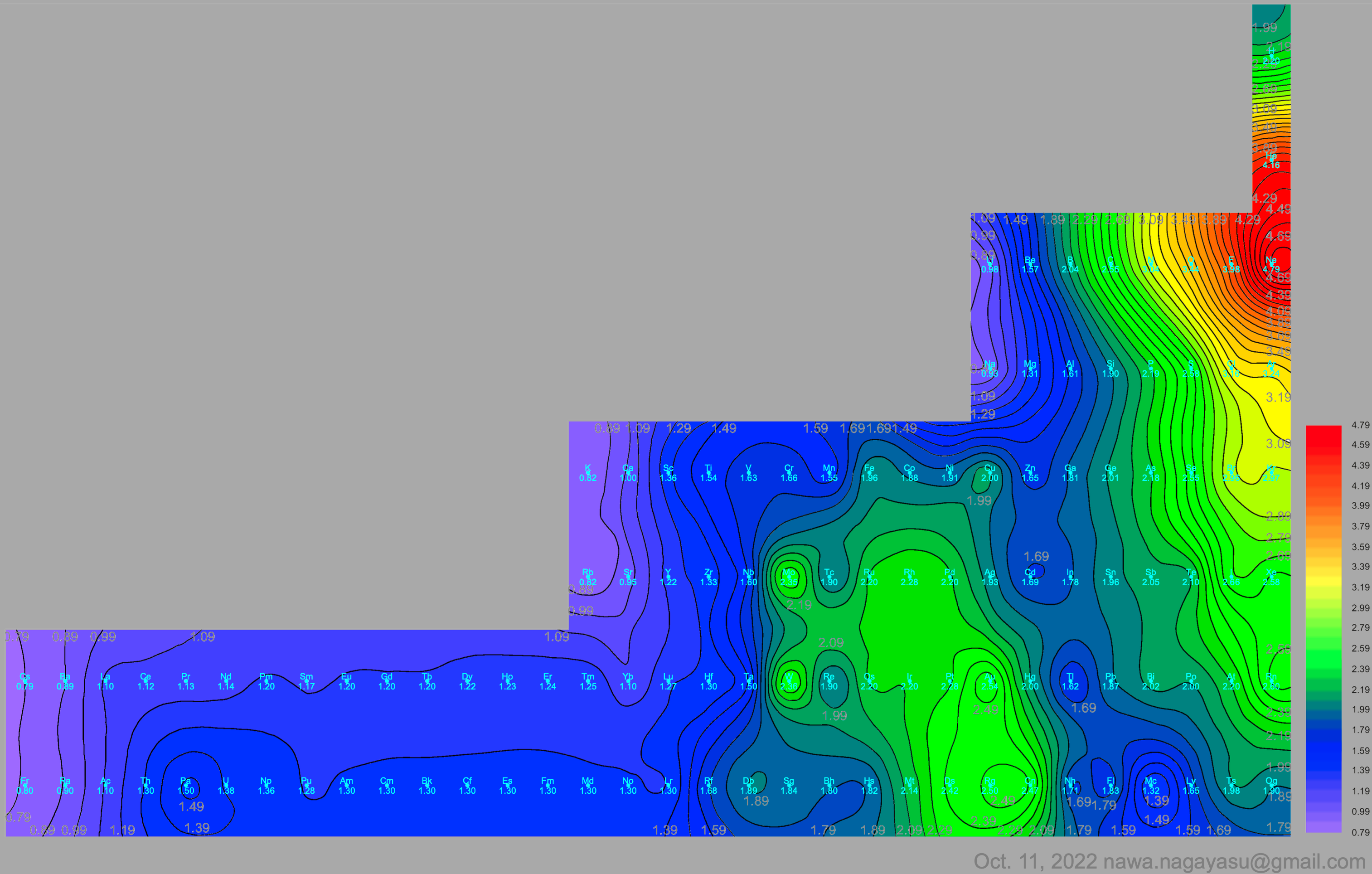
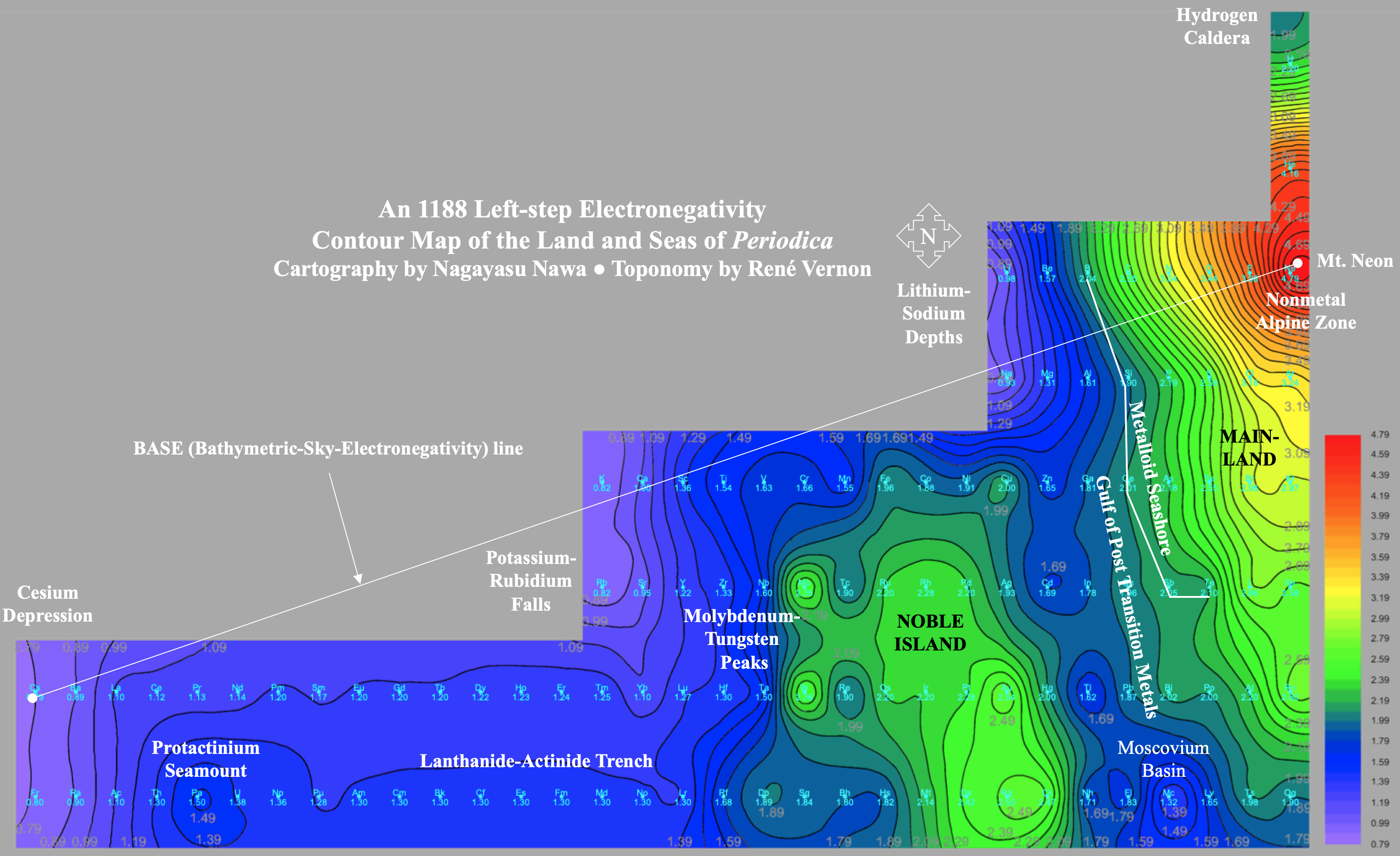
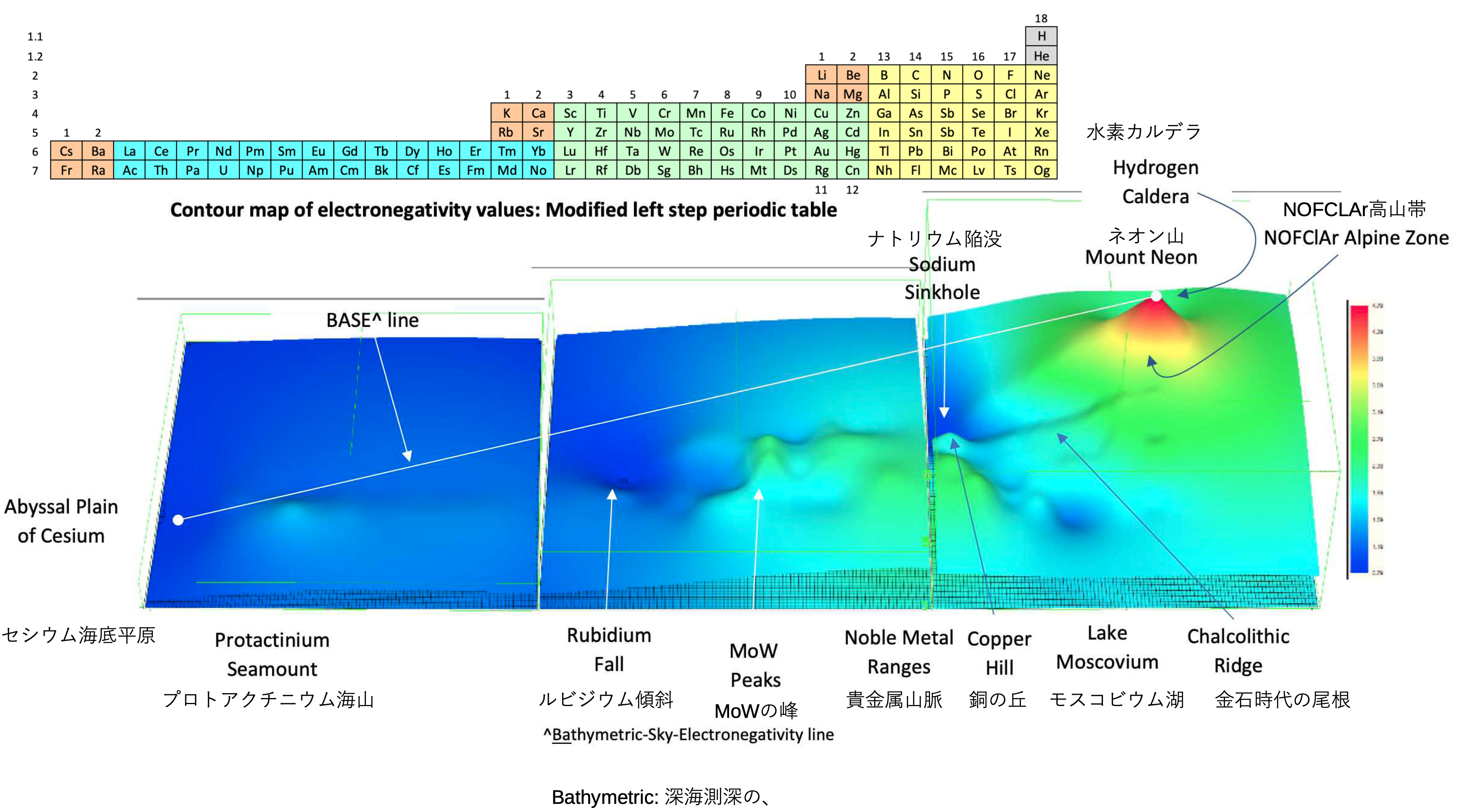
Nawa Nagayasu has mapped electronegativity to Mendeleeve's formulation:
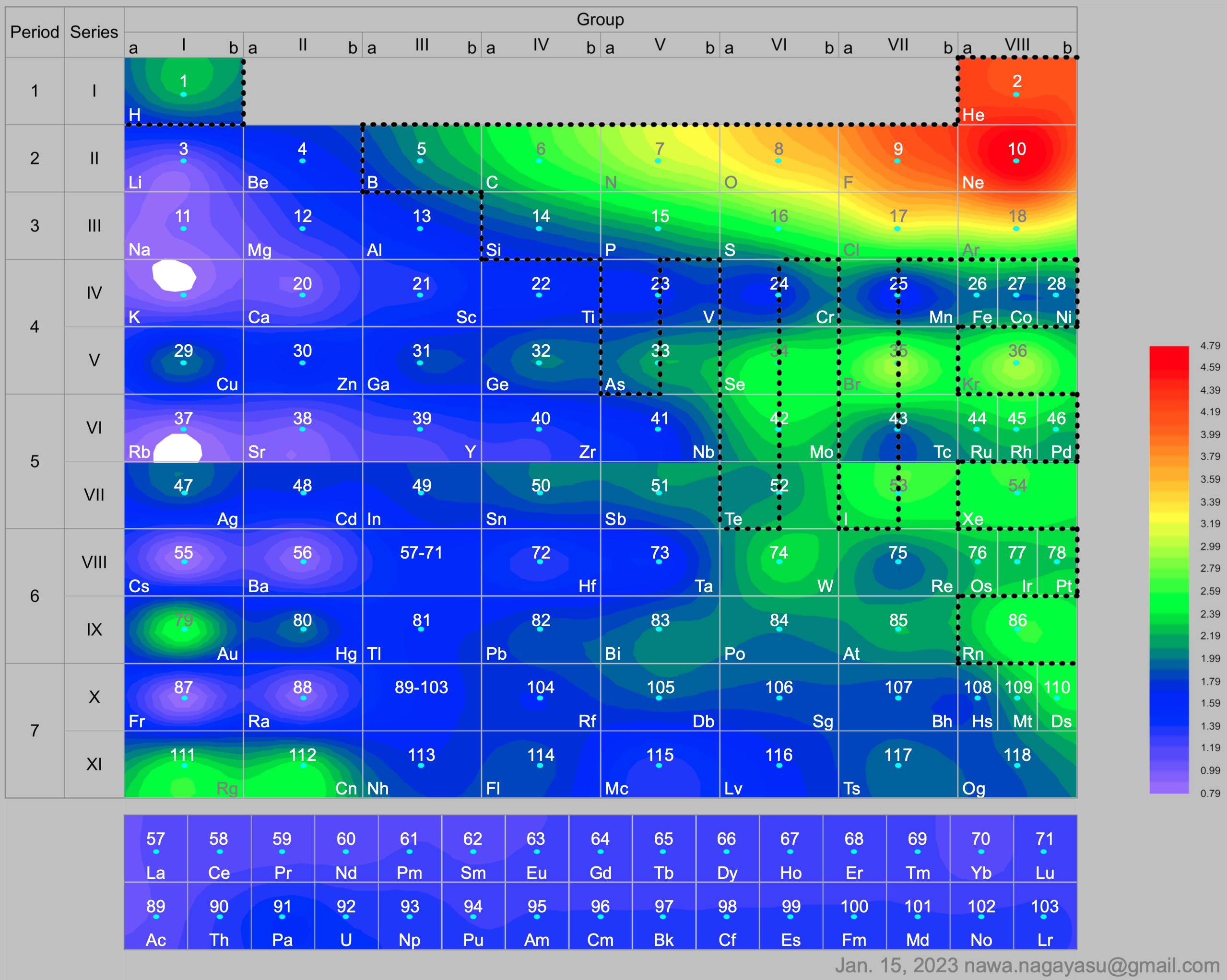
Nawa Nagayasu has mapped electronegativity onto other formulations, Julio's Binode Spiral:
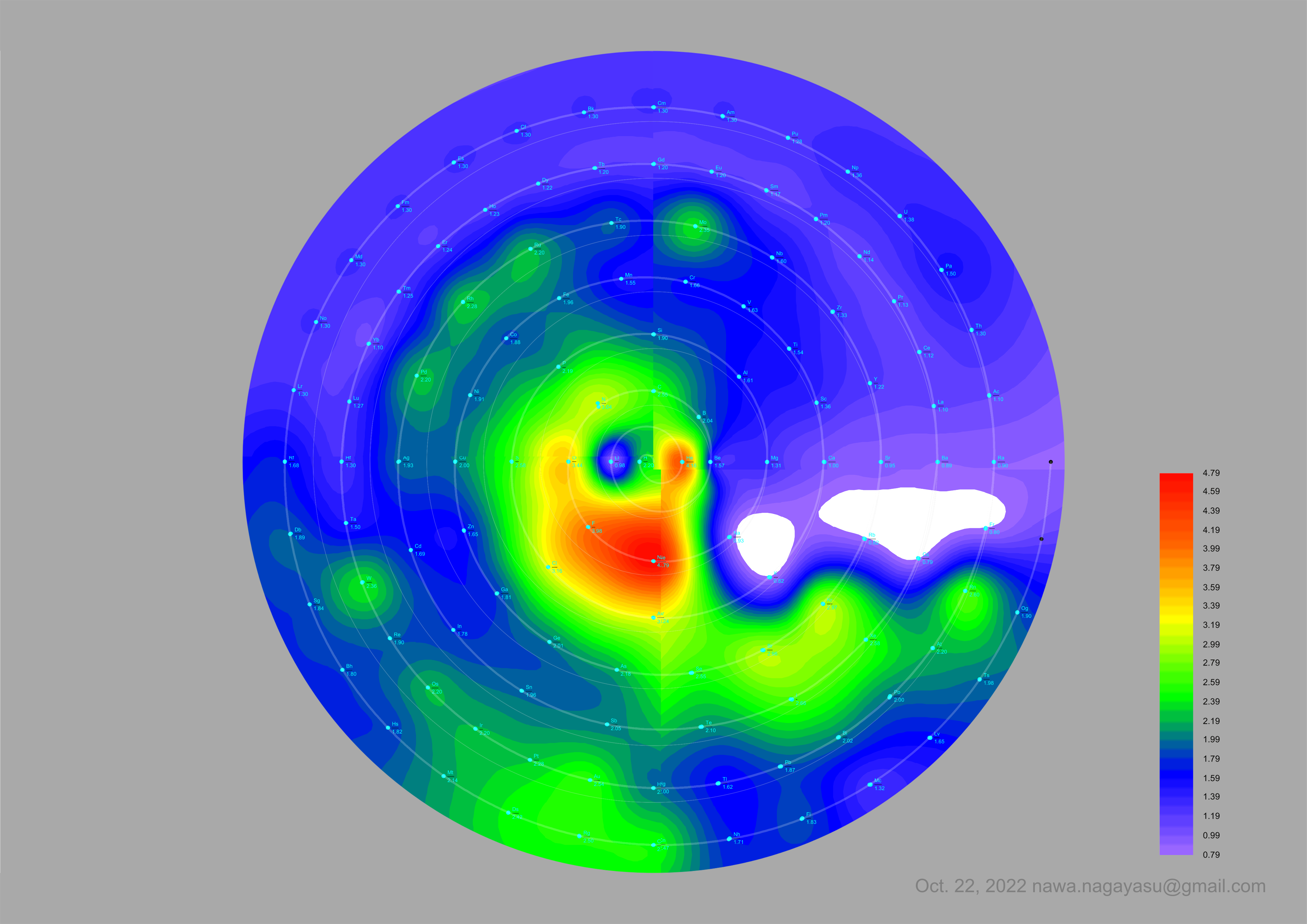
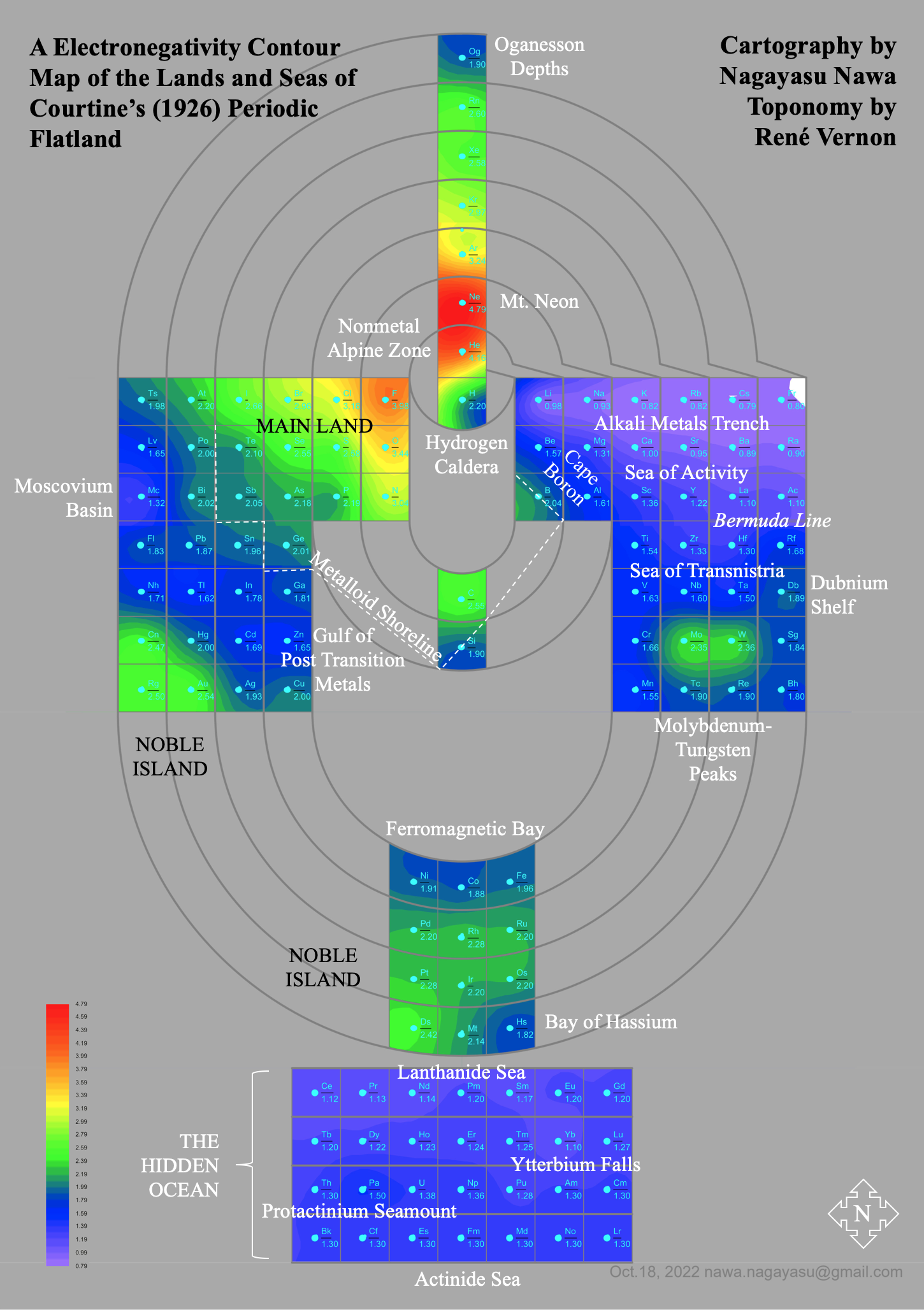
and the "conventional", short, medium and long forms of the periodic table with hydrogen above and between B & C which show the botom-right-to-top-left electronegativity trend:
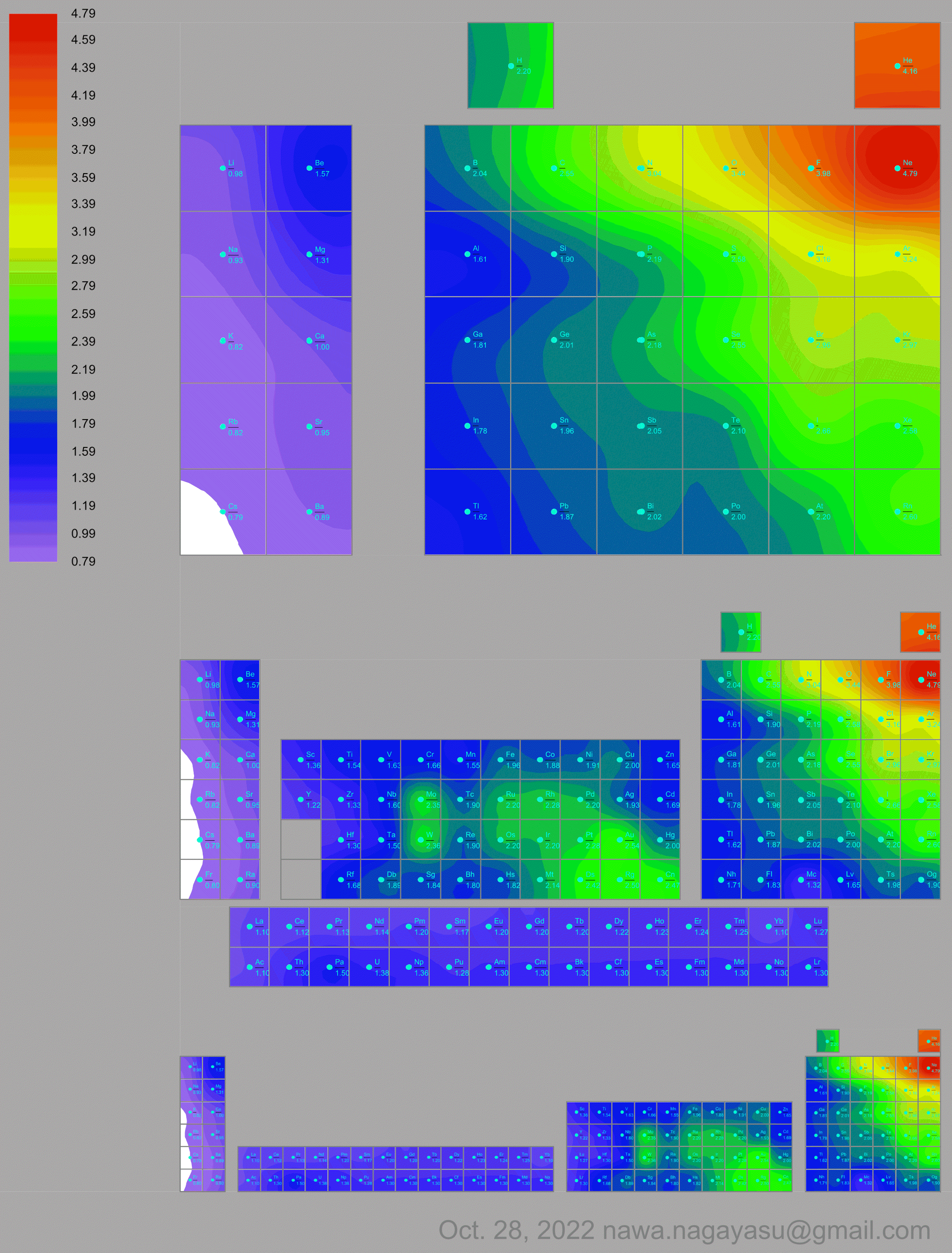
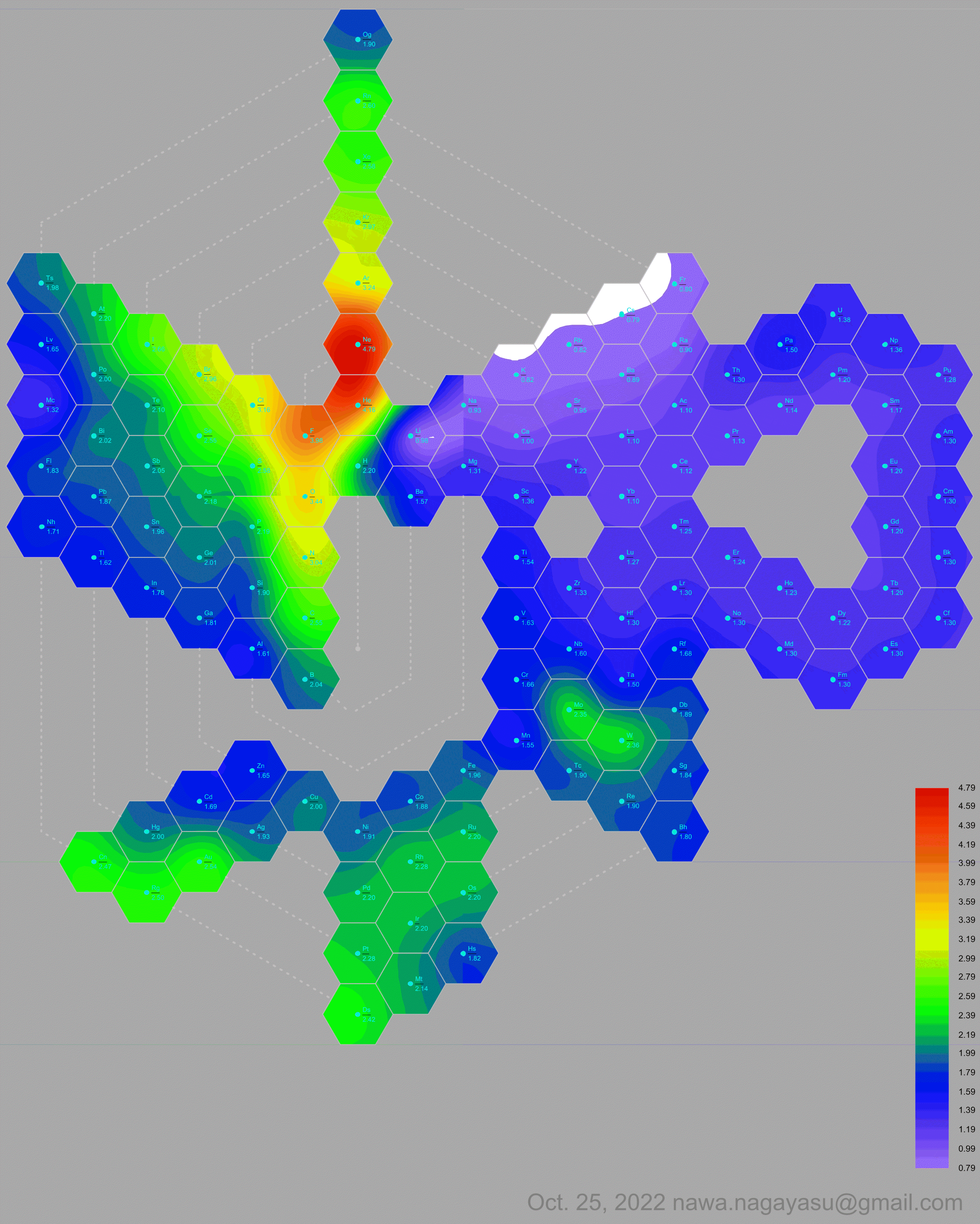
René Vernon's 777 Periodic Wedding Cake:
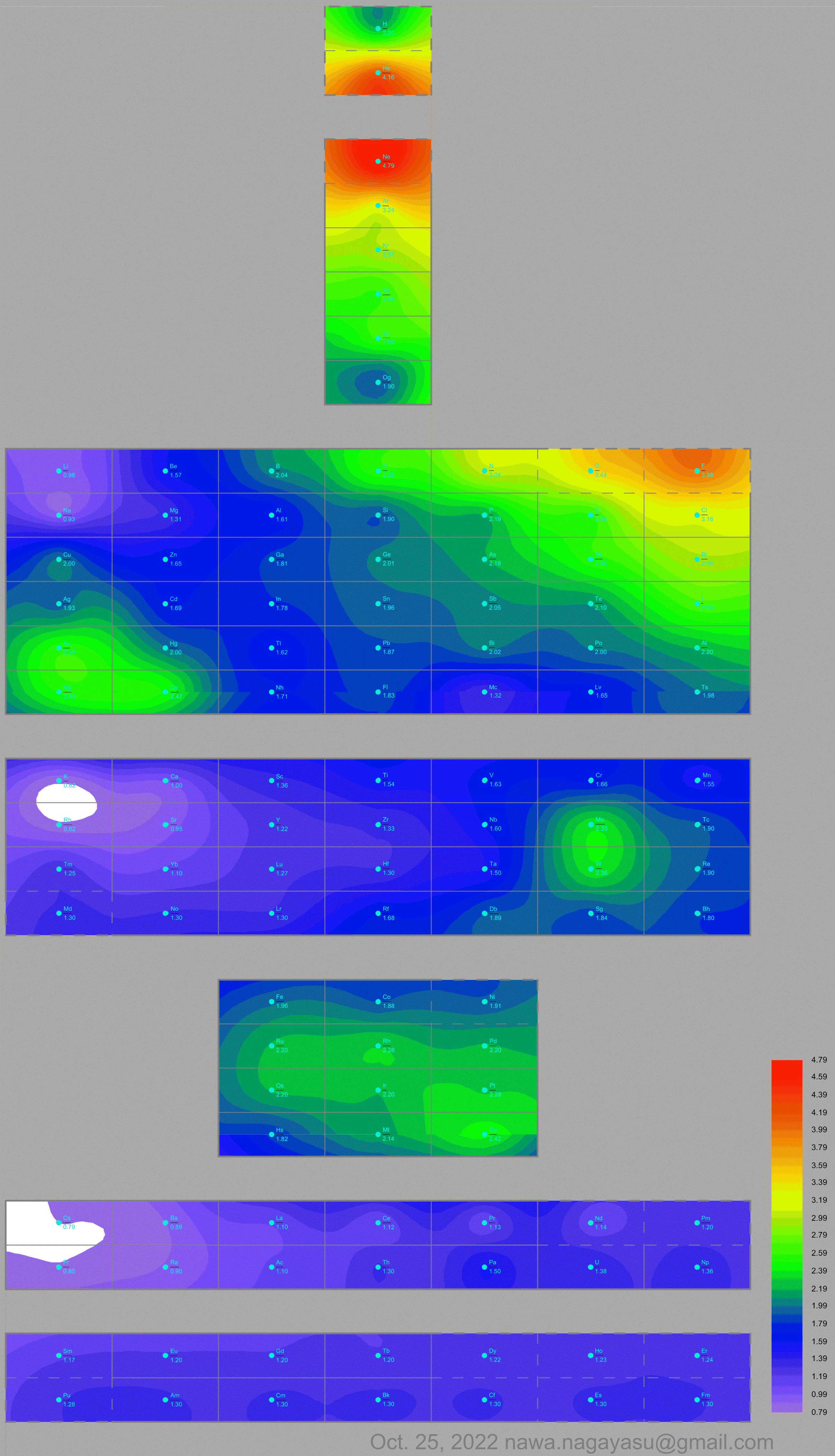
Valery Tsimmerman's ADOMAH formulation:
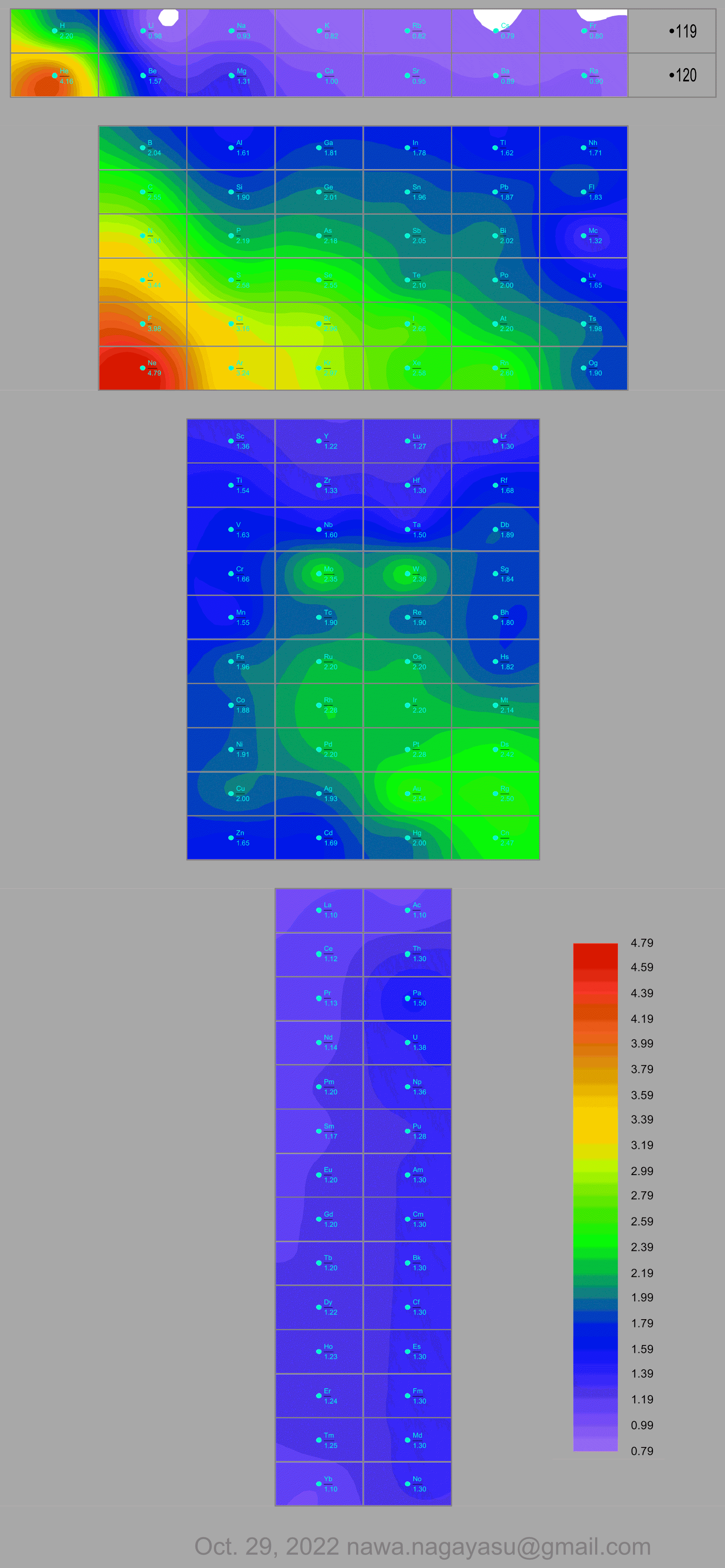
Valery Tsimmerman's ADOMAH tetrahedron (in a glass cube) formulation:
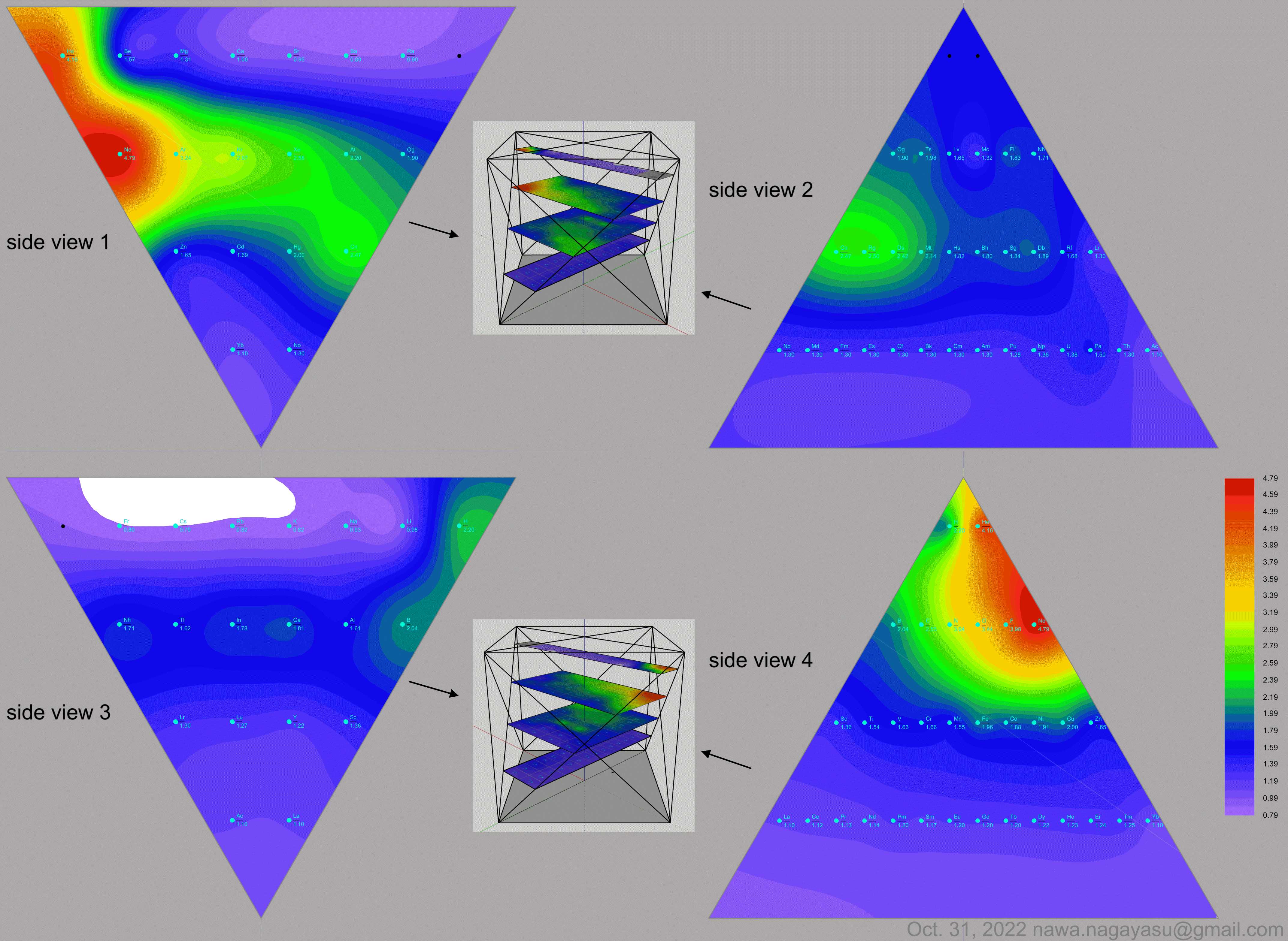
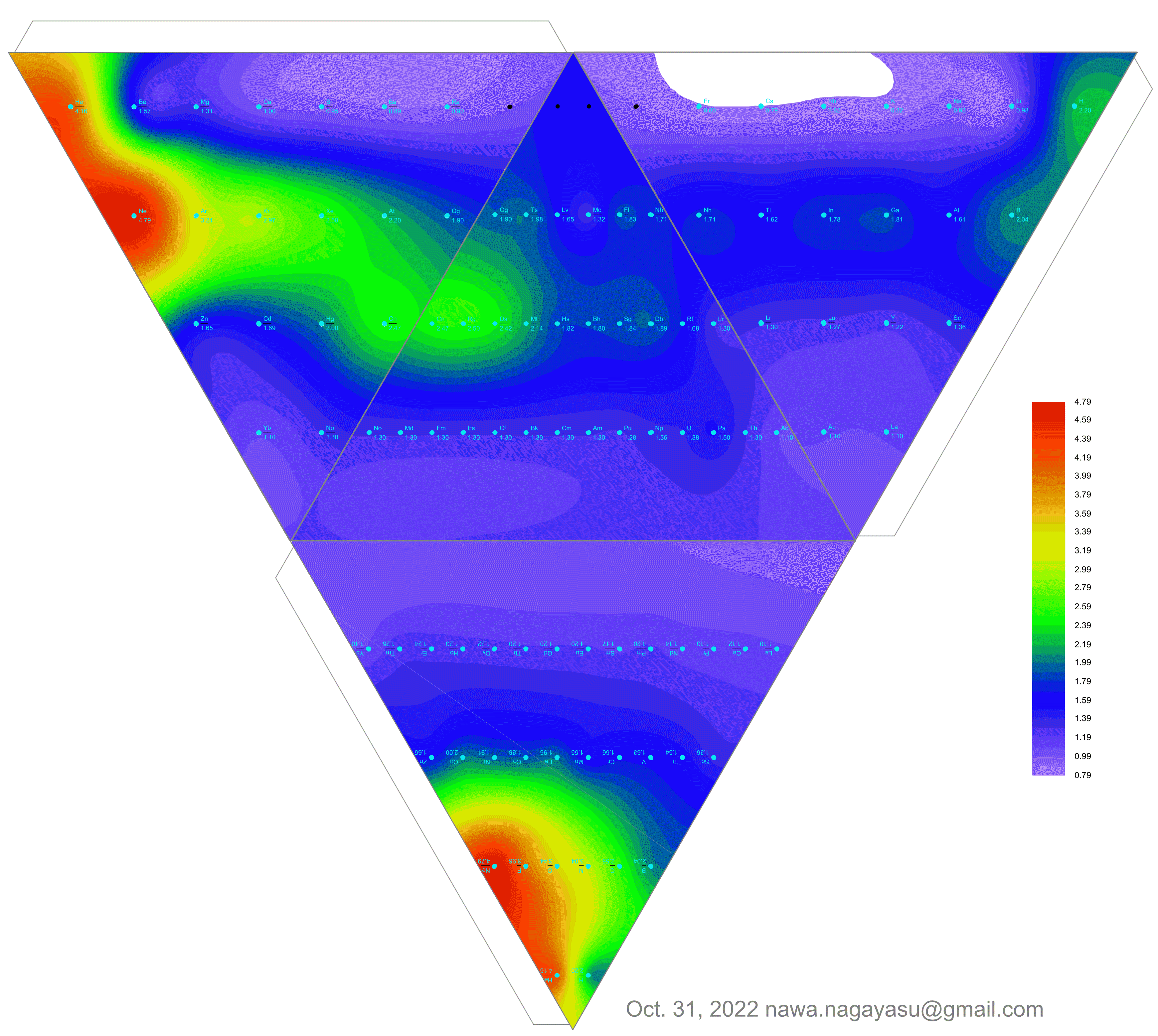
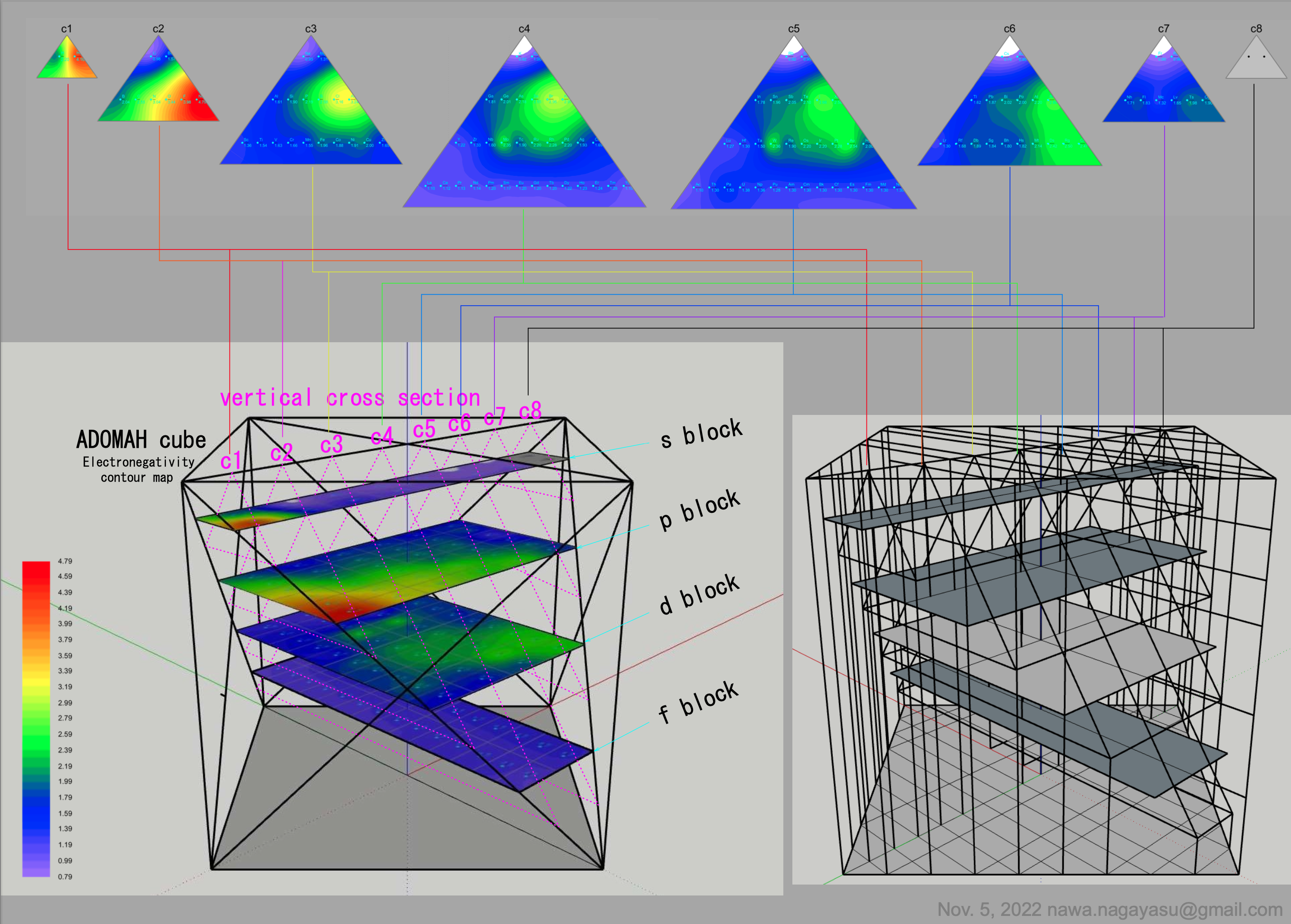
| Year: 2022 | PT id = 1250, Type = formulation |
Makeyev's Relativity Matrix of the Elements of Matter (MOEM)
By Alexander K. Makeyev, a member of the Moscow Society of Naturalists, multidisciplinary researcher and inventor; the author of Theory Relativity of Reality: The Relativity Matrix of the Elements of Matter (MOEM)
"Periodic table of elements vertical form. The periods of atomic levels of dense matter of matter correctly end at the element of the group of alkaline earth metals. Symbols of timelessness-non-existence / time-being are displayed in front of hydrogen; loose matter of vacuum and electrostatics and magnetism of ether; dense matter of neutronium (neutron nuclei of atoms and neutron stars)."
Literature:
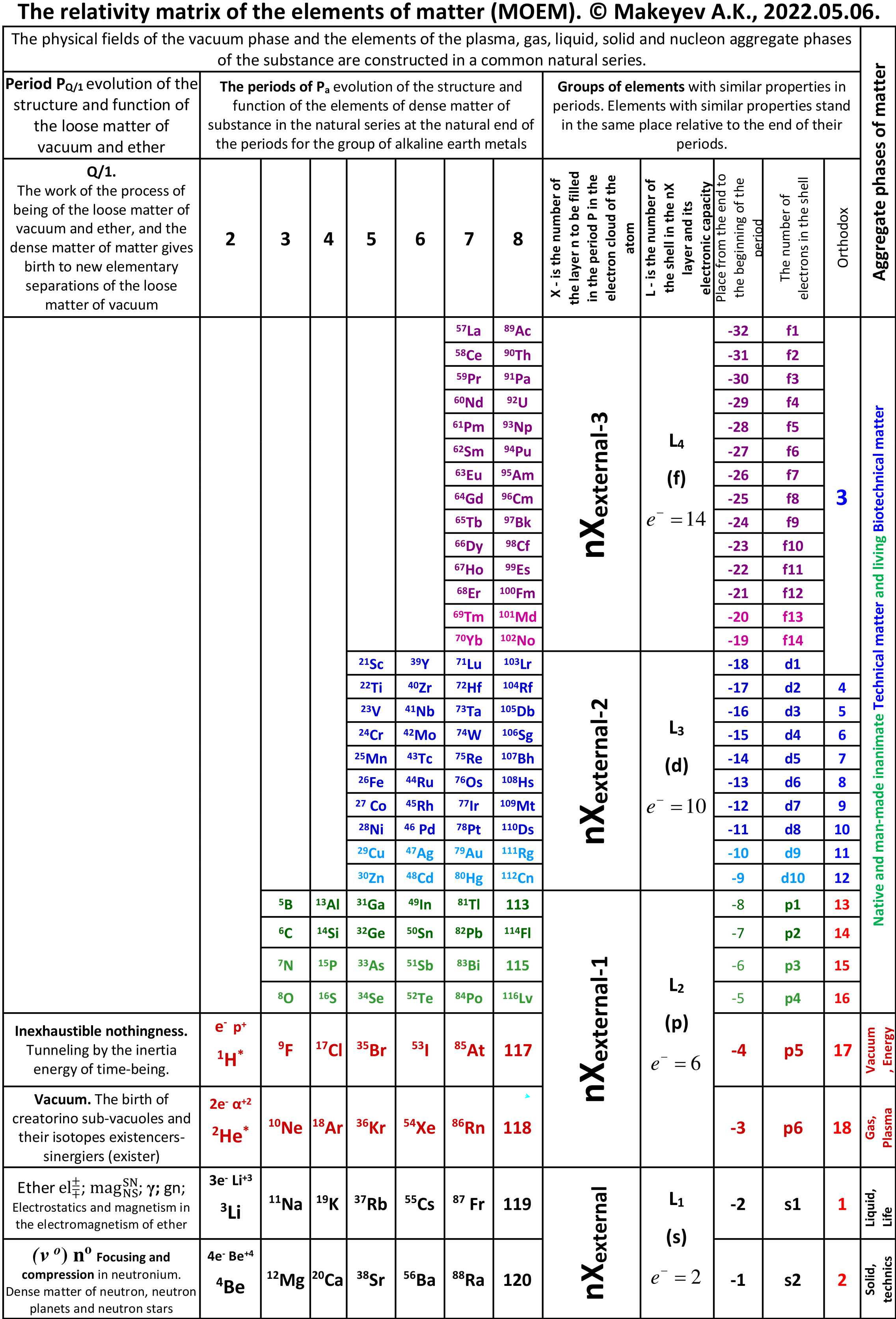
| Year: 2022 | PT id = 1252, Type = formulation |
Vernon's Yin Yang of The Periodic Table
René Vernon writes:
"I was prompted to [develop] this item after reading Eric Scerri's open access article: In Praise of Triads. The nub of Eric’s article is to argue for the LST on the grounds of triad regularity, first-row-anomaly regularity, and consistency with QM.
"It occurs to me that efforts to introduce more regularity to the PT invariably introduce new irregularities elsewhere. For example, as far as triad regularity goes, the left step table (LST) with He over Be introduces its own anomaly in that no element in period 1 (H, He) is part of a triad whereas this is not the case for all periods thereafter. In contrast, all periods of the traditional table have at least one element that is part of a triad.
"As far as QM goes, this tells us that there is a theoretical regularity to the PT. This regularity can be used to inform e.g. the LST, ADOMAH or Julio’s binodes. But such depictions do not reflect the factual relationships we see amongst the chemistry of the elements as well as is the case for the conventional form. A most obvious example is that the LST, while being more consistent with QM, disrupts the bottom-left to top-right trend in metallic to nonmetallic character seen in conventional tables.
(I must caveat that I'm referring to the chemistry of the elements in conditions regularly occurring on Earth. For example, it has been reported that under sufficiently high pressures the elements change their EN and electron configurations. If so, this suggests a need for a different table at high pressure.)
At this point, the chemistry educators enter the picture. They move the s-block to left. Helium is relocated over Ne on the basis of its nobility. (This could change if a few compounds of He were to be synthesized). Somewhat similarly, La was discovered well before Lu, so it ended up under Y, and most folks see no good reason to replace La with Lu. Sure, in the 32-column form, the result is a split d-block but the infrequency with which the 32-column form appears is such that most people are not bothered. The result is the conventional table.
Philosophically, while the n+l based LST might represent the most general form of table, the conventional table appears to currently represent the most pragmatic derivation for chemists and chemistry educators.
Since the PT is classification rather than theory, and there will thus always be hard cases at the boundaries, there will invariably be minor variations in the depiction of the conventional table with respect to e.g. the placement of H, the composition of group 3, or the length of the f block.
And there will always be tables such as MR that focus on particular perspectives of relationships among the the elements.
I’ve tried to sketch what's going in the attached image:"
| Year: 2022 | PT id = 1254, Type = formulation data |
99 Elements Sorted by Density & Electronegativity
René Vernon writes:
"A little while I ago I noticed that a scatter plot of EN (revised Pauling) and density values of the elements resulted in a nice distribution, as per the table below.
"According to Hein and Arena (2013) nonmetals have low densities and relatively high EN values; the table bears this out. Nonmetallic elements occupy the top left quadrant, where densities are low and EN values are relatively high. The other three quadrants are occupied by metals. Of course, some authors further divide the elements into metals, metalloids, and nonmetals although Odberg argues that anything not a metal is, on categorisation grounds, a nonmetal.
Note 25 says:
(a) Weighable amounts of the extremely radioactive elements At (element 85), Fr (87), and elements with an atomic number higher than Es (99), have not been prepared.
(b) The density values used for At and Fr are theoretical estimates.
(c) Bjerrum (1936) classified "heavy metals" as those metals with densities above 7 g/cm^3.
(d) Vernon (2013) specified a minimum electronegativity of 1.9 for the metalloids.
| Year: 2022 | PT id = 1257, Type = formulation data |
Tutti Frutti Periodic Table
René Vernon who writes:
"As a potentially powerful teaching and learning instrument: a Tutti Frutti periodic table. I feel younger folk [will] be delighted by it. The overlay of electron configurations and blocks was designed by a colleague of mine."
| Year: 2022 | PT id = 1259, Type = formulation |
Deming's 1923 Periodic Table, Updated by Vernon
René Vernon writes:
"Here is the 21st century version of Deming's 1923 formulation. Lacking elegance perhaps, but that’s messy chemistry for you. 25 columns wide rather than 18 or 32. Split s, p and d blocks. The connecting lines are based on four sources:
"I used [the term] frontier metals to refer to the post-transition metals, since the latter term has never applied well to Al. The frontier adjective comes from a line by Russell and Lee in which they refer to Bi and Po occupying frontier territory on the PT, adjacent to the nonmetals (2006, p. 419).
"As far as metalloids are concerned, Dingle nicely summarized their status: "With ‘no-doubt' metals on the far left of the table, and no-doubt non-metals on the far right…the gap between the two extremes is bridged first by the poor (post-transition) metals, and then by the metalloids— which, perhaps by the same token, might collectively be renamed the 'poor non-metals'." (2017, p. 101)
Ref: Dingle 2017, The Elements: An Encyclopedic Tour of the Periodic Table, Quad Books, Brighton Russell AM & Lee KL 2005, Structure-Property Relations in Nonferrous Metals, John Wiley & Sons, Hoboken, NJ
| Year: 2022 | PT id = 1266, Type = data |
Electrons, Periodic Table of
Brian Gregory's Periodic Table of Electrons. Brian writes:
"I like sand, purple, denim and fuchsia, color-coded by the differentiating electron."
| Year: 2022 | PT id = 1275, Type = formulation |
777 Periodic Wedding Cake
René Vernon's version of Courtine’s flat projection (1926) of his periodic tower, the 777 Periodic Wedding Cake:
| Year: 2022 | PT id = 1276, Type = formulation data review |
Periodic Table of Periodic Tables
René Vernon has collected the PT formulations in this PT database and classifed them as:
Short, Triangular, Medium, Long, Continious, Folding, Spatial & Unclassified
and tabulated them by date. The 'working document' is currently a word file.
| Year: 2023 | PT id = 1286, Type = formulation review |
Six Stages of The Convergence of The Periodic System
Bran, A.M., Stadler, P.F., Jost, J. et al. The six stages of the convergence of the periodic system to its final structure. Commun Chem 6, 87 (2023). https://doi.org/10.1038/s42004-023-00883-9
Abstract (abridged):
"We show, by analysing the space between 1800 and 2021, that the system has converged towards its current stable structure through six stages, respectively characterised by the finding of elements (1800–1826), the emergence of the core structure of the system (1826–1860), its organic chemistry bias (1860–1900) and its further stabilisation (1900–1948), World War 2 new chemistry (1948–1980) and the system final stabilisation (1980–)."
Periodic tables representative of each period in history. Families of similar elements (sets sharing colour) shown in each table summarise the patterns and do not necessarily imply continuity nor simultaneity of the families throughout the period:
| Year: 2023 | PT id = 1288, Type = formulation data |
Holistic View of Metals & Nonmetals: Exploded View
From Organising the metals and nonmetals: An update by René Vernon from the chemrxiv preprint server.
Rene writes:
Abstract: This paper updates my 2020 article, Organising the metals and nonmetals in which I advocated for parsing the periodic table into four kinds of metals and four of nonmetals. This framework is retained and updated, and augmented with some additional chemistry-related and philosophical observations.
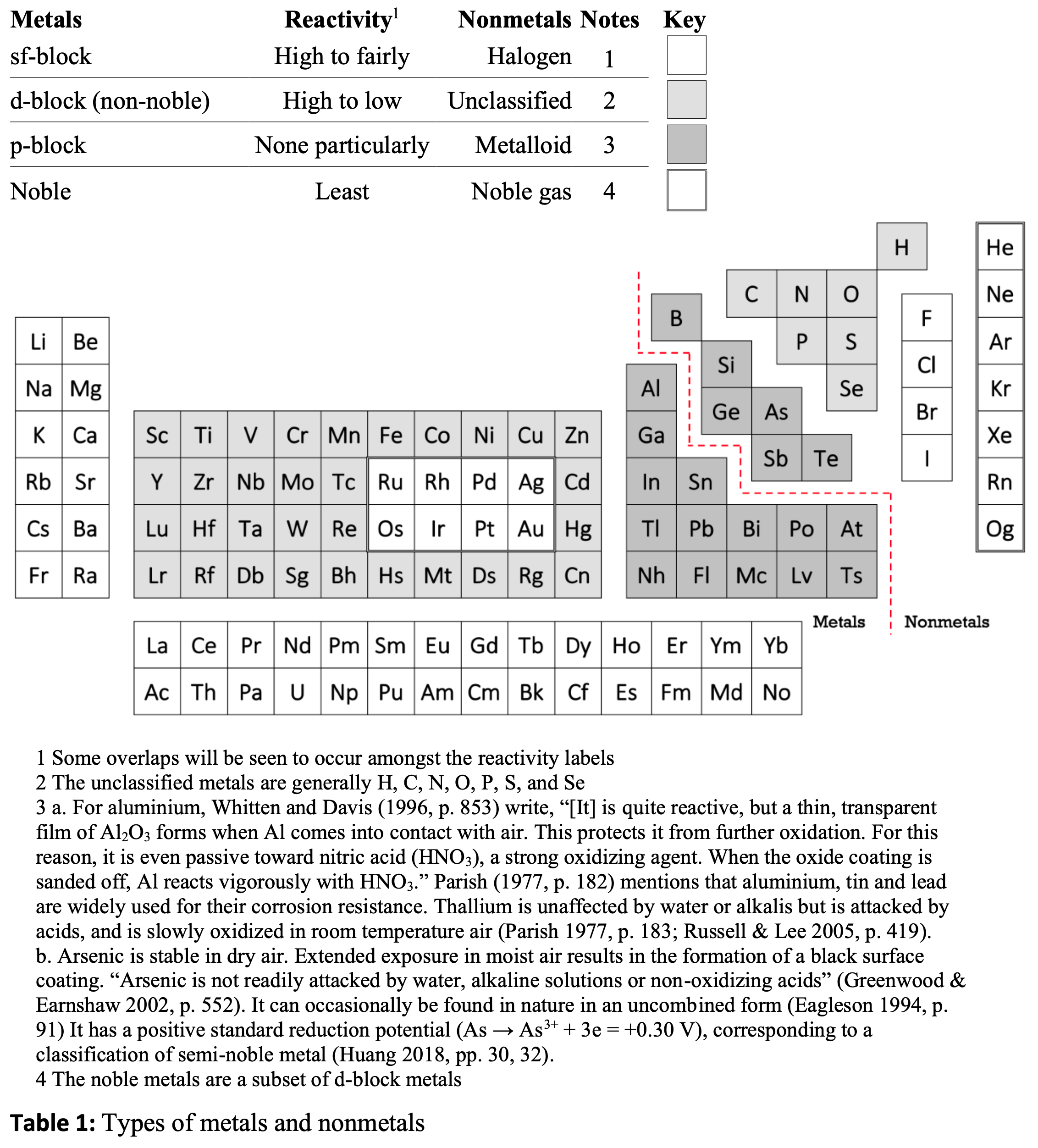
| Year: 2023 | PT id = 1289, Type = formulation data review |
Chemdex: Valence & Oxidation Number Trends
From Mark Winter's review paper Chemdex: quantification and distributions of valence numbers, oxidation numbers, coordination numbers, electron numbers, and covalent bond classes for the elements Dalton Trans., 2024,53, 493-511 https://doi.org/10.1039/D3DT03738J.
The images below show the Valence number (VN) and oxidation number (ON) proportions as percentages for the elements; and Periodic tables displaying valence number proportions (%). (There are few data for Pm and no data for Fr and elements beyond Es.)
The position of H and the group numbers are addressed in the paper.
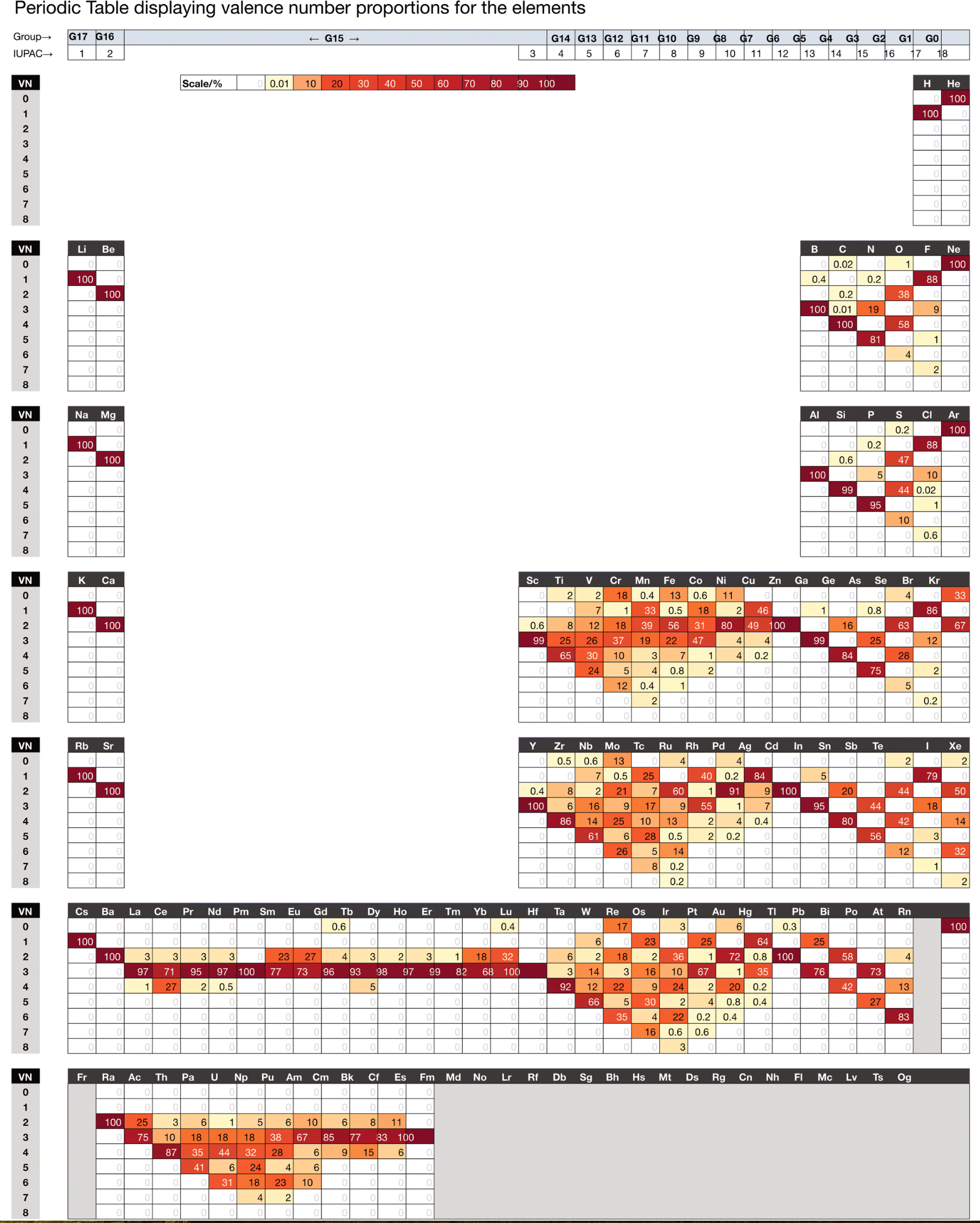
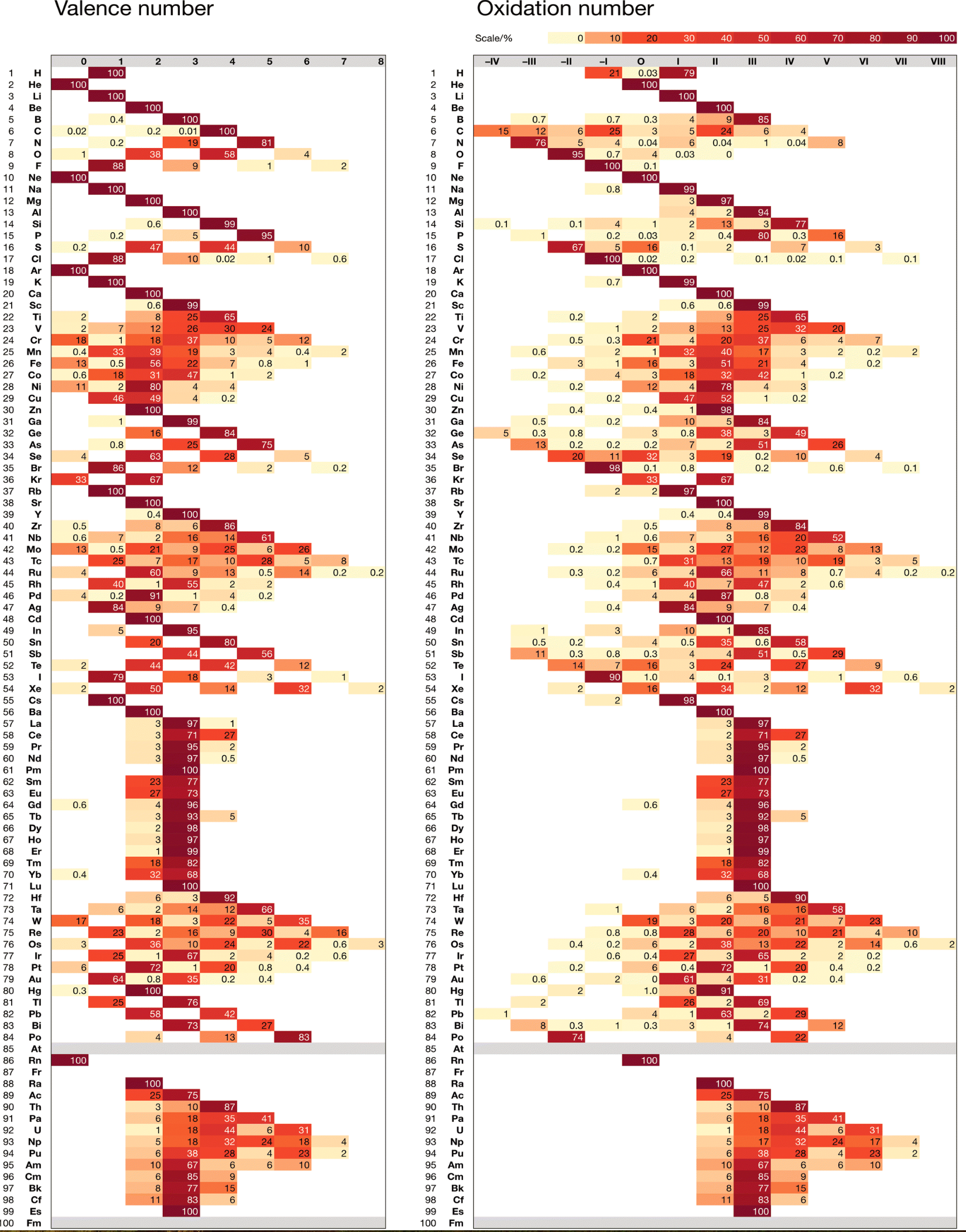
| Year: 2024 | PT id = 1299, Type = misc data |
Can I Lick It? Periodic Table
From Reddit, a "A cool guide to element lickability".
Mark Leach writes:
"I think I would colour calcium yellow as it bubbles hydrogen gas when added to water."
Thanks to René Vernon for the tip!
| Year: 2024 | PT id = 1303, Type = formulation |
Bilateral Symmetry in the Periodic Binodic Table
René Vernon, who developed these ideas, writes:
This table is adapted from the work of Gutiérrez-Samanez (2020), who discusses mathematising the chemical periodic system as a grid, which leads to a quadratic function or “binódica function” formed by pairs of periods or binodos (dyads).
The difference is that whereas Gutiérrez-Samanez showed the first pair of periods as H-He and Li-Be, this table shows the first period as e-n and H-He. Here, e is the electron and n is the neutron. Each pair of periods is shown pancake style rather than in a single row. The formula for the length of each paired period or binode is 2n2 = 2, 8, 16, 32.
The idea of paired periods has a long history; it seems to have originated with Werner in 1905.
According to Jensen:
"The temptation to read more into the shape of the table than is really there is almost overwhelming. Even someone as great as Werner was tempted (1905). Having postulated a missing element between H and He, he decided to perfect the symmetry of his table by guaranteeing that rows of differing length always occurred in pairs. Consequently, he further postulated a row of three missing elements lying above the H-X-He row."
Rydberg (1913, pp. 12–13) used a formula 4n^2 for the number of elements in the paired periods: 4, 16, 32, 64. This formula is also used by Gutiérrez-Samanez.
Paired periods were also used by Janet (1928), Saz (1931), Achimov (1946) and Baca Mendoza (1953).
References

| Year: 2024 | PT id = 1307, Type = formulation 3D spiral |
Cylindrical Periodic Table with Seven Vertical Columns
The Cylindrical Periodic Table with Seven Vertical Columns by Laith H. M. Al-ossmi, College of Engineering, University of Thi-Qar, Iraq; Thi-Qar University Pres. Read the full paper here.
Abstract: In this article, a new model of the periodic table in cylindrical form wrapped around its outer circumference is presented, departing from the traditional periodic table of elements adopted by the International Union of Pure and Applied Chemistry (IUPAC). The cylinder is designed to encompass seven periodic periods, with elements distributed throughout based on their atomic order. This design allows for six vertical columns on the surface of the cylinder to represent the distribution of elements.


| Year: 2024 | PT id = 1311, Type = formulation spiral |
Rodríguez Peña & García Guerra's Periodic Spiral of The Elements
Rodríguez Peña, M., García Guerra, J.Á. The periodic spiral of elements. Found Chem (2024). https://doi.org/10.1007/s10698-024-09510-4
Abstract There are 2 main problems with the current periodic table: artificial breaks from a given noble gas to the next alkali metal (along with the common protrusion of the "f" block) and hydrogen placed in the alkali group, although this gas also exhibits halogen properties. This paper proposes arranging chemical elements in a square spiral with hydrogen at the centre. This element is also above lithium but passes above fluorine to connect with helium, representing its dual alkali and halogen nature effectively. Then the spiral moves outwards in a counter-clockwise direction, avoiding artificial breaks and following the natural direction of reading for the "s" and "p" blocks elements placed at the bottom of the spiral. Furthermore, this proposed square spiral improves upon previous Janet's and Benfey's representations with a more regular shape to draw, an effective depiction of the dual nature of hydrogen, and easily identifiable orbital blocks without the need for protrusions.
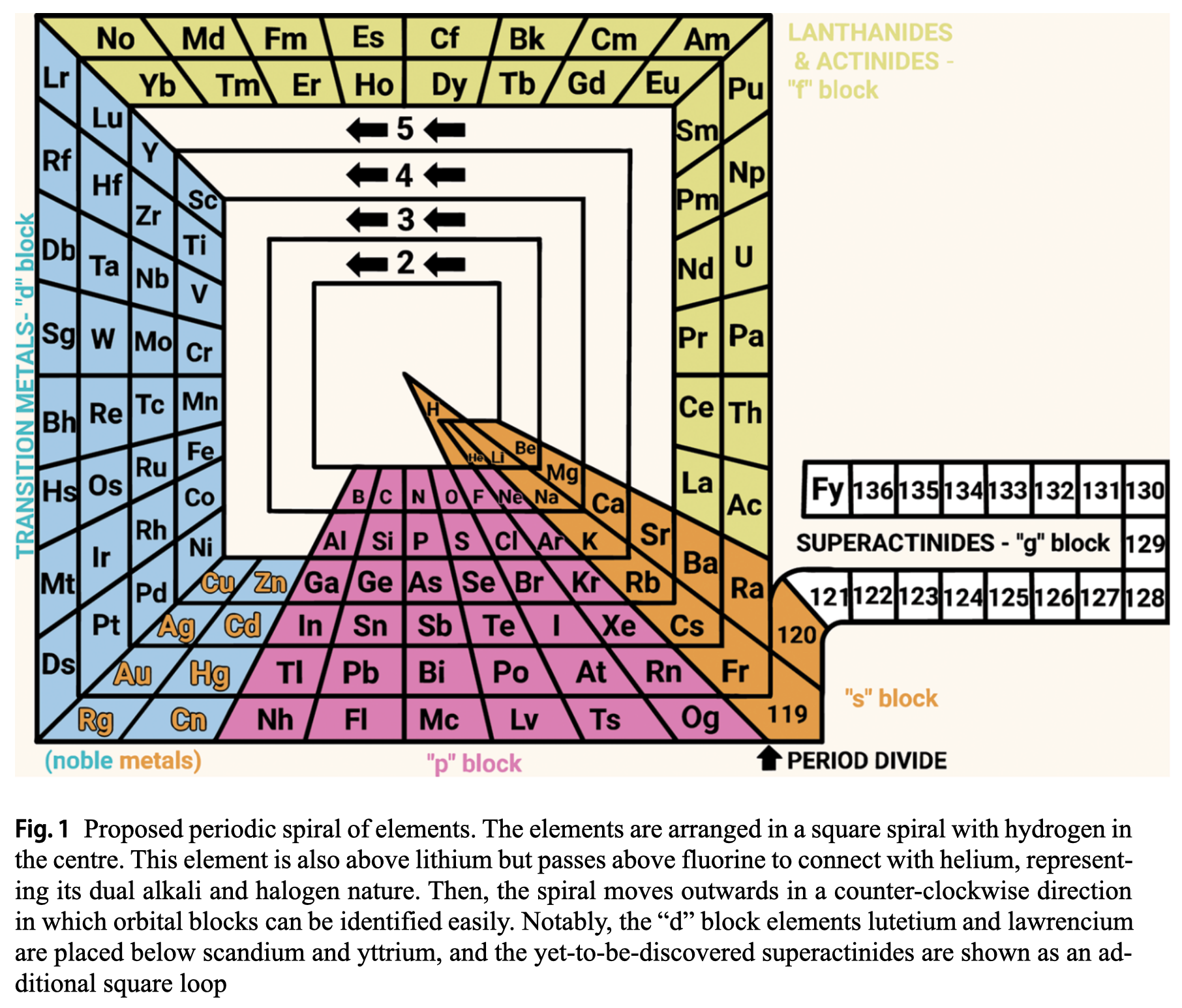
| Year: 2024 | PT id = 1315, Type = formulation 3D spiral |
Dufour’s Elementree in 2D by Vernon
A 2 dimension (flat) drawing of Dufour’s 3 dimensional Elementree by René Vernon.
René Vernon writes:
"I was surprised by its lack of symmetry in Dufour’s Elementree, caused by the awkward placement of He, and the assignment of H as floating above Li and Be. Hydrogen is as much subject to the periodic law as any other element. Without aligning H over Li, and He over Be, I am not sure that Elementree can be made symmetrical."

| Year: 2024 | PT id = 1319, Type = misc |
Elements of Fire & Light
René Vernon presents Elements of Fire and Light: The Majesty of The Periodic Table


| Year: 2025 | PT id = 1344, Type = formulation |
AI Periodic Table: WARNING!
From Chemistry World:
"While images of the periodic table generated by a range of different AIs appear plausible at first glance, such as this one by Microsoft’s Copilot, a closer look reveals multiple errors. Calls are being made to outlaw the use of AI for drawing chemical structures in journal publications"
Thanks to Prof. Richard Hull for the tip!
| Year: 2025 | PT id = 1345, Type = element |
Actinium: The Most Annoying Element for the Rare-Earth Industry?
Koen Binnemans writing on Linkedin:
Actinium: The Most Annoying Element for the Rare Earth Industry?
Rare-earth elements (REEs) are often accompanied by radioactive uranium and thorium in their ores. This is particularly problematic for monazite, which can contain more than 15 wt% thorium dioxide. The REE mineral steenstrupine, which occurs in large quantities in Greenland, is so rich in uranium that it can even be mined as a uranium ore. Due to strict safety regulations governing the handling of naturally occurring radioactive materials (NORM), the thorium content of REE ores poses major challenges for REE producers. Thorium is treated as radioactive waste, and its disposal can be very expensive.
It is important to realize that most radioactivity issues are not caused directly by thorium or uranium themselves, since their primary isotopes (thorium-232, uranium-235, and uranium-238) are all very long?lived, but by their radioactive daughter nuclides formed through decay chains.
Most of these radioactive daughter elements have chemical properties sufficiently different from those of the REEs that they can be removed using conventional hydrometallurgical techniques.
However, actinium presents a particular challenge because its chemical properties are similar to those of the REEs, especially lanthanum. The isotope actinium-227, formed by the decay of uranium-235, has a half-life of 22 years. During REE separation, actinium tends to follow lanthanum. In a solvent-extraction circuit, actinium accumulates in the SX battery along with the lanthanum stream.
Although the concentration of actinium is usually very low, lanthanum must be purified as thoroughly as possible, because one important application of lanthanum is in scintillator detectors for ionizing radiation (e.g. (LaBr3:Ce3+ or LaCl3:Ce3+). If lanthanum is contaminated with radioactive actinium, the resulting detector will exhibit significant background noise and therefore poor performance. Another issue is that REE concentrates produced at mining sites may exceed legal radioactivity limits for export to REE refineries due to the presence of actinium.
Therefore, REE processing companies have developed processes to remove actinium from REE concentrates or feed solutions for SX operations. Limited information is available in the scientific literature, but more can be found in patent documents. For instance, read the following patent of CARESTER, https://lnkd.in/evXsXzDB
It should also be noted that actinium has useful applications: actinium?225 is used in radiopharmaceuticals for the precision treatment of tumors. See: PANTERA
SOLVOMET R&I Centre SIM2 KU Leuven

Oak Ridge National Laboratory (Wikipedia) Blue Cerenkov radiation emitted by a sample of actinium-225
| Year: 2026 | PT id = 1365, Type = formulation |
Pairs and Squares Periodic Table
"Pairs and Squares" Periodic Table by Leonid A. Levin of Boston University
Leonid writes:
"All the tables [in the PT Database] share a common problem: their irregularity exceeds by far their periodicity.
"In my youth I was greatly bothered by this. Yet, taking advantage of a few patterns in atomic orbitals allows a complete elimination of all irregularities, putting all periods in a perfect pattern.This perfectly regular rendition would be much more comfortable, especially at one's first high school exposure to this Science symbol.I am attaching such "Pairs and Squares" rendition with a brief explanation."
"Read more in the full 'Pairs and Squares' paper, here."
| Year: 2026 | PT id = 1382, Type = formulation spiral |
Tableau_Périodique des Elements
La Corrésale writes:
"I have been developing an alternative geometric representation of the periodic table, and I would greatly appreciate your perspective on it.
"The model is a radial (hexagonal) construction with hydrogen and helium positioned at the center, and successive concentric layers corresponding to principal quantum numbers (n = 1-7). The layout follows the Madelung (Klechkowski) filling order, so that the s, p, d, and f blocks arise naturally from the geometric structure.
"Lanthanides and actinides are fully integrated into the continuous sequence rather than separated.
"My intention is not to replace the conventional rectangular table, but to offer a complementary representation that makes the electronic layering and energetic hierarchy more visually explicit. The structure effectively behaves like a “macro-atom,” with shells accumulating outward in a way that mirrors electronic shell construction. Certain periodic trends, such as radial progression and diagonal relationships, appear to become more intuitive in this configuration.
"I am aware that many alternative periodic tables exist, and I have tried to avoid unnecessary aesthetic distortion while preserving structural coherence and regular tiling. I would be very interested to know whether you see any pedagogical or conceptual merit in such a representation, or any structural issues I may have overlooked."
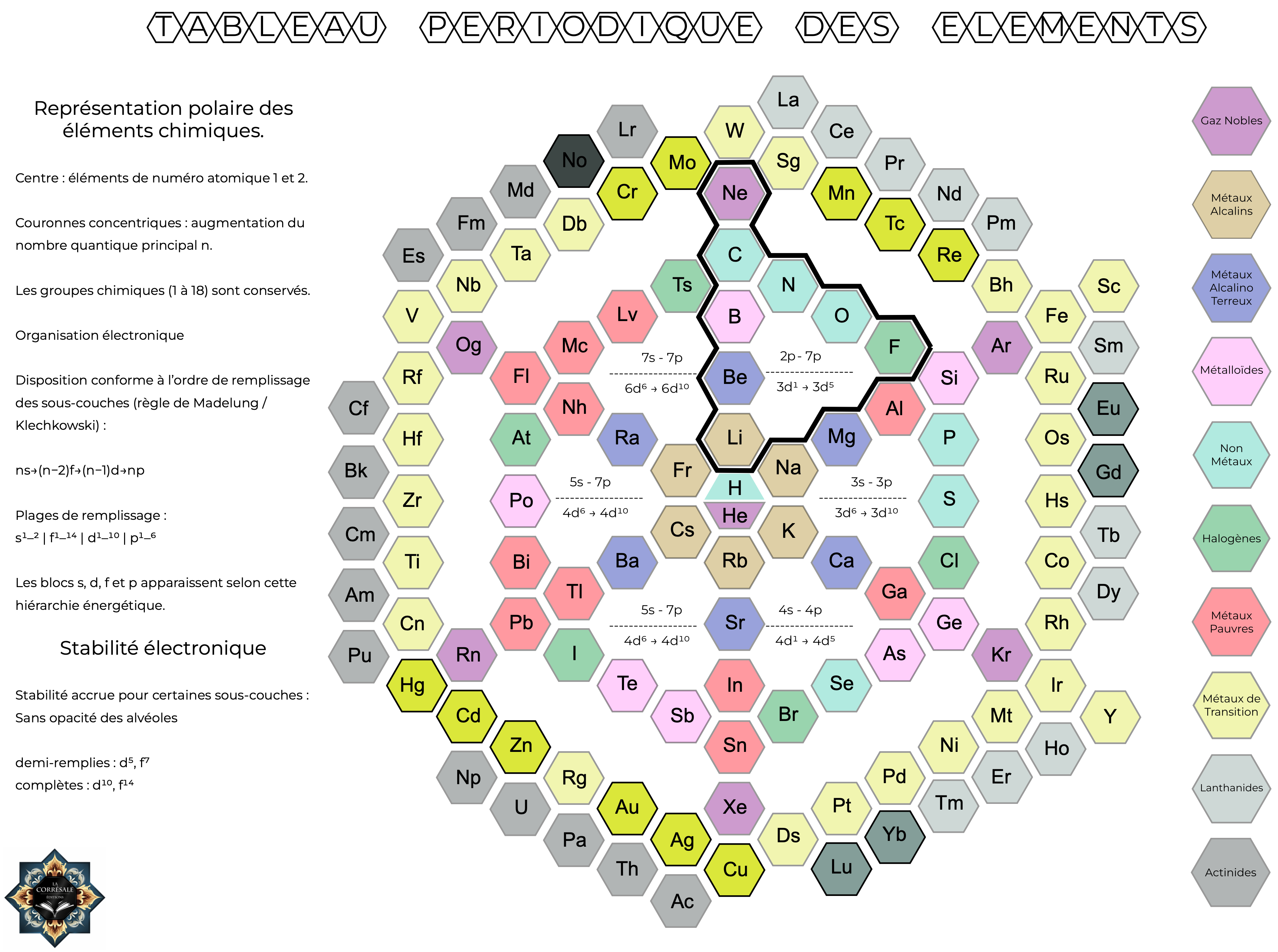
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.